Abstract
Objective
To evaluate the effect of N-benzyl-4-bromobenzamide (NBBA) on lipopolysaccharide (LPS)-induced IL-6 and prostaglandin E2 (PGE2) production in human gingival fibroblasts (HGFs).
Material and Methods
The benzamide compound was synthesized. The condition for IL-6 production of HGFs after induction with LPS was optimized. The HGFs were incubated with NBBA (10 µg/ml) for 30 min before LPS (1 μg/ml) was added. After 24 h of incubation time, the culture media were harvested and their IL-6 and PGE2 contents were determined using an enzyme-linked immunosorbent assay. Prednisolone (PDS) and NS-398 were used as positive controls. Statistical analysis of the IL-6 and PGE2 contents was performed using the ANOVA test followed by the Tukey multiple-comparisons test to compare replicate means. p < 0.001 was considered statistically significant.
Results
The maximum IL-6 production was achieved when HGFs were exposed to 1 μg/ml of LPS for 24 h, which was inhibited by the IL-6 immunosuppressant PDS. The benzamide compound, NBBA, exhibited a potent anti-IL-6 activity with inhibition of 35.6 ± 0.5%, significantly different from in the LPS-induced HGFs (p < 0.001). In addition, it inhibited 75.6 ± 0.52% PGE2 production. Cell viability was not significantly affected by treatment with NBBA at a concentration <10 µg/ml (p < 0.001).
Conclusions
NBBA exhibited an inhibitory effect on the production of IL-6 and PGE2 in LPS-induced HGFs. It could serve as a compound with inhibiting inflammatory activity in periodontal disease.
Key Words: Amide, Benzamide, Gingival fibroblasts, IL-6, Prostaglandin E2, Lipopolysaccharide, Anti-inflammation
Introduction
Both microbial factors and the host immune response have been implicated in the etiology of the chronic oral inflammatory disease, periodontitis [1]. Periodontitis is the most frequent bone-resorptive infectious disease and is thought to be most commonly caused by Gram-negative bacterial infection [2]. Infectious pathogens and pathological conditions induce inflammation, which results in bone resorption and periodontal destruction. Lipopolysaccharide (LPS) is a component of the cell wall of Gram-negative bacteria which exhibits a correlation with the severity of periodontal disease [3,4]. Human gingival fibroblasts (HGFs) are the principal cell type in the soft connective tissues of the periodontium, and they interact directly with bacteria and bacterial products such as LPS [5]. It had been suggested that HGFs play a critical role in the periodontal response to LPS, in part through the production of proinflammatory cytokines and metalloproteases [6,7]. IL-6 and prostaglandin E2 (PGE2) are produced not only in inflammatory cells (monocytes and macrophages) but also in HGFs [8,9]. IL-6 plays an important role in the pathogenesis of periodontal diseases due to its promotion of bone resorption, possibly by stimulating osteoclast precursor recruitment and differentiation [10]. Therefore, in LPS-induced HGFs, IL-6 seems to stimulate alveolar bone resorption, leading to the pathogenesis of periodontal disease [11]. PGE2, in particular, has several functions in vasodilation, the enhancement of vascular permeability and pain and the induction of osteoclastogenesis, so it plays an important role in the inflammatory response and alveolar bone resorption in periodontal disease [12].
Benzamide derivatives are associated with a broad spectrum of biological activities including anti-inflammation [13] and anti-osteoclastogenesis for experimental arthritis [14] and anti-HIV-1 [15]. They have been shown to have noncytotoxic activity. When dosed orally, the compound PF-4950834 potently inhibits neutrophil migration in a carrageenan-induced acute inflammation model [13].
There are reports on the anti-inflammatory properties of benzamide derivatives, e.g. NDMC101 inhibits osteoclastogenesis which also ameliorates paw swelling and inflammatory bone destruction [14]. Its efficacy is associated with the inhibition of such transcription factors as NF-κB and NFATc1 as well as multiple protein kinases, including p38, ERK and JNK [14]. AZD6703 is a clinical p38α MAP kinase inhibitor for the treatment of inflammatory diseases [16]. PF-4950834 blocks stromal cell-derived factor-1α-mediated chemotaxis of T lymphocytes in vitro and the synthesis of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in activated human endothelial cells in vitro [13]. AACBA dose-dependently reduces LPS-induced plasma IL-6 release and prevents or reverses carrageenan-induced paw edema and mechanical hypersensitivity [17]. The compound 2,4-dihydroxy-N-(4-hydroxyphenyl) benzamide most strongly inhibits the production of nitric oxide, TNF-α, PGE2 and cyclooxygenase-2 (COX-2) in LPS-induced RAW264.7 cells, differentiated U937 cells and peritoneal macrophages [18].
Therefore, a library of benzamide was synthesized by combinatorial chemistry (data not shown). From this screening, it was found that N-benzyl-4-bromobenzamide (NBBA) is an anti-inflammatory compound. The aim of this study was to investigate its ability to inhibit IL-6 and PGE2 production.
Materials and Methods
Synthesis of NBBA
All chemicals used for the synthesis of the compound were purchased from Sigma (St. Louis, Mo., USA). 4-Bromobenzoic acid (0.50 g, 2.5 mmol), dicyclohexylcarbodiimide (0.52 g, 2.5 mmol) and 4-dimethylaminopyridine were dissolved in dry dichloromethane (CH2Cl2; 10 ml). The reaction mixture was stirred at room temperature for 30 min, a solution of benzylamine (327 μl, 3.0 mmol) in dry CH2Cl2 (5 ml) was then added, and the resulting mixture was stirred for 12 h at the same temperature. The precipitated N,N-dicyclohexyl urea was removed by filtration and the filtrate was evaporated under reduced pressure to give a crude product. This crude product was purified by column chromatography using CH2Cl2-MeOH as the eluting solvent to give the desired compound.
Prednisolone (PDS, Sigma), an effective anti-inflammatory agent, was used as a positive control to inhibit IL-6 production. Stock solutions of NBBA, PDS and a COX-2 inhibitor, NS-398 (Sigma), were prepared in DMSO (Sigma) at a concentration of 10 mg/ml and stored at −20°C until further use. At the start of each experiment, all compounds were diluted in DMEM (Gibco, Grand Island, N.Y., USA) with the final DMSO concentrations not exceeding 0.1%.
Primary Culture of HGFs
The HGF cultures were prepared by harvesting healthy gingival tissues from 3 volunteers at the time of clinical crown lengthening and processing, as described previously [19]. This study was approved by the Ethics Committee, Faculty of Dentistry, Srinakharinwirot University, Bangkok, Thailand. Before treatment, the study objectives were explained to each patient, and informed written consent was obtained. The gingival tissues were washed 2-3 times with PBS, cut into small pieces, placed on 35-mm tissue culture dishes, and covered with a sterilized glass coverslip. DMEM, supplemented with 10% heat-inactivated FBS (Seromed-Biochrom, Berlin, Germany) and 1% antibiotic-antimycotic solution (Gibco), was used as a culture medium. The cultures were maintained at 37°C in a humidified incubator (ThermoForma Series II Water-Jacketed CO2 Incubator, Forma Scientific, Marietta, Ohio, USA) with 5% CO2, until monolayers of confluent cells were formed. After trypsinization, the HGFs were allowed to regrow, and they routinely expanded using 0.05% trypsin (Gibco) in PBS containing 0.053 mM EDTA in 75-cm2 tissue culture flasks. These HGFs were used at passages 3-7 in all experiments.
Cytotoxic Assay
The effects of the test compounds and control media (DMEM or DMEM-DMSO) on fibroblast viability were determined using a sulphorhodamine B (SRB) sodium salt assay [20]. Each well of a 96-well tissue culture plate (Corning Inc., N.Y., USA) was seeded with 1 × 104 cells in 0.1 ml complete medium and incubated at 37°C for 24 h. The medium was then removed and replaced with DMEM or DMEM-DMSO supplemented with NBBA or drugs. After 24 h, cultured cells were fixed with trichloroacetic acid for 60 min and then stained for 30 min with 0.4% (w/v) SRB sodium salt (Sigma) dissolved in 1% acetic acid. The unbound dyes were removed by washing 4 times with 1% acetic acid, and protein-bound dye was then extracted with 10 mM unbuffered Tris base (pH 10.5) for 5 min. Optical density (OD) values were determined at 490 nm using a microplate reader (Tecan U.S., Durham, N.C., USA).
Optimized Condition for IL-6 Production by LPS-Induced HGFs
To optimize an experimental condition, the condition necessary for stimulating LPS to induce the secretion of IL-6 in HGFs was evaluated. The HGFs were seeded at 1 × 105 cells/ml in 96-well plates (100 μl/well) in complete medium and allowed to adhere overnight at 37°C. The medium was subsequently removed and each well was washed once with DMEM. In the time-course of the experiments, cells were incubated with DMEM with or without 1 μg/ml LPS for 1-48 h. In the concentration-response experiments, cells were incubated for 24 h with 0.625, 1.25, 2.5, 5, 10 and 20 μg/ml LPS. The culture media were harvested at the indicated times and stored at −80°C before analysis of their IL-6 content using an enzyme-linked immunoabsorbent assay (ELISA). This condition was used to evaluate the effects of NBBA and drugs on the production of IL-6 in the subsequent experiments.
Inhibition Effects of NBBA or Drugs on LPS-Induced IL-6 Production
The HGFs (1 × 105 cells/ml) were seeded in 96-well plates (100 μl/well) in completed medium and the cells were allowed to adhere overnight at 37°C. The medium was subsequently removed and each well was washed once with DMEM. Cells were incubated with NBBA (10 μg/ml) for 30 min before adding LPS (1 μg/ml). After 24 h of incubation, the culture media were harvested and for their IL-6 contents were determined with ELISA. The effects of these substances on LPS-stimulated IL-6 production by HGFs were calculated (as ng/ml), and converted to a percentage of the amount in the control medium (DMEM-0.1% DMSO) with LPS. PDS, the immunosuppressive drug, was used as a positive control. The effects of this drug on LPS-stimulated IL-6 production by the HGFs were first determined.
ELISA for IL-6 Determination
To detect cellular production of IL-6, an ELISA was used according to Shirai et al. [21]. In brief, a microtiter plate was coated with 100 μl/well of 0.5 ng/ml monoclonal anti-human IL-6 antibody (Sigma) in carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at room temperature. The wells were washed with 0.05% Tween in PBS (PBS/Tween) and blocked with 1% BSA in PBS for 2 h. Samples and standards were diluted with 0.1% BSA in PBS and about 100 μl of sample was added to each well. After washing with PBS/Tween, 100 µl/well of biotinylated anti-human IL-6 antibody (Zymed, San Francisco, Calif., USA) in 1% BSA was added before incubating the plates for 1 h. Each well was washed with PBS/Tween, 100 µl/well of streptavidin horseradish peroxidase (Zymed) was added, and the mixture was further incubated for 1 h before washing again with PBS/Tween. A substrate solution of 100 µl/well was added and incubated for 25 min before stopping the reaction with 1 M H2SO4 (100 µl/well). The absorbance was determined at 450 nm with a microplate reader. In each experiment, the steps were conducted with 3 wells and the standard deviation was calculated.
ELISA for PGE2 Determination
The concentration of PGE2 in the supernatants was measured by ELISA according to the manufacturer's instructions (Cayman Chemical, Ann Anbor, Mich., USA). In each experiment, the steps were conducted with 3 wells and the standard deviation was calculated for evaluation.
The effects of these substances on LPS-stimulated PGE2 production by HGFs were calculated (as ng/ml), and converted to a percentage of the amount in the control medium (DMEM-0.1% DMSO) with LPS. NS-398, the COX-2 inhibitor, was used as a positive control.
Statistical Analysis
All experiments were performed in triplicate. The data are presented as means ± standard error and were analyzed using SIGMASTAT (SPSS Inc., Chicago, Ill., USA). One-way repeated-measurement analysis of variance (ANOVA), followed by the post hoc Holm-Sidak test (when appropriate) and the Student paired t test were used for statistical analysis. The level of statistical significance was set at p = 0.001.
Results
Synthesis of NBBA
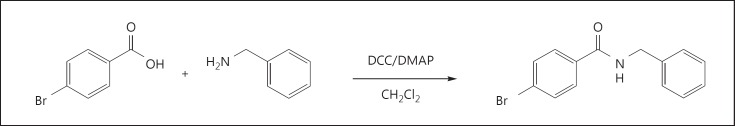
NBBA (0.61 g, 87%; fig. 1) was obtained as a white solid with mp 167-169°C. IR: νmax 3,315, 3,084, 1,635, 1,550, 1,483, 1,322, 1,257, 1,011, 847, 732, 701 and 670 cm-1; 1H NMR (400 MHz, CDCl3): δ 4.55 (d, J = 5.4 Hz, 2H, NHCH2), 6.89 (br s, 1H, NH), 7.29 (m, 5H, Ph-H), 7.49 (d, 2H, J = 8.5 Hz H-3 and H-5) and 7.61 (d, 2H, J = 8.4 Hz, H-2 and H-6); HRESIMS m/z 311.9998 [M + H]+ (calculated for C14H12BrNONa, 311.9994).
Fig. 1.
Structure and synthesis of NBBA.
Cytotoxic Effect of NBBA
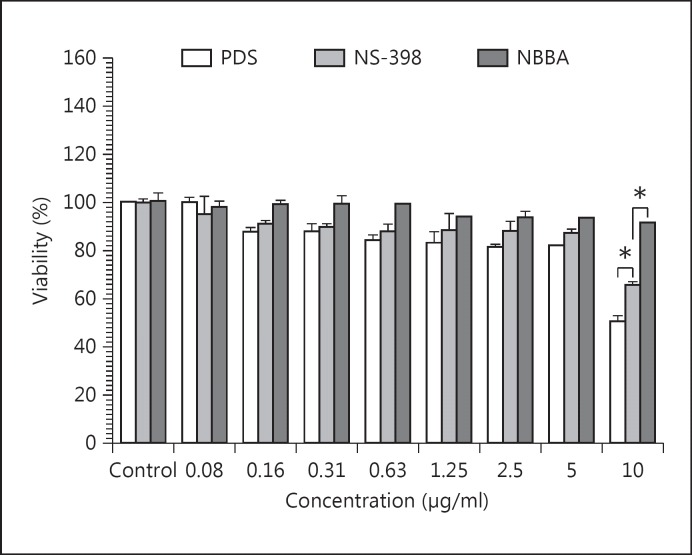
In this study, there was no dose-dependent effect of NBBA on the viability of HGFs (fig. 2). Incubation of HGFs with 10 μg/ml of PDS and NS-398 for 24 h altered cell viability to 50.87 ± 2.08% and 65.92 ± 1.22% of that of the control, respectively. The results revealed that both drugs were toxic to HGFs at the concentration of 10 μg/ml HGFs. Furthermore, PDS was toxic to HGFs in a dose-dependent manner. At concentrations of 5-10 μg/ml, the cell viability of HGFs was reduced and not acceptable 20-65% (fig. 2).
Fig. 2.
Effect of PDS, NS-398 and NBBA on cell viability of HGFs. HGFs were incubated with PDS, NS-398 or NBBA (0.08-20 μg/ml) for 24 h. After incubation, the cell viabilities were measured by the SRB method. Data are representative of 3 experiments and expressed as mean ± SEM.
Inhibitory Effects of NBBA and PDS on LPS-Induced IL-6 and PGE2 Production
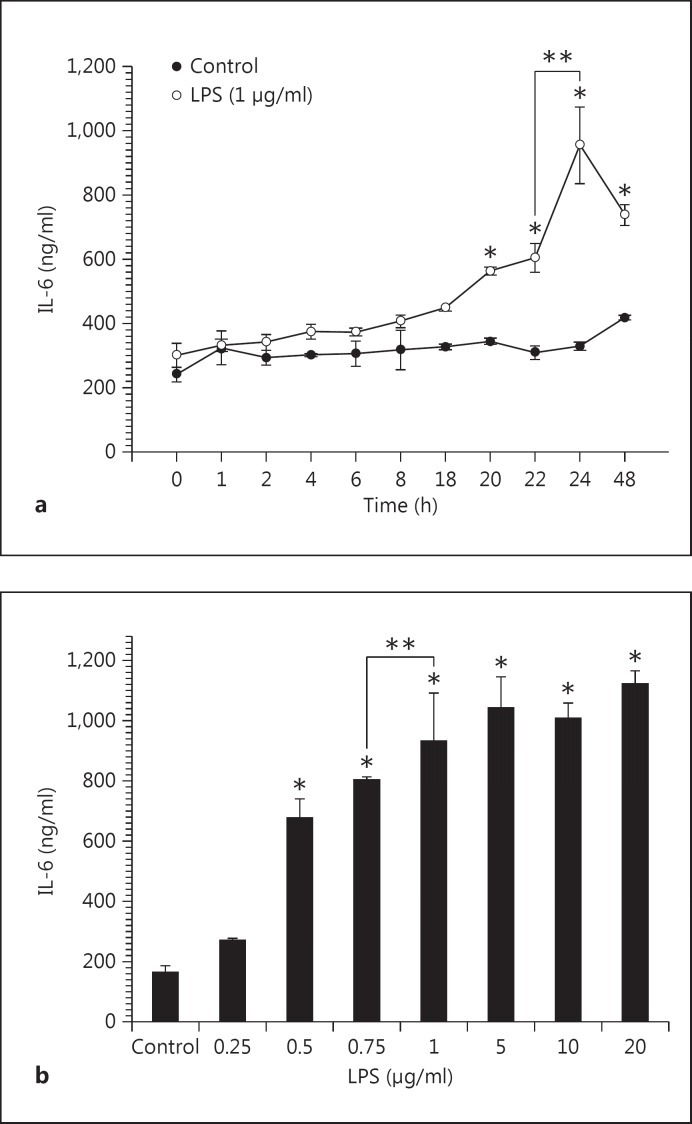
In the time-course of the experiments (fig. 3a), IL-6 was released into the culture medium at 4 h and continued for up to 24 h. A significant difference in the amount of IL-6 released after stimulating with LPS was observed after 20 h (564.0 ng/ml in LPS-induced HGFs vs. 344.4 ng/ml in the control; p < 0.001) and after 22-24 h (604.0 and 954.7 mg/ml in the LPS-induced subgroup vs. 308.9 and 329.3 in the control subgroup; p < 0.001). The amount of IL-6 significantly decreased after long-term incubation of 48 h (954.7 mg/ml) when compared with incubation of 24 h (737.3 mg/ml; p < 0.05; fig. 3a). Because the highest IL-6 production was obtained after incubating for 24 h, this duration was used in subsequent experiments. In the concentration-response experiments, HGFs cultured with different concentrations of LPS (0.5-20 μg/ml) produced significantly higher levels of IL-6 compared with the control (without LPS stimulation; p < 0.001; fig. 3b). We therefore used 1 μg/ml of LPS and incubated the cells for 24 h as the condition to induce the cytokine production in the subsequent experiments.
Fig. 3.
Time course (a) and concentration response (b) of LPS on IL-6 production in HGFs. HGFs were incubated with LPS (1 μg/ml) for the indicated times (0-48 h) or with increasing concentrations (0.25-20 μg/ml) of LPS for 24 h.
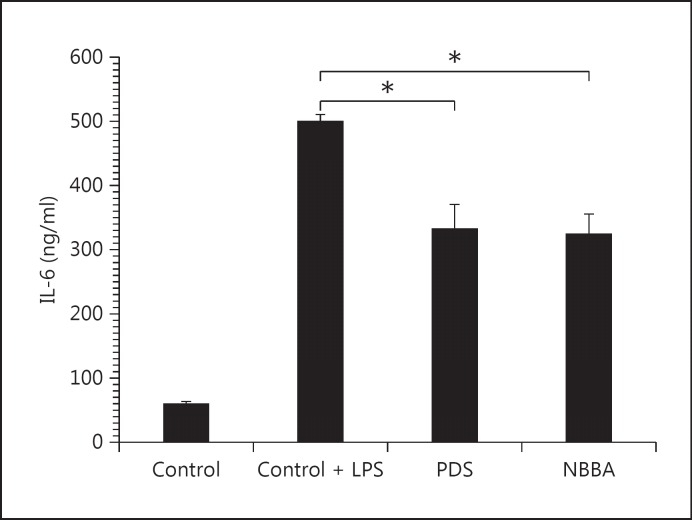
LPS-induced IL-6 production in HGFs was inhibited by pretreatment with NBBA in a dose-dependent manner (data not shown), reaching about 65% of that of the control at the highest doses of NBBA (10 μg/ml). Incubation of HGFs with 2.5 μg/ml and 5 μg/ml of NBBA inhibited IL-6 production by 42.83 ± 3.27% and 53.55 ± 2.11% of that of the LPS-induced control, respectively. The % control of NBBA was not significantly different from PDS (324.2 vs. 332.0 ng/ml; p = 0.832; fig. 4). From our results, the IL-6 production was reduced by 33.6% upon treatment with 10 μg/ml PDS. It was significantly lower with the PDS treatment compared with the control group (332.0 vs. 500 mg/ml in the LPS-induced HGFs; fig. 4).
Fig. 4.
Effect of substances on IL-6 production in LPS-induced HGFs. HGFs were incubated with 10 μg/ml PDS or 10 μg/ml NBBA, for 30 min before LPS (1 μg/ml) was added. After 24 h of incubation time, the supernatants were evaluated for IL-6 levels by ELISA.
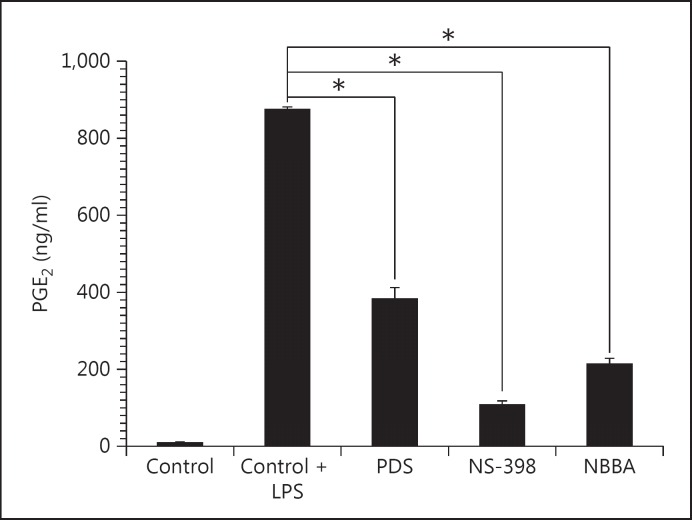
In addition, NBBA also had strong p value anti-PGE2 in LPS-induced HGFs with 24.41 ± 0.05% of the control value (213.1 ng/ml in NBBA vs. 873.3 ng/ml in LPS-induced HGFs; p < 0.001). NBBA had higher anti-PGE2 activity than PDS but less activity than NS-398 (COX-2 inhibitor) at the same concentration (213.1 ng/ml in NBBA vs. 383.3 ng/ml in PDS vs. 106.6 ng/ml in NS-398; p < 0.001; fig. 5). The inhibitory effects of IL-6 (324.0 ng/ml) and PGE2 production (213.1 mg/ml) of NBBA were significantly different from the control media induced with LPS alone (500.0 and 873.3 mg/ml, respectively; p < 0.001).
Fig. 5.
Effect of substances on PGE2 production in LPS-induced HGFs. HGFs were incubated with 10 μg/ml PDS, COX-2 inhibitor NS-398 or 10 μg/ml NBBA for 30 min before LPS (1 μg/ml) was added. After 24 h of incubation time, the supernatants were evaluated for IL-6 levels by ELISA.
Discussion
In this study, LPS induced IL-6 production by HGFs in a time-dependent manner. At the concentration of 1 μg/ml, LPS stimulated the secretion of IL-6 within 20 h of incubation. This confirms the previously reported effects of LPS-stimulated IL-6 production by healthy HGFs [8,22]. The maximum IL-6 production was achieved when exposed to 1 μg/ml of LPS for 24 h, which was inhibited by the IL-6 immunosuppressant PDS. The benzamide compound, NBBA, exhibited potent anti-IL-6 activity with a % inhibition of 35.6 that was significantly different from the LPS-induced HGFs (p < 0.001). In addition, it inhibited 75.6% PGE2 production. Since IL-6 and PGE2 are involved in the development of inflammation by promoting bone resorption via stimulation of osteoclast precursor recruitment and differentiation [10,23], these mediators are indicators of the inflammatory process. The regulating gingival inflammation could be one way to prevent and control the progression of periodontitis. In addition, it was worth nothing that increasing the concentration of NBBA (0.08-100 μg/ml) did not decrease the viability of HGFs. NBBA was thus a nontoxic, anti-inflammatory agent to HGFs.
The LPS-stimulated IL-6 production in HGFs was reduced by treatment with PDS, as noted earlier. A toxic effect of this drug on HGFs was found at concentrations of 5-10 μg/ml in this study. PDS has been shown to exhibit a dose-dependent, anti-proliferative effect on human conjunctival fibroblasts [24]. A previous study showed that PDS was coupled to human albumin in order to selectively deliver this steroid drug to macrophages without causing side effects in pathological conditions [25]. Drugs developed by the pharmaceutical industry have thus far been associated with toxicity and side effects, which is why natural substances are of increasing interest.
In HGFs, we found that the anti-IL-6 property of NBBA was also effective when compared with the corresponding control drugs. Many benzamide derivatives have been synthesized and evaluated for their biological properties. The bromobenzamide (N-(3,5-dibromobenzo-2,1,3-thiadiazolyl-4)-2-[(4-chlorophenylsulfonyl)amino]-5-bromobenzamide) has been reported to possess an antiparasitic agent [26]. N-(4-bromobenzoyl)-S,S-dimethyliminosulfurane is an effective penetration enhancer through the skin by its biotransformation into an active enhancer [27]. We have shown that NBBA exhibits an anti-inflammatory activity by inhibiting the production of IL-6 and PGE2 in LPS-induced HGFs. At an equivalent amount, NBBA is a potent inhibitor when compared to the conventionally used drugs, NS-398 or PDS.
Conclusion
We have shown that NBBA has potential as an anti-inflammatory agent for treating chronic inflammation including periodontal disease, used on its own or possibly in combination with other drugs. It may serve as a lead compound with its inhibiting property for IL-6 and PGE2 production.
Acknowledgements
This work was supported by the Commission on Higher Education, Ministry of Education, Thailand Research Fund (TRF) and Srinakharinwirot University Fund, Thailand. Support from the Center of Excellence for Innovation in Chemistry (PERCH-CIC) is gratefully acknowledged.
References
- 1.Holt SC, Ebersole J, Felton J, et al. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 2.Taubman MA, Valverde P, Han X, et al. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi K, Shitashige M, Yanai M, et al. Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation. 1996;20:555–568. doi: 10.1007/BF01487046. [DOI] [PubMed] [Google Scholar]
- 4.Okamura H, Yamaguchi M, Abiko Y. Enhancement of lipopolysaccharide-stimulated PGE2 and IL-1beta production in gingival fibroblast cells from old rats. Exp Gerontol. 1999;34:379–392. doi: 10.1016/s0531-5565(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch CA, Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res. 1991;26:144–154. doi: 10.1111/j.1600-0765.1991.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 6.Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–266. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- 7.Bodet C, Andrian E, Tanabe S, et al. Actinobacillus actinomycetemcomitans lipopolysaccharide regulates matrix metalloproteinase, tissue inhibitors of matrix metalloproteinase, and plasminogen activator production by human gingival fibroblasts: a potential role in connective tissue destruction. J Cell Physiol. 2007;212:189–194. doi: 10.1002/jcp.21018. [DOI] [PubMed] [Google Scholar]
- 8.Reddi K, Henderson B, Meghji S, et al. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine. 1995;7:287–290. doi: 10.1006/cyto.1995.0034. [DOI] [PubMed] [Google Scholar]
- 9.Ara T, Honjo K, Fujinami Y, et al. Preventive effects of a Kampo medicine, Orento, on inflammatory responses in lipopolysaccharide treated human gingival fibroblasts. Biol Pharm Bull. 2010;33:611–616. doi: 10.1248/bpb.33.611. [DOI] [PubMed] [Google Scholar]
- 10.Tamura T, Udagawa N, Takahashi N, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci USA. 1993;90:11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishimi Y, Miyaura C, Jin CH, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 12.Noguchi K, Ishikawa I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontol 2000. 2007;43:85–101. doi: 10.1111/j.1600-0757.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan LE, Davies MS, Kahn LE, et al. Biochemical, cellular, and anti-inflammatory properties of a potent, selective, orally bioavailable benzamide inhibitor of Rho kinase activity. J Pharmacol Exp Ther. 2010;333:707–716. doi: 10.1124/jpet.110.166033. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CP, Huang HS, Hsu YC, et al. A benzamide-linked small molecule NDMC101 inhibits NFATc1 and NF-kappaB activity: a potential osteoclastogenesis inhibitor for experimental arthritis. J Clin Immunol. 2012;32:762–777. doi: 10.1007/s10875-012-9660-9. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Ao Z, Jayappa KD, et al. Characterization of antiviral activity of benzamide derivative AH0109 against HIV-1 infection. Antimicrob Agents Chemother. 2013;57:3547–3554. doi: 10.1128/AAC.00100-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DS, Cumming JG, Bethel P, et al. The discovery of N-cyclopropyl-4-methyl-3-[6- (4-methylpiperazin-1-yl)-4-oxoquinazolin-3(4H)-yl]benzamide (AZD6703), a clinical p38alpha MAP kinase inhibitor for the treatment of inflammatory diseases. Bioorg Med Chem Lett. 2012;22:3879–3883. doi: 10.1016/j.bmcl.2012.04.116. [DOI] [PubMed] [Google Scholar]
- 17.Broom DC, Matson DJ, Bradshaw E, et al. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-amino-pyrrolidin-1-yl)methyl]-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther. 2008;327:620–633. doi: 10.1124/jpet.108.141853. [DOI] [PubMed] [Google Scholar]
- 18.Kim MH, Son YJ, Lee SY, et al. JAK2-targeted anti-inflammatory effect of a resveratrol derivative 2,4-dihydroxy-N-(4-hydroxyphenyl)benzamide. Biochem Pharmacol. 2013;86:1747–1761. doi: 10.1016/j.bcp.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Mariotti A, Cochran DL. Characterization of fibroblasts derived from human periodontal ligament and gingiva. J Periodontol. 1990;61:103–111. doi: 10.1902/jop.1990.61.2.103. [DOI] [PubMed] [Google Scholar]
- 20.Lin ZX, Hoult JR, Raman A. Sulphorhodamine B assay for measuring proliferation of a pigmented melanocyte cell line and its application to the evaluation of crude drugs used in the treatment of vitiligo. J Ethnopharmacol. 1999;66:141–150. doi: 10.1016/s0378-8741(98)00199-8. [DOI] [PubMed] [Google Scholar]
- 21.Shirai A, Holmes K, Klinman D. Detection and quantitation of cells secreting IL-6 under physiologic conditions in BALB/c mice. J Immunol. 1993;150:793–799. [PubMed] [Google Scholar]
- 22.Kent LW, Rahemtulla F, Hockett RD, Jr, et al. Effect of lipopolysaccharide and inflammatory cytokines on interleukin-6 production by healthy human gingival fibroblasts. Infect Immun. 1998;66:608–614. doi: 10.1128/iai.66.2.608-614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiji M, Nagasawa T, Hormdee D, et al. Internal prostaglandin synthesis augments osteoprotegerin production in human gingival fibroblasts stimulated by lipopolysaccharide. Clin Exp Immunol. 2007;149:327–334. doi: 10.1111/j.1365-2249.2007.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frantz A, Becker J. Establishing human, conjunctival fibroblast cultures as a test system for evaluating ophthalmic drugs (in German) Klin Monbl Augenheilkd. 1996;208:181–187. doi: 10.1055/s-2008-1035193. [DOI] [PubMed] [Google Scholar]
- 25.Fiume L, Chiricolo M, Busi C, et al. A conjugate of prednisolone with albumin is pharmacologically active in macrophages. Pharm Acta Helv. 1989;64:351–352. [PubMed] [Google Scholar]
- 26.Mikhailitsyn FS, Kozyreva NP, Veretennikova NL, et al. The search for new antiparasitic agents. 14. The synthesis, acute toxicity and anthelmintic activity of N-(3, 5-dibromobenzo-2, 1, 3-thiadiazolyl-4)-2-[(4-chlorophenylsulfonyl)amino]-5-bromobenzamide (in Russian) Med Parazitol (Mosk) 1995;2:30–32. [PubMed] [Google Scholar]
- 27.Sintov AC, Zhang PJ, Michniak-Kohn BB. Cutaneous biotransformation of N-(4-bromobenzoyl)-S,S-dimethyliminosulfurane and its product, 4-bromobenzamide, leading to percutaneous penetration enhancement of drugs: initial evidence using hydrocortisone. J Control Release. 2009;133:44–51. doi: 10.1016/j.jconrel.2008.09.084. [DOI] [PubMed] [Google Scholar]