Abstract
Objective
The aim of this work was to study the effect of 7 days of strict glycemic control with insulin on glomerular function and structure in streptozotocin (STZ)-diabetic rats.
Materials and Methods
Three groups of adult male Fischer rats were studied: controls (n = 15), diabetics (n = 15), and insulin-treated diabetics (n = 15). Diabetes was induced by treating the rats with STZ (55 mg/kg i.p.). One week after the induction of diabetes, blood glucose, protein excretion rate (PER), glomerular filtration rate (GFR), and renal plasma flow (RPF) were estimated in each group. Furthermore, morphometric analysis was performed to estimate the tuft volume and changes in mesangial matrix area. The results are expressed as the mean ± SEM.
Results
STZ diabetes caused significant increases in GFR (0.89 ± 0.1 to 1.21 ± 0.1 mL/min/100 g; p < 0.01) and RPF (1.78 ± 0.37 to 3.32 ± 0.6 mL/min/100 g; p < 0.05). Furthermore, the diabetic rats had higher glomerular volumes but mesangial matrix areas similar to controls. Insulin treatment prevented the increases in blood glucose (4.5 ± 0.2 mM), PER (66.1 ± 7.8 mg/day), GFR (0.6 ± 0.07 mL/min/100 g), and RPF (1.72 ± 0.36 mL/min/100 g), but did not prevent glomerular hypertrophy (21.7% increase), but induced mesangial matrix expansion (25% increase).
Conclusions
Insulin prevented the diabetes-induced hyperfiltration and proteinuria, but did not prevent glomerular growth, and induced mesangial expansion. Hyperglycemic episodes could be partly responsible for persistent glomerular growth and accelerated mesangial growth.
Key Words: Insulin, Glomerular function, Mesangial matrix area
Introduction
There is increasing evidence that glycemic disorders such as rapid daily glucose fluctuations might play an important role in diabetic complications [1]. Phlorizin treatment achieved normoglycemia and prevented proteinuria and hyperfiltration in short-term diabetes in rats [2]; however, it did not prevent glomerular hypertrophy, suggesting that functional and structural changes were independent of each other. Indeed, renal structural abnormalities are known to precede the development of proteinuria, hypertension, and reduced renal function in patients with type 1 and 2 diabetes, and progression to glomerular sclerosis can occur in the absence of proteinuria or changes in glomerular filtration or in renal plasma flow (RPF) [3]. Therefore, the objective of the current study was to investigate the effect of strict glycemic control with repeated insulin doses and its effect on glomerular function and morphology.
Materials and Methods
Animals
Forty-five male Fischer rats (8 weeks old; Harlan Laboratories Inc., Indianapolis, IN, USA) were placed in a room with an alternate 12-h light and dark cycle, at 22.3 ± 0.3°C and 31.2 ± 0.8% humidity. The rats had free access to water and standard rat chow (Special Diets Services, Witham, UK). All animals were cared for in accordance with the Guidelines for the Care and Use of Laboratory Animals, and all experimental protocols used in this study were approved by the Research Administration Committee at Kuwait University. The rats were divided into 3 groups of 15 each: controls, diabetic rats, and diabetic rats treated with insulin (DI).
Induction of Diabetes
Diabetes was induced by an intraperitoneal (i.p.) injection of 55 mg/kg of streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, USA). Controls were given (vehicle) 50 mM trisodium-citrate buffer (sodium citrate tribasic dehydrate; Sigma-Aldrich), pH 4.5. After STZ injection, the animals were placed individually in metabolic cages, and diabetes was confirmed 16 h later if pronounced glucosuria and polyuria had developed. Before the animals were sacrificed, their fasting blood glucose was measured using a glucometer (Glucotrend 2; Roche, Mannheim, Germany) in blood samples taken from the tail. In a group of 6 DI rats, the blood glucose was measured every 12 h until the day of sacrifice.
Insulin Treatment
DI rats were treated with insulin (Mixtard 30 HM; Novo Nordisk, Bagsvaerd, Denmark; 30 and 70%, 100 IU/mL), starting 24 h after STZ injection, until the day of sacrifice. The rats were given a subcutaneous injection of 1-2 units of insulin (dissolved in 0.1-0.3 mL of sterile 0.9% NaCl) at 8:00 p.m. just before feeding and 0.5-1 units at 8:00 a.m. if the blood glucose exceeded 8 mM, for a period of 6 days.
Protein Excretion Rate
The urine samples were collected over 24 h from rats placed in metabolic cages. The protein concentration was measured using a modified Lowry assay as previously described [2]. The protein excretion rate (PER) was calculated from the urinary protein concentration (mg/mL) and urine flow rate (mL/24 h).
Renal Hemodynamics
The RPF and glomerular filtration rate (GFR) were estimated by measuring the renal plasma clearances of para-aminohippuric acid (CPAH) and inulin (Cinulin), respectively. The procedure used for inulin clearance was the same as described earlier [2]. In addition, the clearance of PAH was simultaneously measured for each rat. Priming doses of PAH (8 mg/kg) and inulin (160 mg/kg) were given intravenously. PAH and inulin (mg/min) were then infused at a constant rate by adjusting their concentrations in the perfusate to 4.5 and 18 mg/mL, respectively. After a 30-min equilibration period, 4 urine samples were collected. At the mid-point of each urine collection period an arterial blood sample was collected in dry heparinized tubes. In diabetic rats the infusion rate was increased to compensate for their higher urine flow rates. Concentrations of inulin [4] and PAH [5] were measured in urine and plasma samples after precipitation with 10% trichloroacetic acid (Sigma-Aldrich). Clearance values were expressed in mL/min/100 g of initial body weight.
Morphometry
The right kidneys were removed 7 days after STZ (n = 6), vehicle treatment (n = 6), or insulin treatment (n = 6). The kidney wet and dry weights were measured. The dry weights of the right kidneys were taken after being placed in an oven at 105°C for 72 h. The left kidneys were perfusion fixed as described previously [2]. Four-micrometer-thick sections were cut using a microtome (MICROM HM 325; Thermo Scientific, Walldorf, Germany) and placed on APES-coated slides. The sections were stained with periodic acid-Schiff (PAS; Schiff's reagent; Sigma-Aldrich) for measurement of the total glomerular tuft area (GTA) and mesangial matrix area (MMA) [6]. A total of 15-20 glomeruli showing their tuft attached at the hilum were selected randomly from all renal cortical zones in each animal. The morphometry of glomeruli was performed with a CAS 200 cell analysis system (Becton & Dickinson Image Cytometry Systems, Franklin Lakes, NJ, USA). The contour of the tuft midsection was manually traced, and the total GTA (mm2) was calculated. The glomerular tuft volume (GTV) was estimated from:
GTV = b/k × (GA) 3/2,
where b = 1.38, a shape coefficient for a sphere, and k = 1.1, a size distribution coefficient [7]. The MMA in each glomerular tuft was measured using a threshold method that selectively highlighted all PAS-positive areas within the tuft as previously described [2].
Statistics
All the data were analyzed by one-way ANOVA to establish differences between groups and are expressed as the mean ± SEM. When an F value obtained from ANOVA indicated that the means of 2 populations were significantly different, comparisons between the 2 groups were further tested using an unpaired 2-tailed Student t test for equal or unequal variances according to the equality of variance test. The software used was SPSS 11 for Windows. For all statistical tests, a p value <0.05 was considered significant.
Results
Effects of Insulin Treatment on Body Weight, Blood Glucose, Urine Flow, Protein Excretion, and Kidney Weight
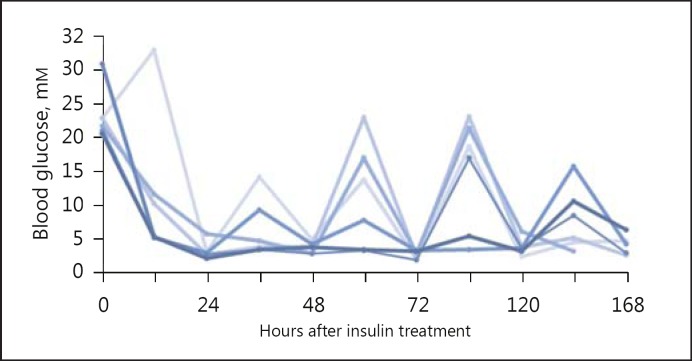
Overt diabetes (blood glucose >16 mM) developed within 16 h after STZ injection, and the 7-day treatment with insulin successfully normalized the blood glucose level to 4.5 + 0.2 mM, which was not significantly different from the controls (5.6 + 0.3 mM; p = 0.1; Table 1); however, the insulin-treated group showed large fluctuations in blood glucose levels (Fig. 1). Insulin treatment prevented the diabetes-induced body weight loss (Table 1) and the increase in urinary PER (66.1 ± 7.8 vs. 48.9 ± 5.8 mg/day in controls; Table 1). The urine flow rate was lower than that in untreated diabetic rats (15.6 ± 2.1 vs. 63.4 ± 6.4 mL/day; p < 0.001). However, insulin did not totally abolish diabetic diuresis since the urine flow rate was still significantly higher than in controls (p < 0.01; Fig. 1). In addition, insulin treatment prevented the diabetes-induced increase in percentages of kidney wet and dry weights to initial body weight (Table 1). Normoglycemia achieved by replacing insulin prevented the whole kidney hypertrophy that occurs at 1 week of diabetes.
Table 1.
Body and kidney weights and blood and urinary changes in control, diabetic, and DI rats
| C (n = 14) | D (n = 15) | DI (n = 10) | |
| Bwt0, g | 196.5 ± 3.2 | 192 ± 2.0 | 217.6 ± 4.5* |
| Bwtf, g | 208 ± 6.5 | 177.8 ± 4.9# | 210.9 ± 5.4 |
| BG, mmol/L | 5.6 ± 0.3 | 27.7 ± 1.4*** | 4.5 ± 0.2+++ |
| V, mL/24 h | 6.5 ± 1.04 | 63.4 ± 6.4*** | 15.6 ± 2.1**, +++ |
| PER, mg/24 h | 48.9 ± 5.8 | 121.1 ± 11.2*** | 66.1 ± 7.8+++ |
| Wet wt/Bwt0 | 0.39 ± 0.01 | 0.43 ± 0.01* | 0.39 ± 0.02 |
| Dry wt/Bwt0 | 0.085 ± 0.003 | 0.095 ± 0.001* | 0.085 ± 0.003+ |
Bwt0 = Initial body weight; Bwtf = final body weight; BG = blood glucose; PER = protein excretion rate; wet wt/Bwt0 = ratio of kidney wet weight to Bwt0; dry wt/Bwt0 = ratio of kidney dry weight to Bwt0; C = control; D = diabetic; DI = insulin-treated diabetic.
p <0.05
p <0.01
p < 0.001 compared to controls
p < 0.05
p < 0.001 compared to diabetics
p < 0.05
p < 0.01 compared to initial weight using the paired 2-tailed Student t test.
Fig. 1.
Daily fluctuations of blood glucose with insulin treatment of STZ-diabetic rats.
Effects of Insulin Treatment on Glomerular Morphometric Changes Early in Diabetes
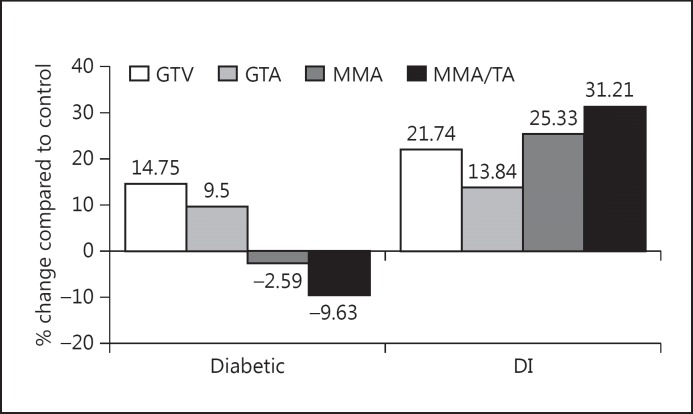
The effects of insulin treatment on glomerular morphology in 1-week-old diabetic rats are shown in Figures 2 and 3. The GTV (592 ± 49 μ3) and GTA (6,006 ± 60 μ2) were significantly larger (21.7%) in DI than in control rats (13.8%; p < 0.001) and were not different from those observed in untreated diabetic rats (Fig. 2, 3). Therefore, insulin did not prevent the growth of the glomerular capillary tuft seen in 1-week-old diabetic rats. However, the MMA (247 ± 4 μ2), which did not show any change at 1 week in this model of diabetes in Fisher rats, was significantly larger (p < 0.001) in DI rats than in controls (25%), resulting in a higher MMA as a percentage of the GTA (to 4 ± 0.2% from 3.1 ± 0.1%), which represented a significant increment (31%; p < 0.001). Serum glucose levels, urine volume, and PER were not different when compared with controls with insulin treatment, yet the MMA was significantly greater in DI rats than in both control and diabetic rats.
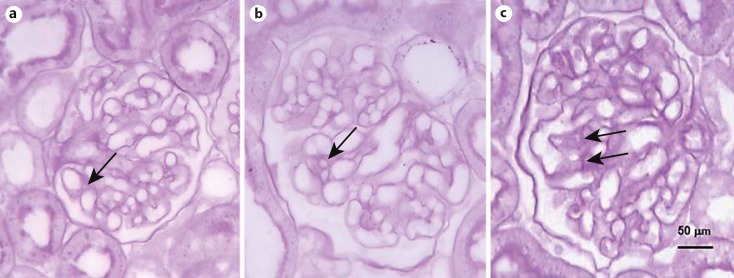
Fig. 2.
Glomerular morphometric changes in diabetic and DI rats. The PAS-positive area exhibited no change in 1-week-old diabetics (b) when compared with controls (a). The MMA was larger in DI (c) than in control and diabetic rats. The arrows point to the mesangial matrix. Five-micrometer paraffin sections. PAS stain. ×400.
Fig. 3.
Glomerular morphometric changes in 1-week-old diabetic rats and DI rats when compared with controls. MMA/TA = Ratio of MMA to tuft area.
Effects of Insulin Treatment on Renal Functions Early in Diabetes
One-week-old diabetic rats had significantly higher Cinulin (0.84 ± 0.1 mL/min/100 g; Table 2; p < 0.01) and CPAH (2.41 ± 0.43 mL/min/100 g; p < 0.05) than control rats (0.47 ± 0.02 and 1.34 ± 0.27 mL/min/100 g, respectively). However, the ratio of Cinulin to CPAH (an estimate of filtration fraction) was not significantly different (Table 2) in 1-week-old diabetic rats (0.43 ± 0.06) compared with control rats (0.38 ± 0.05). DI rats had a Cinulin of 0.6 ± 0.07 mL/min/100 g and CPAH of 1.72 ± 0.36 mL/min/ 100 g, which were not significantly different from the controls (inulin, p = 0.17; PAH, p = 0.3; Table 2). The filtration fraction was unchanged by the insulin treatment of diabetic rats. The mean arterial pressure was similar in all of the experimental groups studied (Table 2).
Table 2.
Inulin and PAH clearances in the experimental groups
| C (n = 5) | D (n = 5) | DI (n = 5) | |
|---|---|---|---|
| Cinulin, mL/min/100 g | 0.47 ± 0.02 | 0.84 ± 0.1** | 0.6 ± 0.07 |
| CPAH, mL/min/100 g | 1.34 ± 0.27 | 2.41 ± 0.43* | 1.72 ± 0.36 |
| Cinulin/CPAH | 0.43 ± 0.06 | 0.38 ± 0.05 | 0.4 ± 0.07 |
| Bwt0, g | 203.2 ± 5.6 | 196.2 ± 1.5 | 213.6 ± 4.4 |
| MBP, mm Hg | 126.6 ± 3.3 | 123.9 ± 4.2 | 124.0 ± 6.5 |
| V, mL/min | 0.011 ± 0.003 | 0.02 ± 0.003** | 0.02 ± 0.01 |
C = Controls; D = diabetic rats; DI = insulin-treated diabetic rats; Cinulin = clearance of insulin; CPAH = clearance of PAH; Bwt0 = initial body weight; MBP = mean blood pressure; V = urine flow rate during clearance experiments.
p < 0.05
p < 0.01 compared to controls.
Discussion
The main new finding of this study was that strict glycemic control with repeated doses of insulin does not prevent glomerular growth, and leads to glomerular mesangial expansion, an observation that confirmed the presence of a trophic effect of insulin and that did not occur when glycemia was controlled with an SLGT2 inhibitor [2]. Similar to the findings in this in vivo study, previous in vitro studies [8] showed an increase in mesangial matrix protein synthesis with exposure to insulin and 5 mM glucose. Insulin also stimulates the expression of early growth response genes (erg-1) in mesangial cells, indicating its possible contribution to mesangial proliferation [9]. In experimental diabetes [10] mesangial matrix expansion was not prevented with insulin treatment; however, blood glucose in that study was moderately controlled. The main growth factor responsible for the proliferation of mesangial cells and the deposition of extracellular matrix in glomeruli during diabetes is transforming growth factor-beta (TGF-β) [11], which is increased in the glomeruli of diabetic rats and humans [12]. Hyperinsulinemia activates cation channels, NADPH, and the production of reactive oxygen species (ROS) in podocytes [13]. Local release of ROS can lead to enhanced mesangial production of TGF-β and mesangial matrix expansion; however, insulin treatment was shown to decrease renal cortical expression TGF-β in diabetic rats [14]. On the other hand, fluctuation of glucose, which was shown to increase ROS in type 1 [15] and type 2 [16] diabetic patients, may promote the production of glomerular TGF-β and, hence, favor the development of sclerosis [17]. Large variations in blood glucose occurred in this study. These were associated with the intermittent nature of the insulin dosage and may have induced mesangial expansion, since glucose fluctuations were found to promote fibrogenesis, independently of the mean glucose level [18]. The fluctuations in blood glucose could be due to the decrease in levels of amylin, a hormone that complements the action of insulin in postprandial blood glucose control [18]. Amylin was shown to be significantly decreased in STZ-diabetic rats [19]. The intermittent nature of the glucose control achieved in these rats reduced their exposure to hyperglycemia, but also resulted in exposure to episodes of hyperinsulinemic hypoglycemia, which may have contributed to mesangial expansion. More prolonged or continuous infusion treatment with insulin (4-8 weeks) may be required to reverse the glomerular structural changes observed in these diabetic rats [14].
Insulin treatment prevented whole kidney growth, hyperperfusion, hyperfiltration, and proteinuria, but did not prevent glomerular tuft growth. The current results are therefore similar to those observed by Carraro et al. [20]. Since these effects of insulin are similar to those of phlorizin [2], persistent glomerular growth is unlikely to be due to a trophic effect of insulin itself but relate instead to other trophic factors. Renal IGF-I levels are increased 2 days after the induction of diabetes but are rapidly reversed to normal with insulin treatment [21]. Therefore, glomerular enlargement might result from the interaction of the injected insulin with IGF-I receptors [22]. However, increased expression of renal cortical IGF-1 receptors observed in diabetes is normalized within 1 week of insulin treatment [23] and, thus, unlikely to account for the sustained glomerular enlargement. Trophic cytokines such as basic fibroblast growth factor released from infiltrating macrophages may also contribute to diabetic glomerular growth [24]. Indeed, glomerular infiltration with macrophages is seen as early as 1 day after the induction of diabetes. Since insulin treatment prevents infiltration [24] trophic factors derived from macrophages are unlikely to explain the glomerular hypertrophy that persists during insulin treatment. The lack of C-peptide in this model of type 1 diabetes may contribute to glomerular injury [25] since treatment with C peptide reversed diabetes-induced glomerular growth [26]. Its continued absence during insulin replacement therapy may contribute to sustained glomerular enlargement through the activation of intracellular kinases or through growth factors (such as vascular endothelia growth factor).
In the present study, insulin treatment, similar to the SGLT inhibitor phlorizin [2], prevented the diabetes-induced hyperperfusion and hyperfiltration and reversed these changes with an unaltered filtration fraction, indicating that adjustments in preglomerular resistance were responsible. Since insulin had an effect similar to that of phlorizin, it is likely that most of the normalization of preglomerular resistance was associated with the normalization of blood glucose and not with direct effects of insulin on the renal vessels. Normalization of blood glucose with insulin (or phlorizin) affects preglomerular resistance by reducing proximal reabsorption, increasing the distal delivery, and altering the tubule-glomerular feedback signal [27]. In diabetic rats, normal GFR and preglomerular resistance can be achieved by increasing distal delivery without changes in blood glucose [27]. This latter finding indicates that altered tubule-glomerular feedback, rather than direct effects of insulin [28] or glucose [29] on the renal vessels, is responsible for most of the changes in preglomerular resistance that occur in diabetes and during its treatment with insulin.
With insulin treatment, glomerular tuft growth, and hence the increase in glomerular filtration surface area, was not prevented, yet Cinulin and RPF were normalized. Therefore, despite persistent elevated glomerular capillary filtration surface areas, insulin treatment was enough to prevent the increase in Cinulin, probably through the normalization of preglomerular resistance and glomerular capillary pressure rather than through changes in the filtration surface area.
The early mild proteinuria detected in the diabetic rats was mainly associated with increased filtration, as evident by the high correlation observed between PER and GFR [2]. Treatment with insulin corrected proteinuria without altering glomerular hypertrophy but decreasing hyperfiltration. These data could indicate that most of the observed increase in PER during early STZ diabetes and its reversal with insulin treatment were likely to be due to changes in GFR.
Insulin treatment reduced hyperglycemia and attenuated glomerular functional changes, as was previously shown with phlorizin [2]. Short-term intermittent insulin treatment did not prevent diabetic glomerular capillary tuft hypertrophy and, in addition, promoted glomerular mesangial expansion as a harbinger of glomerulosclerosis. Variations in blood glucose that were difficult to avoid during intermittent insulin replacement therapy could be responsible for sustained tuft hypertrophy and mesangial matrix expansion in glomeruli of 1-week-old DI rats. Although the latter finding might bear no relationship to clinical findings on the risks of strict insulin control of blood glucose, it might indicate that in some circumstances intensive intermittent insulin treatment could have a deleterious effect on the diabetic kidney.
Conclusions
Short-term glycemic control with insulin induced expansion of the mesangial matrix and did not prevent glomerular tuft hypertrophy in STZ diabetes. Whole kidney (mostly tubular) hypertrophy, hyperperfusion, hyperfiltration, and proteinuria observed within 1 week of inducing STZ diabetes were normalized with insulin treatment, indicating that glomerular structural and functional changes are not always related.
References
- 1.The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malatiali S, Francis I, Barac-Nieto M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp Diabetes Res. 2008:305–403. doi: 10.1155/2008/305403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Non-albuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 4.Bojensen E. A method for determination of inulin in plasma and urine. Acta Medica Scand Suppl. 1952;266:275–282. doi: 10.1111/j.0954-6820.1952.tb13376.x. [DOI] [PubMed] [Google Scholar]
- 5.Bratton AC, Marshall EK. A new coupling component for sulfanilamide determination. J Biol Chem. 1939;128:537–550. [Google Scholar]
- 6.Ketteler M, Westenfeld R, Gawlik A, et al. Acute glomerular upregulation of ornithine decarboxylase is not essential for mesangial cell proliferation and matrix expansion in anti-Thy-1-nephritis. Nephrol Dial Transplant. 2000;15:16–22. doi: 10.1093/ndt/15.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Awazu M, Omori S, Ishikura K, et al. The lack of cyclin kinase inhibitor p27 (Kip1) ameliorates progression of diabetic nephropathy. J Am Soc Nephrol. 2003;14:699–708. doi: 10.1097/01.asn.0000051726.41601.c0. [DOI] [PubMed] [Google Scholar]
- 8.Abrass CK, Spicer D, Raugi GJ. Induction of nodular sclerosis by insulin in rat mesangial cells in vitro: studies of collagen. Kidney Int. 1995;47:25–37. doi: 10.1038/ki.1995.3. [DOI] [PubMed] [Google Scholar]
- 9.Solow BT, Derrien A, Smith JA, et al. Angiotensin II inhibits insulin-induced egr-1 expression in mesangial cells. Arch Biochem Biophys. 1999;370:308–313. doi: 10.1006/abbi.1999.1389. [DOI] [PubMed] [Google Scholar]
- 10.Stackhouse S, Miller PL, Park SK, et al. Reversal of glomerular hyperfiltration and renal hypertrophy by blood glucose normalization in diabetic rats. Diabetes. 1990;39:989–995. doi: 10.2337/diab.39.8.989. [DOI] [PubMed] [Google Scholar]
- 11.Chang AS, Hathaway CK, Smithies O, et al. Transforming growth factor-β1 and diabetic nephropathy. Am J Physiol Renal Physiol. 2016;310:F689–F696. doi: 10.1152/ajprenal.00502.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T, Nakamura T, Noble NA, et al. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1848. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim EY, Anderson M, Dryer SE. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol. 2012;302:F298–F307. doi: 10.1152/ajprenal.00423.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang D, Guan H, Liu J, et al. Early intensive insulin therapy attenuates the p38 pathway in the renal cortex and indices of nephropathy in diabetic rats. Endocrine J. 2012;59:81–90. doi: 10.1507/endocrj.ej11-0057. [DOI] [PubMed] [Google Scholar]
- 15.Wentholt IME, Kulik W, Michels RPJ, et al. Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia. 2008;51:183–190. doi: 10.1007/s00125-007-0842-6. [DOI] [PubMed] [Google Scholar]
- 16.Monnier L, Mas E, Ginet C, Michel F, et al. Glucose fluctuations during postprandial periods and, more generally, during glucose swings exhibited a more specific triggering effect on oxidative stress than chronic sustained hyperglycemia. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 17.Polhill TS, Saad S, Poronnik P, et al. Short-term peaks in glucose promote renal fibrogenesis independently of total glucose exposure. Am J Physiol Renal Physiol. 2004;287:F268–F273. doi: 10.1152/ajprenal.00084.2004. [DOI] [PubMed] [Google Scholar]
- 18.Levetan C, Want LL, Weyer C, et al. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26:1–8. doi: 10.2337/diacare.26.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa A, Harris V, McCorkle SK, et al. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. J Clin Invest. 1990;85:973–976. doi: 10.1172/JCI114528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carraro M, Mancini W, Artero M, et al. Albumin permeability in isolated glomeruli in incipient experimental diabetes mellitus. Diabetologia. 2000;43:235–241. doi: 10.1007/s001250050035. [DOI] [PubMed] [Google Scholar]
- 21.Flyvbjerg A. Role of growth hormone, insulin-like growth factors and IGF-binding proteins in renal complications of diabetes. Kidney Int Suppl. 1997;60:S12–S19. [PubMed] [Google Scholar]
- 22.Zhao WQ, Chen GH, Chen H, et al. Secretion of annexin II via activation of insulin receptor and insulin-like growth factor receptor. J Biol Chem. 2003;278:4205–4215. doi: 10.1074/jbc.M210545200. [DOI] [PubMed] [Google Scholar]
- 23.Werner H, Shen-Orr Z, Stannard B, et al. Experimental diabetes increases insulin-like growth factor I and II receptor concentration and gene expression in kidney. Diabetes. 1990;39:1490–1497. doi: 10.2337/diab.39.12.1490. [DOI] [PubMed] [Google Scholar]
- 24.Sassy-Prigent C, Heudes D, Mandet C, et al. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–475. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- 25.Marks V. What's new in diabetes: incretins and C-peptide. Med Princ Pract. 2011;20:101–102. doi: 10.1159/000321278. [DOI] [PubMed] [Google Scholar]
- 26.Samnegard B, Jacobson SH, Jaremko G, et al. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int. 2001;60:1258–1265. doi: 10.1046/j.1523-1755.2001.00964.x. [DOI] [PubMed] [Google Scholar]
- 27.Vallon V, Richter K, Blantz RC, et al. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- 28.Juncos LA, Ito S. Disparate effects of insulin on isolated rabbit afferent and efferent arterioles. J Clin Invest. 1993;92:1981–1985. doi: 10.1172/JCI116792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus RG, England R, Nguyen K, et al. Renal expression of the insulin-responsive glucose transporter GLUT4 in experimental diabetes mellitus. Am J Physiol. 1994;267:F816–F824. doi: 10.1152/ajprenal.1994.267.5.F816. [DOI] [PubMed] [Google Scholar]