Abstract
Objective
To identify the prognostic score that is the best predictor of outcome in patients hospitalized with decompensated liver cirrhosis.
Material and Methods
In this prospective study, 126 patients were enrolled and followed up for 29 months. For each patient, prognostic scores were calculated; these included the Child-Turcotte-Pugh score (CTP score), CTP creatinine-modified I score, CTP creatinine-modified II score, Model for End-Stage Liver Disease (MELD score), MELD model for end-stage liver disease sodium-modified score, Integrated MELD score, updated MELD score, United Kingdom MELD, and the MELD score remodeled by serum sodium index (MESO index). Cox regression analysis was used to assess the ability of each of the scores for predicting mortality in patients with alcoholic cirrhosis. Their discriminatory ability was evaluated using receiver operating characteristic (ROC) curve analysis.
Results
The updated MELD score had the highest predictive value (3.29) among the tested scores (95% CI: 2.26–4.78). ROC curve analysis demonstrated that the MELD score of 22.50 (AUC = 0.914, 95% CI: 0.849–0.978; p < 0.001) had the best discriminative ability for identifying patients with a high risk of mortality; the next best was the MESO index of 16.00 (AUC = 0.912, 95% CI: 0.847–0.978; p < 0.001).
Conclusion
The risk of mortality was highest in patients with the highest updated MELD score, and those with MELD scores >22.50 and a MESO index >16.00.
Key Words: Cirrhosis, Prognostic scores, Outcome, Transplantation, Survival
Introduction
Prognosis in alcoholic liver cirrhosis is better than in cirrhosis due to other etiologies, but much depends on the patient's ability to abstain from alcohol consumption, socioeconomic factors, and the availability of family support [1]. Prognosis is also reported to be worse in women [2]. The 5-year survival is about 60% in patients who abstain versus 40% in those who continue to consume alcohol [3]. In the later stages of disease, when signs of decompensation such as persistent ascites and jaundice are dominant features, abstinence has less influence on prognosis [4]. At that stage, both in alcoholic cirrhosis and in alcoholic hepatitis, mortality is the highest within the 1-year follow-up, while with active alcoholic patients it can be measured in weeks [5]. The best indicators of prognosis are the histological findings on liver biopsy; poor prognostic features include zone 3 fibrosis, perivenular fibrosis, and alcoholic hepatitis [6].
In clinical practice, a number of scoring systems are also used to estimate prognosis. The most important prognostic scores are the Child-Turcotte-Pugh (CTP) score, developed in 1973 by Pugh's modification of the Child-Turcotte score [7], and the Model for End-Stage Liver Disease (MELD) score, which was originally developed to predict 3-month survival in cirrhotic patients undergoing transjugular intrahepatic portosystemic shunt [8]. In 2003, the CTP score was remodeled by the inclusion of the serum creatinine level in the formula [9], which has improved its predictive accuracy and justified the wider use of the CTP score in day-to-day clinical practice [10,11].
In February 2002, the organ allocation system for liver transplantation in the USA started to base its prioritization technique on the MELD score [12]. The MELD score has been shown to be a valid, independent predictor of short-term as well as the long-term survival of patients with end-stage liver disease [13]. Recently, the MELD score was remodeled by the inclusion of the serum sodium in the calculation of the numerical value.
There is much diversity among patients with alcoholic liver disease and it is therefore advantageous to include a large number of potential indicators in the scoring system used to determine prognosis. According to Kamath et al. [13], among the various prognostic scores used to assess mortality in alcoholic cirrhosis, the CTP score is a very reliable indicator.
Alcoholic cirrhosis is essentially a disease of addiction which, in many cases, is very difficult to control and treat, hence the importance of monitoring and evaluating the patient. Patients need to abstain from alcohol for at least 6 months, and only then can they be presented to the surgical team for the transplant preparation. There is much controversy regarding the length of the abstinence period and the optimal time for transplantation. Surgeons are also confronted by ethical issues; for example, should transplantation be offered to the severely ill alcoholic patient in the terminal stage who has not adhered to recommendations regarding abstinence, and whose only hope of cure is a liver transplant [14].
Organ and tissue transplantation is successful only if everyone involved in the process, including physicians and medical institutions, respect and consider the best interests of the patients. It is also very important to honor the ethical, moral, and religious values of society [15]. In terms of the optimal timing for liver transplantation in patients with alcoholic cirrhosis, Veldt et al. [16] emphasized the importance of achieving abstinence from alcohol. Abstinence can significantly change the prognosis in the patient; it leads to stabilization of the patient's condition and results in lowering the CTP score to A, which is not an indication for liver transplantation. This shows preserved functional reserve of the liver, and these patients have significantly longer survival. Veldt et al. [16] found that patients who continued to drink alcohol after hospital treatment for advanced liver cirrhosis died within 6–10 months. The authors concluded that those patients who do not improve their condition despite abstinence are the ones for whom transplantation is indicated.
The objectives of this study were: (1) to examine which prognostic markers are good indicators of decompensation of alcoholic liver cirrhosis, (2) to find out which prognostic score is the best predictor of mortality in patients with decompensated alcoholic liver cirrhosis in hospital conditions, (3) to examine which of the remodeled CTP scores and derived MELD scores has the best sensitivity and specificity in the determining a deadly outcome in patients with terminal alcoholic liver cirrhosis, and (4) which of these scores can be actively applied in monitoring alcoholic patients listed for liver transplantation for the purpose of better positioning of the potential recipient and reducing mortality of patients listed for the liver transplantation.
Subjects and Methods
This was a prospective study. Eighty-seven patients with alcoholic liver cirrhosis (males: 79, females: 8) and 39 with nonalcoholic liver cirrhosis (males: 19, females: 20) were followed up during 29-month period. In the nonalcoholic cirrhosis group, those who did not consume alcohol, the causes of terminal liver cirrhosis included hepatitis B infection, hepatitis C infection, cryptogenic cirrhosis, primary biliary cirrhosis, and Wilson disease. The exclusion criteria were malignant disease of the liver or of any other organ, preexisting renal disease, and decompensated heart failure.
For each patient, laboratory biochemical parameters were determined and prognostic scores were calculated during hospitalization. In-hospital mortality was also monitored. Blood was collected for estimation of serum aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase (GGT), total bilirubin, serum sodium, and international normalized ratio (INR). The demographic, clinical, and biochemical characteristics of the 2 groups of patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the patients with alcoholic liver cirrhosis compared to other patients
| Parameter | Alcoholic cirrhosis n = 87 | Nonalcoholic cirrhosis n = 39 | P |
|---|---|---|---|
| Age, years | 54.46 ± 10.31 | 59.71 ± 11.56 | 0.0191 |
| Sex | |||
| Male | 79 (90.8) | 19 (48.7) | <0.0012 |
| Female | 8 (9.2) | 20 (51.3) | |
| Disease duration, years | 2.69 ± 1,68 | 2.93 ± 2.29 | 0.9683 |
| Hospitalization, days | 7.98 ± 6.53 | 6.74 ± 3.80 | 0.8243 |
| ICU, days | 6.00 ± 6.03 | 4.84 ± 3.15 | 0.9073 |
| AST, U/L | 114.84 ± 188.53 | 152.15 ± 293.09 | 0.1583 |
| ALT, U/L | 44.21 ± 47.17 | 94.47 ± 218.18 | 0.9973 |
| GGT, U/L | 317.24 ± 478.05 | 210.95 ± 504.67 | 0.0083 |
| TBIL, µmol/L | 106.98 ± 133.28 | 63.08 ± 81.21 | 0.0143 |
| Na, mmol/L | 135.52 ± 3.75 | 135.26 ± 3.51 | 0.7711 |
| INR | 1.77 ± 0.52 | 1.63 ± 0.39 | 0.0793 |
Values are provided as means ± SD or n (%). ICU, intensive care unit; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin; Na, sodium; INR, international normalized ratio of prothrombin time.
t test.
χ2 test.
Mann-Whitney test.
The prognostic scores that were evaluated included the following: CTP score, CTP creatinine-modified I score (CTP crea I score), CTP creatinine-modified II score (CTP crea II score), Model for End-Stage Liver Disease (MELD) score, MELD sodium-modified (MELD Na score), integrated score, updated MELD score, United Kingdom MELD score, and MELD score remodeled by serum sodium index (MESO index).
Statistical Analysis
Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). For descriptive statistics, the data are presented as the arithmetic mean ± SD, median and interquartile range, or in the form of absolute or relative numbers. Data were tested for normality using the Kolmogorov-Smirnov test. A t test was used for comparisons of the means of normally distributed data and the Mann-Whitney U test for data that were not normally distributed. The Kaplan-Meier survival analysis in relation to the variables examined was applied. The log-rank test was used to compare average survival in relation to the tested parameters. Cox regression analysis was used to assess the ability of each of the scores for predicting mortality in alcoholic cirrhosis patients. Discriminatory ability was evaluated using receiver operating characteristic (ROC) curve analysis [17].
Results
The mean age was comparable between the groups: 54.46 ± 10.3 years for alcoholic cirrhosis patients and 59.71 ± 11.56 years for nonalcoholic cirrhosis patients. The median follow-up period was 6.5 months (range: 1–29) in the alcoholic group and 6.5 months (range: 1–26) in the nonalcoholic group. Among the biochemical parameters (Table 1), the mean serum GGT value was significantly higher in the alcoholic cirrhosis group than in the nonalcoholic cirrhosis group (317.24 ± 478.05 U/L vs. 210.95 ± 504.67; p = 0.008). The serum bilirubin was also significantly higher in the alcoholic cirrhosis group than in the nonalcoholic cirrhosis group (106.98 ± 133.28 µmol/L vs. 106.98 ± 133.28; p = 0.014). There was no significant difference between the groups in serum sodium level (135.52 ± 3.75 mmol/L vs. 135.26 ± 3.51; p = 0.771), INR prothrombin time (1.77 ± 0.52 vs. 1.63 ± 0.39; p = 0.079), serum aspartate aminotransferase (114.84 ± 188.53 U/L vs. 152.15 ± 293.09; p = 0.158), and serum alanine aminotransferase (114.84 ± 188.53 U/L vs. 152.15 ± 293.09; p = 0.997).
In the alcoholic cirrhosis group, 10/87 (11.8%) patients had CTP score A, 29/87 (34.1%) patients had CTP score B, and 48/87 patients (54.1%) had CTP score C. In the nonalcoholic cirrhosis group, 9/39 (23.1%) patients had CTP score A, 16/39 (41.0%) patients had CTP score B, and 14/39 (35.9%) patients had CTP score C. The difference between the groups was not statistically significant (p = 0.087). Patients in the alcoholic cirrhosis group had significantly higher scores of the following: CTP (z = 2.490; p = 0.013), CTP crea I (z = 2.309; p = 0.021), CTP crea II (z = 2.686; p = 0.007), MELD Na (z = 2.152; p = 0.031), and MELD score (z = 2.101; p = 0.036; Table 2).
Table 2.
Score values in patients with alcoholic liver cirrhosis
| Scores | Alcoholic liver cirrhosis |
p2 | |
|---|---|---|---|
| yes | no | ||
| n = 87 | n = 39 | ||
| CTP | 9.86 ± 2.64 | 8.61 ± 2.351 | 0.013 |
| CTP crea I | 11.07 ± 3.61 | 9.34 ± 3.35 | 0.021 |
| CTP crea II | 10.91 ± 3.47 | 9.13 ± 3.09 | 0.007 |
| MESO index | 15.05 ± 6.23 | 12.04 ± 5.72 | 0.056 |
| MELD Na | 21.53 ± 8.10 | 17.99 ± 7.74 | 0.031 |
| iMELD | 40.56 ± 11.35 | 38.37 ± 11.27 | 0.750 |
| MELD | 20.61 ± 7.93 | 16.58 ± 6.97 | 0.036 |
| UK-MELD | 54.52 ± 5.41 | 52.42 ± 4.84 | 0.262 |
| Updated MELD | 4.28 ± 1.08 | 3.76 ± 0.94 | 0.086 |
CTP, Child-Turcotte-Pugh score; CTP crea I, CTP creatinine-modified I score; CTP crea II, CTP creatinine-modified II score; MELD, Model for End-Stage Liver Disease score; MELD Na, MELD sodium-modified score; MESO index, MELD score remodeled by serum sodium index; iMELD, integrated MELD score; Updated MELD, updated MELD score; UK-MELD, United Kingdom MELD score.
Arithmetic mean ± SD.
Mann-Whitney test.
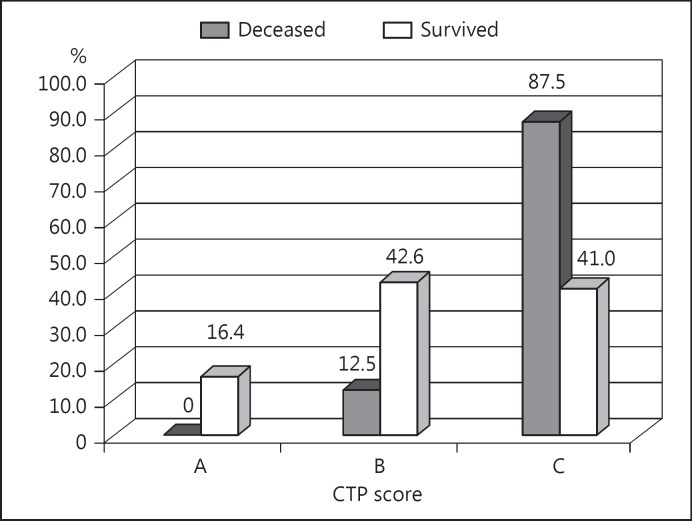
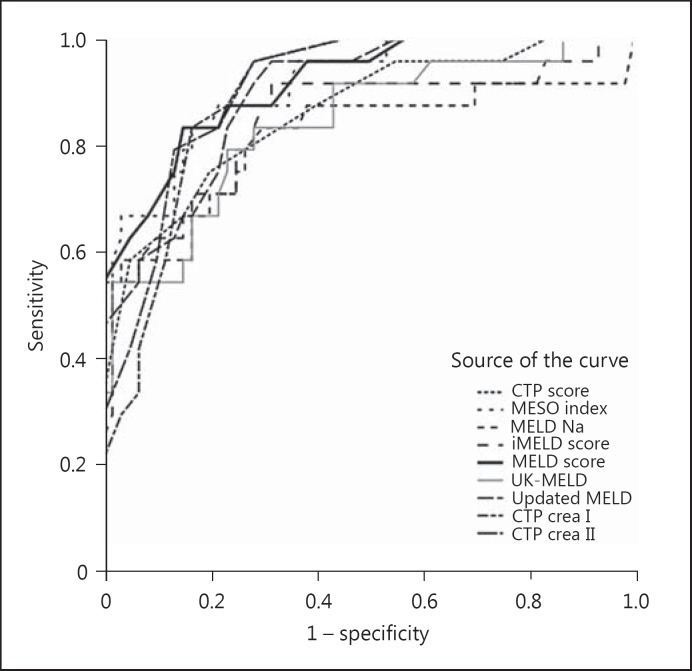
During the follow-up period, 24/87 (27.6%) patients died in the alcoholic cirrhosis group versus 13/39 (33.3%) in the nonalcoholic group; the difference was not statistically significant (p = 0.658). In the alcoholic liver cirrhosis group, the CTP score C was significantly more common among the patients who died than in those who survived (87.5 vs. 41.0%; χ2 = 18.466; p < 0.001; Fig. 1). All the scoring systems that were evaluated showed significantly higher values in the patients who died than in those who survived (Table 3). In the univariate model of Cox regression analysis, all the tested scores were statistically significant independent predictors of mortality in patients with alcoholic liver cirrhosis (Table 4); the highest predictive value was shown by the updated MELD score (HR = 3.29, 95% CI: 2.26–4.78; Table 4). ROC curve analysis demonstrated that, among the tested markers, the MELD score (AUC = 0.914; 95% CI: 0.849–0.978; p < 0.001) and MESO index (AUC = 0.912; 95% CI: 0.847–0.978) had the best discriminative ability in patients with alcoholic liver cirrhosis (Fig. 2; Table 5).
Fig. 1.
Distribution of Child-Turcotte-Pugh (CTP) scores in patients with alcoholic liver cirrhosis as compared to outcome of the follow-up.
Table 3.
Values of the tested scores in patients with alcoholic liver cirrhosis in relation to outcome of the follow-up
| Scores | Outcome of the follow-up |
p | ||
|---|---|---|---|---|
| deceased n = 24 | survived n = 63 | |||
| CTP | 12.21 ±2.13 | 8.95 ± 2.231 | <0.001 | |
| CTP crea I | 14.54 ±2.55 | 9.73 ± 3.03 | <0.001 | |
| CTP crea II | 14.46 ±2.64 | 9.53 ± 2.70 | <0.001 | |
| MESO index | 21.95 ±6.39 | 12.42 ± 3.75 | <0.001 | |
| MELD Na | 27.22 ±10.73 | 19.26 ± 5.38 | <0.001 | |
| iMELD | 50.27 ±10.96 | 36.86 ± 9.15 | <0.001 | |
| MELD | 29.33 ±7.96 | 17.18 ± 4.61 | <0.001 | |
| UK-MELD | 59.55 ±5.35 | 52.54 ± 3.98 | <0.001 | |
| Updated MELD | 5.44 ±1.14 | 3.83 ± 0.64 | <0.001 | |
CTP, Child-Turcotte-Pugh score; CTP crea I, CTP creatinine-modified I score; CTP crea II, CTP creatinine-modified II score; MELD, Model for End-Stage Liver Disease score; MELD Na, MELD sodium-modified score; MESO index, MELD score remodeled by serum sodium index; iMELD, integrated MELD score; Updated MELD, updated MELD score; UK-MELD, United Kingdom MELD score.
Arithmetic mean ± SD.
Table 4.
Cox's logistic regression in patients with alcoholic liver cirrhosis – univariate analysis
| HR | 95% CI | P | |
|---|---|---|---|
| CTP | 1.81 | 1.44 – 2.27 | <0.001 |
| CTP crea I | 1.46 | 1.27 – 1.67 | <0.001 |
| CTP crea II | 1.58 | 1.36 – 1.84 | <0.001 |
| MESO index | 1.23 | 1.16 – 1.31 | <0.001 |
| MELD Na | 1.14 | 1.08 – 1.20 | <0.001 |
| iMELD | 1.12 | 1.08 – 1.16 | <0.001 |
| MELD | 1.18 | 1.12 – 1.24 | <0.001 |
| UKELD | 1.30 | 1.19 – 1.42 | <0.001 |
| Updated MELD | 3.29 | 2.26 – 4.78 | <0.001 |
CTP, Child-Turcotte-Pugh score; CTP crea I, CTP creatinine-modified I score; CTP crea II, CTP creatinine-modified II score; MELD, Model for End-Stage Liver Disease score; MELD Na, MELD sodium-modified score; MESO index, MELD score remodeled by serum sodium index; iMELD, integrated MELD score; Updated MELD, updated MELD score; UK-MELD, United Kingdom MELD score.
Fig. 2.
ROC curve analysis demonstrated that the most discriminative ability among the tested markers in patients with alcoholic liver cirrhosis had the MELD score AUC = 0.914 (95% CI: 0.849–0.978; p < 0.001). CTP, Child-Turcotte-Pugh score; CTP crea I, CTP creatinine-modified I score; CTP crea II, CTP creatinine-modified II score; MELD, Model for End-Stage Liver Disease; MELD Na, MELD sodium modified; MESO index, MELD score remodeled by serum sodium index; iMELD, integrated MELD score; Updated MELD, updated MELD score; UK-MELD, United Kingdom MELD score.
Table 5.
ROC curve analysis of the tested scores in patients with alcoholic liver cirrhosis
| Parameter | Limit value (cutoff) | Sensitivity,% | Specificity% | AUS | 95% CI | P |
|---|---|---|---|---|---|---|
| CTP | 11.50 | 66.7 | 85.0 | 0.861 | 0.770 – 0.951 | <0.001 |
| CTP crea I | 12.50 | 83.3 | 83.3 | 0.895 | 0.830 – 0.961 | <0.001 |
| CTP crea II | 10.50 | 95.8 | 71.7 | 0.906 | 0.845 – 0.968 | <0.001 |
| MESO | ||||||
| index | 16.00 | 83.3 | 83.3 | 0.912 | 0.847 – 0.978 | <0.001 |
| MELD Na | 22.10 | 83.3 | 70.0 | 0.814 | 0.692 – 0.937 | <0.001 |
| iMELD | 39.85 | 91.7 | 68.3 | 0.845 | 0.740 – 0.950 | <0.001 |
| MELD | 22.50 | 83.3 | 85.0 | 0.914 | 0.849 – 0.978 | <0.001 |
| UKELD | 50.50 | 79.2 | 76.7 | 0.839 | 0.740 – 0.938 | <0.001 |
| Updated | ||||||
| MELD | 4.15 | 91.7 | 71.7 | 0.894 | 0.826 – 0.963 | <0.001 |
CTP, Child-Turcotte-Pugh score; CTP crea I, CTP creatinine-modified I score; CTP crea II, CTP creatinine-modified II score; MELD, Model for End-Stage Liver Disease score; MELD Na, MELD sodium-modified score; MESO index, MELD score remodeled by serum sodium index; iMELD, integrated MELD score; Updated MELD, updated MELD score; UK-MELD, United Kingdom MELD score. 1 score; CTP crea II, CTP creatinine-modified II score; MELD, Model for End-Stage Liver Disease score; MELD Na, MELD sodium-modified score; MESO index, MELD score remodeled by serum sodium index; iMELD, integrated MELD score; Updated MELD, updated MELD score; UK-MELD, United Kingdom MELD score.
Discussion
In this study, patients with alcoholic cirrhosis had significantly higher levels of serum GGT and serum bilirubin than patients with nonalcoholic cirrhosis. Serum sodium levels did not differ significantly between the groups. The INR was higher in alcoholic cirrhosis patients, but the difference was not statistically significant. Xie et al. [18], found that in patients with alcoholic cirrhosis, aspartate aminotransferase and GGT levels were significantly higher than in the control group, and that serum bilirubin, an integral part of the MELD score, was not an independent predictor of mortality [18], which differed from the results of our current study. This difference can be explained by differences in the patient population. In the abovementioned study, the authors analyzed a more heterogeneous group of patients with respect to the decompensation stage of the terminal cirrhosis, while our study focused only on patients with decompensated cirrhosis.
In the present study, in both groups, the most common indication for admission to hospital was ascites with edema. Although the alcoholic cirrhosis group had a large proportion (>50%) of patients with CTP score C, the difference from the nonalcoholic group was not statistically significant, probably because patients in both groups had been admitted with similar indications. Hence, this finding could indicate that the CTP score is useful indicator of decompensation of terminal cirrhosis, regardless of etiology. The remodeled CTP scores – CTP crea I and CTP crea II scores – were also found to be excellent indicators of the decompensation of alcoholic cirrhosis, as they were statistically significantly higher in the group of alcoholic patients in a phase of decompensated disease. This result points out the role of creatinine in qualifying of the disease decompensation by scores that include it in their formula. Among the MELD scores, the basic MELD score and MELD Na score were well correlated with decompensation of alcoholic cirrhosis.
In our study, ascites and peripheral edema were present in 29% of cases, and were the most common reasons for the hospitalization of patients with alcoholic cirrhosis. Earlier studies have also found that ascites and peripheral edema are the most common manifestations of decompensated alcoholic cirrhosis. A Danish study that included 466 alcoholic patients with end-stage liver disease found that these 2 features were present in 55% of patients admitted for treatment [19]. The significance of such manifestations of decompensated cirrhosis is not only seen in the role in rank modulation of CTP score. Ascites is an indicator of poor prognosis in patients with alcoholic cirrhosis, regardless of the CTP score, and even a good response of ascites to treatment does not improve the prognosis [20].
On the other hand, scores in the MELD group (basic MELD and MELD Na) do not include ascites in their formula. Their good correlation with decompensated disease in the alcoholic cirrhosis group could be due to the presence of high bilirubin levels in these patients. The high MELD Na scores in patients with decompensated alcoholic cirrhosis patients with ascites indicates that the role of serum sodium levels in the assessment of decompensation is also very important. Theoretically and clinically, hyponatremia indicates advanced liver disease, poor prognosis [21,22,23,24], and is often associated with complications of terminal cirrhosis [21,25].
The 27.6% mortality in the alcoholic cirrhosis group during follow-up was not significantly higher than 33.3% in the nonalcoholic cirrhosis group. However, among the patients with alcoholic cirrhosis, the CTP score C was significantly more common in patients who died than in those who survived. This finding indicated that the CTP score is a good predictor of mortality in patients with decompensated alcoholic cirrhosis. Similar results were presented in the study by Papatheodoridis et al. [11]. A systematic review by Cholongitas et al. [26] compared the Child-Pugh classification and MELD score in prognosis of terminal cirrhosis. This study proposed to keep using the CTP score for individual assessment of liver disease in daily clinical practice, while the MELD score may be best suited as a verifiable system in prioritizing candidates for liver transplantation.
Cox regression analysis showed that all the prognostic scores had good independent predictive value for mortality in patients with terminal alcoholic cirrhosis. The best predictive value was shown by the updated MELD score (HR = 3.29, 95% CI: 2.26–4.78). The updated MELD score includes all of the parameters of the MELD score in a remodeled mathematical relationship, and performs better than the baseline MELD score by more than 2-fold. After analyzing formula for calculating the updated MELD score, it becomes clear that the formula gives greater weight to the INR value than to bilirubin and creatinine. In the formula, a value of 1 is added to the log of the values of INR, bilirubin, and creatinine. This added value of 1 is closer to the numerical value of INR than to the numerical values of creatinine and bilirubin, which are significantly less influenced by the addition of the number 1. Although the difference was not statistically significant, patients in the alcoholic cirrhosis group had a higher INR value than the nonalcoholic group. After comparing the MELD and the updated MELD formulas, it can be seen that the latter formula gives greater weight to the value of serum bilirubin. If we keep in mind that serum bilirubin is significantly higher in alcoholic cirrhosis patients than in the nonalcoholic cirrhosis patients, we can position the significance of this parameter not only in the alcoholic group but also in the formula for calculating the updated MELD score, and therefore in the quality of predictability of the updated MELD score.
With ROC analysis [17], we identified a MELD score of 22.50 (AUC: 0.914) as having the best sensitivity and specificity for predicting mortality alcoholic cirrhosis patients. The next best was a MESO index of 16.00 (AUC: 0.912). The value of the MESO index lies in its mathematical simplicity, which clearly demonstrates the coexistence of 2 independent indicators of outcome – the MELD score and the serum sodium level. Rapid decrease in serum sodium increases the MESO index, which is a valuable indicator of shortened survival [27,28]. ROC analysis showed that the updated MELD score has high sensitivity but low specificity, which diminishes its value compared to the MELD score. On the other hand, the CTP score is characterized by high specificity, equivalent to that of the MELD score, but its sensitivity is significantly lower. Xie et al. [18] compared the CTP score and the MELD score for prediction of short-term survival in patients with terminal alcoholic cirrhosis and concluded that the MELD score is the better predictor.
In the group of CTP scores, the best features were demonstrated by the CTP crea II score. The CTP crea II score has been affirmed in several studies to be a good predictor of mortality. Papatheodoridis et al. [11] demonstrated that the CTP crea II score is fully equal to the MELD score in predictions of 3- and 6-month survival. Durand et al. compared the basic CTP score and the MELD score, the 2 most commonly applied scores in clinical hepatology, and reported that in the evaluation of short-term survival, the basic CTP score demonstrated better features than the MELD score [29,30].
Our findings suggest that new prognostic scores could be proposed for the evaluation of patients with terminal alcoholic cirrhosis. However, further multicenter studies with large numbers of patients and longer follow-up durations are required to confirm our findings.
Conclusion
In this study, a high CTP score and the CTP crea I and CTP crea II scores were good indicators of decompensation of alcoholic cirrhosis. The risk of mortality was highest in patients with the highest updated MELD scores, and those with MELD score >22.50 and MESO index >16.00. Our results suggest that the MESO index could be used as an additional indicator of prognosis; when listed patients have the same MELD score, priority should be given to patients who have a MESO index >16.
References
- 1.Ritson B. Treatment for alcohol related problems. BMJ. 2005;330:139–141. doi: 10.1136/bmj.330.7483.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24:217–232. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- 3.Powell WJ, Jr, Klatskin G. Duration of survival in patients with Laennec's cirrhosis. Am J Med. 1968;44:406–420. doi: 10.1016/0002-9343(68)90111-3. [DOI] [PubMed] [Google Scholar]
- 4.O'Shea RS, Dasarathys S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 5.Orrego H, Blake JE, Blendis LM, et al. Prognosis of alcoholic cirrhosis in the presence and absence of alcoholic hepatitis. Gastroenterology. 1987;92:208–214. doi: 10.1016/0016-5085(87)90861-4. [DOI] [PubMed] [Google Scholar]
- 6.Worner TM, Lieber CS. Perivenular fibrosis as precursor lesion of cirrhosis. JAMA. 1985;253:627–630. [PubMed] [Google Scholar]
- 7.Pugh RN, Murray-Lyon IM, Dawson JI, et al. Transection of the oesophagus for bleeding oesophageal varics. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 8.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 9.Angermayr B, Cejna M, Karnel F, et al. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879–885. doi: 10.1136/gut.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla YK, Kashinath RC, Duseja A, et al. Predicting mortality across a broad spectrum of liver disease – an assessment of model for end-stage liver disease (MELD), Child-Turcotte-Pugh (CTP), and creatinine-modified CTP scores. J Clin Exp Hepatol. 2011;1:161–168. doi: 10.1016/S0973-6883(11)60233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papatheodoridis GV, Cholongitas E, Dimitriadou E, et al. MELD versus Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J Gastroenterol. 2005;11:3099–3104. doi: 10.3748/wjg.v11.i20.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman RB, Jr, Wiesner RH, Harper A, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 13.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 14.Krom RA. Liver transplantation and alcohol: who should get transplants? Hepatology. 1994;20:28S–32S. doi: 10.1016/0270-9139(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 15.Abouna MG. Ethical issues in organ transplantation. Med Princ Pract. 2003;12:54–69. doi: 10.1159/000068158. [DOI] [PubMed] [Google Scholar]
- 16.Veldt BJ, Laine F, Guillygomarch A, et al. Indication for liver transplantation in severe alcohol liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93–98. doi: 10.1016/s0168-8278(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 17.Soreide K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009;62:1–5. doi: 10.1136/jcp.2008.061010. [DOI] [PubMed] [Google Scholar]
- 18.Xie YD, Feng B, Gao Y, et al. Characteristics of alcoholic liver disease and predictive factors of mortality of patients with alcoholic cirrhosis. Hepatobiliary Pancreat Dis Int. 2013;12:594–601. doi: 10.1016/s1499-3872(13)60094-6. [DOI] [PubMed] [Google Scholar]
- 19.Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: a danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 20.Marsano LS, Mendez C, Hill D, et al. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res Health. 2003;27:247–256. [PMC free article] [PubMed] [Google Scholar]
- 21.Angeli P, Wong F, Watson H, et al. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44:1535–1542. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 22.Biggins SW, Rodriguez HJ, Bacchetti P, et al. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 23.Serste T, Gustot T, Rautau PE, et al. Severe hyponatremia is a better predictor of mortality than MELD Na in patients with cirrhosis and refractory ascites. J Hepatol. 2012;57:274–280. doi: 10.1016/j.jhep.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Gianotti RJ, Cardenas A. Hyponatraemia and cirrhosis. Gastroenterol Rep. 2014;10:1–6. doi: 10.1093/gastro/got037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahluwalia V, Wade JB, Thacker L, et al. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J Hepatol. 2013;59:467–473. doi: 10.1016/j.jhep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cholongitas E, Papatheodorids GV, Vangeli M, et al. Systematic review: the model for end stage liver disease – should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Londoño MC, Cardenas A, Guevara M, et al. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut. 2007;56:1283–1290. doi: 10.1136/gut.2006.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42:100–107. doi: 10.1016/j.jhep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Botta F, Giannini E, Romagnoli P, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. 2003;52:134–139. doi: 10.1136/gut.52.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]