Abstract
Objective
To evaluate the clinical sensitivity and specificity of the newly developed Genedia malaria antigen enzyme-linked immunosorbent assay (ELISA) test and to evaluate the diagnostic efficiency of the combined use of the Genedia malaria antigen and antibody ELISA tests to detect Plasmodium vivax in blood samples.
Materials and Methods
In all, 1,070 samples were analyzed: 300 P. vivax-infected patients, 41 samples from posttreatment patients upon follow-up and 729 healthy volunteers. The Genedia malaria antigen ELISA test and the Genedia malaria antibody ELISA 2.0 test were evaluated and compared to polymerase chain reaction and microscopy.
Results
The Genedia malaria antigen ELISA test had a clinical sensitivity of 94.7s% (284/300) and a clinical specificity of 99.3s% (724/729). The Genedia malaria antibody ELISA 2.0 test had a clinical sensitivity of 94.0s% (282/300) and a clinical specificity of 98.4s% (717/729). The Genedia malaria antigen ELISA test was able to detect 13 confirmed P. vivax cases without antibodies against P. vivax, whereas the Genedia malaria antibody ELISA 2.0 test detected 11 confirmed P. vivax cases nonreactive to the Genedia malaria antigen ELISA test, and 25 cases from 41 follow-up samples nonreactive in the Genedia malaria antigen ELISA test. The combined Genedia malaria antigen and antibody ELISA 2.0 tests had a clinical sensitivity of 98.3s% (295/300) and a clinical specificity of 97.9s% (714/729).
Conclusion
The combination of antigen and antibody ELISAs improved the diagnostic sensitivity in P. vivax-confirmed cases in the Republic of Korea.
Key Words: Malaria, Plasmodium vivax, Plasmodium lactate dehydrogenase, ELISA, Antibody
Introduction
Plasmodium vivax, which causes malaria, is indigenous to the Republic of Korea (ROK). Since its recent reemergence in 1993, the number of reported malaria cases rapidly reached its peak at 4,142 cases in 2000 and gradually decreased to 555 cases in 2012 [1]. The transmission of P. vivax malaria through blood transfusions poses a real threat, with 10 new cases of transfusion-transmitted malaria (TTM) recorded in 2000 [2,3]. There are different approaches to prevent TTM between endemic and nonendemic countries. In endemic countries, donor questioning with knowledge of geographical distribution and seasonal variation is most helpful in identifying possibly infected donors [4]. In nonendemic countries, the risk of transmission is minimized through donor deferral together with specific antimalarial antibody screening [4]. Due to the annual prevalence rate compared to endemic countries and the geographic locality of malaria occurrence, the ROK implemented selective screening strategies for malaria risk donors. Based on the annual prevalence of malaria, risk areas for malaria infections were categorized as high risk (>100 cases/100,000 residents), low risk (between 100 and 10 cases/100,000 residents) or regions with possible risk. Based on data from malaria-endemic areas in the ROK, the Korea Centers for Disease Control and Prevention published guidelines for blood donations. Residents in all three categorized areas are restricted from donating blood [5]. As a consequence, malaria is now one of the most common deferral factors for blood donors in the ROK.
Appropriate programs for preventing TTM include effective donor deferral criteria and malaria screening testing of all donations [5]. In a retrospective validation of the 2004 proposed donor selection guidelines in the UK to 5 TTM cases, 1 case was reported to be insufficient in preventing TTM [6,7]. There exist several candidates for malaria screening testing such as traditional microscopy, plasmodial antigen and antibody assays, and molecular tests [7,8]. Of these methods, the malaria antibody test had been adopted by transfusion services and is reported to be effective in detecting malaria infection in travelers returning from overseas and is now widely used to screen blood donors [8,9,10,11,12]. The ROK has instituted malaria antibody screening of blood from donors from malaria risk areas since 2001. As a result, no TTM cases have been reported thus far. Although malaria antibody screening of blood donors from risk areas is widely used as a screening method, the reported sensitivities of the malaria antibody ELISA (enzyme-linked immunosorbent assay) test for P. vivax were unsatisfactory [12,13]. Therefore, negative results from a malaria antibody test could not guarantee that the donor was free of malaria infection, because antibodies against the parasite might not be detectable within the first few days of new malarial transmission [6,7,10,11]. Until now, compared to Plasmodium falciparum, no reliable approved laboratory test is available to screen donated blood for P. vivax [6,7,8].
Here, a new malaria antigen ELISA kit (Genedia malaria antigen ELISA test; Green Cross Co., Yong In, ROK), which was developed to detect pan-Plasmodium lactate dehydrogenase (pLDH) enzyme of P. vivax, was evaluated in the ROK. Our aims in the present study were as follows: (1) to evaluate the clinical sensitivity and specificity of the newly developed Genedia malaria antigen ELISA test and (2) to evaluate the diagnostic efficiency of the combined use of the Genedia malaria antigen and antibody ELISA tests (Genedia malaria antigen ELISA test and Genedia malaria antibody ELISA 2.0; Green Cross Co.) in detecting P. vivax in blood samples.
Subjects and Methods
Subjects
A total of 1,070 samples were collected from April 2005 to November 2014 at Korea University Hospital, ROK. These samples were divided into three groups as follows: 300 P. vivax-infected patients (acute patients), 41 posttreatment patients and 729 healthy controls. The acute patients were confirmed with malarial infection of P. vivax by microscopic examination within 1 week of the appearance of the symptoms. The posttreatment patients were completely treated and followed up monthly for 9 months. The healthy controls were other patients who did not have any prior history of malaria or trips to a malaria-endemic area during the previous 3 years. All patients and control subjects provided written informed consent to participate in the study. The Ethics Committee of Korea University Guro Hospital and Korea Red Cross Blood Center approved the study.
Microscopic Diagnosis
For malaria diagnosis, thick and thin blood films were prepared using standard protocols. Briefly, after Giemsa staining of blood films, the species and density of plasmodial parasites were diagnosed through microscopic examinations (×1,000). Using both thick and thin blood films, the positivity of the samples was determined by reading 200 high-power fields and parasitemia was indirectly calculated using the parasite numbers per 200 white blood cells in the blood film, where the white blood cell counts were determined by the automatic blood cell counter (Beckman Coulter, USA). To diagnose malarial infection, microscopic diagnosis should be confirmed.
Polymerase Chain Reaction Diagnosis
Genomic DNA was extracted from frozen pellets using a blood genomic DNA extraction kit (Bio-Solution, ROK) and stored at −80°C. Circumsporozoite protein (CSP) genes of P. vivax (Belem strain, M11926) were amplified by nested polymerase chain reaction (PCR) using the following oligonucleotide primers, corresponding to nucleotides 266–1,325: CSP-A1 (5′-GTCGGAATTCATGAAGAACT-TCATTCTC-3′), CSP-A3 (5′-GTAGATCTGTCCAAGGCCATAAATTTAA-3′) and CSP-B1 (5′-GAGGACGCCGAAAATAATGGATG-3′). Internal primers were CSP- A2 (5′-TCTAGAGAAAATAAGCTGAAACAACCAGGA-3′) and CSP-B2 (5′-AAGCTTCTAAACTTTATCTAGGTATTCTTTCA −3′), corresponding to base pairs 422–1,072. The first round of amplification was carried out using primer pairs CSP-A1 or CSP-A3 and CSPB1. The second round of nested PCR was carried out in separate tubes, each containing the internal primer pair of CSP-A2 and CSP-B2. In both rounds of PCR, primers were used at a final concentration of 0.1 μM in a 100-μl reaction mixture (10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM each dNTP, 2.5 units of AmpliTaq polymerase; Perkin Elmer Co., Norwalk, Conn., USA). In the first round,10 μl of DNA was used, while 5 μl of template DNA was used in the second round. Reaction mixtures were cycled 30 times with the following parameters: 1 min denaturation at 95°C, 1 min annealing at 53°C and 3 min extension at 72°C in a DNA thermal cycler (Perkin-Elmer Model 9600). Plasmids containing the P. vivax CSP gene and normal healthy control samples were used as positive and negative controls for PCR tests, respectively. Amplified products were size fractionated by electrophoresis on 1.5s% agarose gels containing 0.5 mg/ml ethidium bromide.
Genedia Malaria Antigen and Antibody ELISA Tests
The Genedia malaria antigen ELISA test was provided by the manufacturer (Green Cross Co.) for evaluation. It was developed to quantify pan-pLDH in whole blood samples using pan-pLDH-specific capturing antibodies immobilized in the wells of a 96-well plate. The ELISA test was performed as recommended by the manufacturer. Absorbance was read at a wavelength of 450 nm using an ELISA reader (Bio-Rad CODA System; Bio-Rad, Hercules, Calif., USA). Each sample was tested in duplicate, and optical density (OD) values measured at 450 nm were averaged. The background wavelength at 650 nm was subtracted from the OD values at 450 nm for each sample. For data analysis of dichotomous groups, the cutoff value (CO) for a positive ELISA signal was set to 0.1 plus the mean OD of the negative control (as recommended by the manufacturer). The sample was divided by the CO to obtain the sample/CO value. If the sample/CO value was ≥1.0, the sample was interpreted as positive, whereas a value <1.0 was interpreted as negative.
We used the Genedia malaria antibody ELISA 2.0 test kit (Green Cross Co.) to screen for P. vivax antibodies; this kit was kindly donated by the Green Cross Company. The ELISAs were performed as previously described [14].
Data Analysis
Clinical sensitivity was calculated based on the proportion of positive results for malaria antigen and antibody tests compared to results confirmed by Giemsa staining microscopy analysis and PCR results. Clinical specificity was calculated based on the proportion of negative test results among healthy controls. Posttreatment follow-up cases were also included in the evaluation. Results were compared to those obtained using the P. vivax-specific antibody ELISA alone, with Giemsa stain smear microscopy examination and PCR test results as references.
Results
Clinical Sensitivity Based on Test Results in Confirmed P. vivax Malaria Samples
Both microscopic examination using Giemsa-stained smear and the PCR assay detected malaria in all 300 samples. Clinical sensitivity of microscopic examination using Giemsa-stained smear and PCR assay was 100 and 100s%, respectively.
Clinical Sensitivity and Specificity of Malaria Antigen ELISA Tests
Of the 300 samples from patients confirmed to have P. vivax malaria, the antigen tool detected 284, thereby resulting in a sensitivity of 94.7s%. The mean sample/CO value from subjects with confirmed P. vivax malaria was 25.39 (median 28.7; tables 1, 2). Of the 729 healthy controls without parasite confirmed by microscopy and PCR, 5 (0.7s%) samples tested positive using the Genedia malaria antigen ELISA test. Hence, the assay specificity with healthy controls was 99.3s% (table 1). Among the 41 posttreatment patients, all samples were negative for the malaria antigen ELISA test (table 1).
Table 1.
Malaria antigen and antibody ELISA results were compared in confirmed P. vivax patients and healthy controls
| ELISA |
P. vivax patients |
Posttreatment follow-up |
Healthy controls |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 week |
1 – 3 months |
4 – 6 months |
7 – 9 months |
|||||||
| n (s%) | mean S/CO | n (s%) | mean S/CO | n (s%) | mean S/CO | n (s%) | mean S/CO | n (s%) | mean S/CO | |
| Antigen | ||||||||||
| Positive | 284 (94.7) | 26.7 ± 15.42 | 0 | – | 0 | – | 0 | – | 5 (0.7) | 2.39 ± 0.10 |
| Negative | 16 (5.4) | 0.63 ± 0.17 | 22 (100) | 0.29 ± 0.10 | 16 (100) | 0.30 ± 0.11 | 3 (100) | 0.20 ± 0.06 | 724 (99.3) | 0.19 ± 0.19 |
| Total | 300 (100) | 25.39 ± 16.07 | 22 (100) | 0.29 ± 0.10 | 16 (100) | 0.30 ± 0.11 | 3 (100) | 0.20 ± 0.06 | 729 (100) | 0.2 ± 0.27 |
| Antibody | ||||||||||
| Positive | 282 (94.0) | 14.43 ± 6.15 | 15 (61) | 5.11 ± 5.29 | 9 (56.3) | 4.66 ± 3.96 | 1 (33) | 8.04 | 12 (1.6) | 4.24 ± 3.55 |
| Negative | 18 (6) | 0.35 ± 0.08 | 7 (39) | 0.35 ± 0.08 | 7 (43.8) | 0.43 ± 0.13 | 2 (67) | 0.25 ± 0.02 | 717 (98.4) | 0.27 ± 0.11 |
| Total | 300 (100) | 13.59 ± 6.84 | 22 (100) | 5.58 ± 5.43 | 16 (100) | 3.07 ± 3.72 | 3 (100) | 2.86 ± 4.49 | 729 (100) | 0.34 ± 0.69 |
S/CO = Signal to cutoff ratio.
Table 2.
Agreement between Genedia malaria antigen and Genedia malaria antibody ELISA 2.0 test results for 300 confirmed P. vivax cases
| Malaria antibody ELISA |
|||
|---|---|---|---|
| positive | negative | total | |
| Malaria antigen ELISA | |||
| Positive | 271 (90.3) | 13 (4.3) | 284 (94.7) |
| Negative | 11 (3.6) | 5 (1.6) | 16 (5.3) |
| Total | 282 (94.0) | 18 (6.0) | 300 (100) |
Values are n (s%).
Clinical Sensitivity and Specificity of Malaria Antibody ELISA Tests
Of the 300 samples, the antibody ELISA detected 282 (94.0s%) of confirmed P. vivax malaria. The mean sample/CO value of subjects with confirmed P. vivax malaria was 13.59 ± 6.84 (tables 1, 2). Of the 729 healthy controls without parasite by microscopy and PCR, 12 samples (1.6s%) tested positive using the Genedia malaria antibody ELISA 2.0 test. Hence, the assay specificity was 98.4s% (table 1). Among the 41 posttreatment patients, 25 cases were positive by the antibody ELISA test (table 1).
Relationship between Parasitemia and Sensitivity of Malaria Antigen ELISA Tests
The malaria antigen ELISA test had a sensitivity of 100.0s% (57/57), 98.5s% (134/136), 93.5 (72/77) and 70.0s% (21/30), respectively, in P. vivax samples with parasitemia levels above 5,000, 500–5,000, 50–499 and below 50 parasites/μl (table 3). The mean parasitemia level in negative cases (n = 16) was approximately 127.9 (median 30.7) parasites/μl. The lowest parasitemia level of patients detected by pLDH antigen ELISA was 3.3 parasites/μl in clinical samples (table 3).
Table 3.
Sensitivities of malaria antigen ELISA tests against parasitemia levels in detecting P. vivax infection
| Parasitemia level | Sensitivity, n (s%) | Mean parasitemia level, parasites/μl | Range of parasitemia level, parasites/µl |
|
|---|---|---|---|---|
| positive samples | negative samples | |||
| >5,000 (n = 57) | 57/57 (100.0) | 8,663.1 ± 3116.6 | 5,000 – 16,780 | – |
| 500 – 5,000 (n = 136) | 134/136 (98.5) | 2,315.7 ± 1422.3 | 510 – 5,000 | 510 – 5,000 |
| 50 – 499 (n = 77) | 72/77 (93.5) | 220.9 ± 137.8 | 51 – 500 | 51 – 500 |
| <50 (n = 30) | 21/30 (70.0) | 22.1 ± 16.55 | 3.3 – 50 | 3.3 – 50 |
| Total (n = 300) | 284/300 (94.7) | 2,754.7 ± 3447.1 | 3.3 – 16,780 | 3 – 16,780 |
Relation between P. vivax Antibody and Antigen ELISA Tests
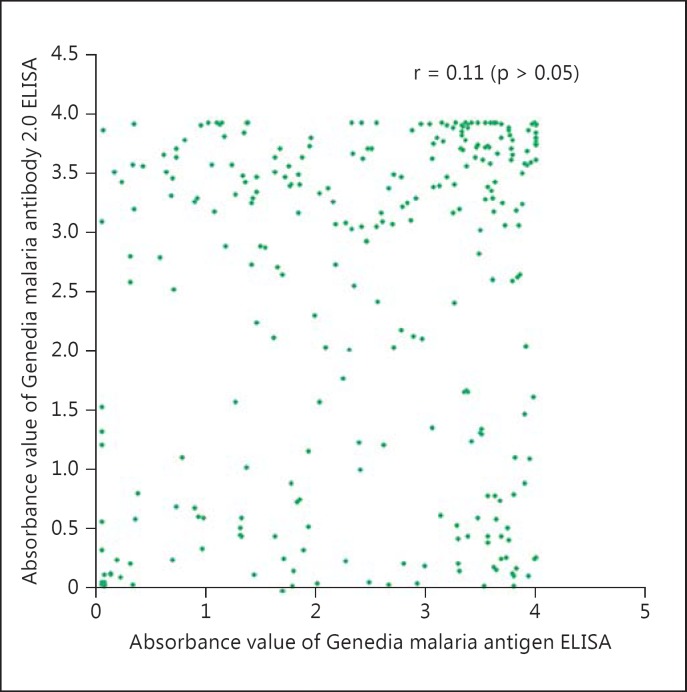
Of the 300 samples from P. vivax-confirmed patients, the agreement between P. vivax antibody and antigen ELISA tests was 92.0s% (table 2). The combined antigen and antibody tests were able to improve the detection sensitivity to 98.4s% in P. vivax-confirmed cases in this study (table 2). There was no significant correlation in absorbance values between P. vivax antibody and antigen ELISA tests (r = 0.11, p > 0.05; fig. 1).
Fig. 1.
Correlations of absorbance values between the Genedia malaria antigen ELISA test and the Genedia malaria antibody ELISA 2.0 test among P. vivax patients with clinical symptoms.
Changes of Clinical Sensitivity of P. vivax Antibody ELISA Tests with a History of Malaria
Of the 41 patient samples collected from 1 to 9 months after recovery following treatment with chloroquine/primaquine, the sensitivity and the mean sample/CO value of the P. vivax antibody ELISA tests were higher in samples collected within 1 week after symptoms than the other samples collected at 1–3, 4–6 and 7–9 months after recovery (table 1).
Discussion
The Genedia malaria antibody ELISA 2.0 test showed similar sensitivities and specificities compared to a previous report [14]. However, in our study, the sensitivity and mean sample/CO values against the malaria antibody seemed to decrease over time with a still high sample/CO value, which was similar to a previous report [15]. The sensitivity of malaria antibody tests is known to be strongly influenced by the length of time after the malaria invasion [9]. In our study, we observed decreased sensitivity of the malaria antibody ELISA according to the time intervals after the exposure. The sensitivity differences of this study could be due to differences of subjects with follow-up samples with or without standard treatments.
The combined use of the malaria antigen and antibody ELISA tests had a higher sensitivity (98.3s%) for P. vivax detection than the prior malaria antibody ELISA test (table 2). The specificity of the Genedia malaria antigen ELISA test was similar to that of existing malaria antibody tests. The improvement in the sensitivity and specificity of this test could facilitate the early detection of acute infections prior to the formation of antibodies. Moreover, it could help to differentiate malaria patients with current ongoing infections from those who have recovered from past infections without infectivity or symptoms.
Although the transmission of malaria is an unusual event in a nonendemic area, transmission is possible by local competent mosquito vectors and asymptomatic patients who travelled abroad [8]. Therefore, there is a risk of TTM not only in malaria-endemic countries but also in low-risk countries. Because of the difficulties in controlling P. vivax infection, malaria is an important and problematic disease in the ROK, where TTM cannot be ignored [15]. TTM occurs when the patient is infected by the same parasite that was present in the donor's blood. TTM causes enormous damage to the receiver, especially in immunocompromised patients. It may occasionally be difficult to diagnose in regions/areas where these infections are not endemic. Even though strong requirements and regulations have been implemented at various blood centers to test for different transmissible diseases to prevent TTM, only a limited number of tests meet the recommended requirements for screening donated blood for P. vivax; most suffer from low sensitivity. To prevent TTM, the majority of nations use blood donor deferral criteria based on interviews to determine previous medical history [5,6]. However, current donor exclusion guidelines have been challenged [6,16], and more effective and evidence-based malaria blood screening tests are required. The current standard method, using thick and thin blood films, has low sensitivity for detecting asymptomatic patients with low parasitemia levels. Detecting malaria antibodies in blood is widely used to screen prospective blood donors to avoid TTM in low-risk areas including the ROK and European countries [8]. The wide range of P. vivax sensitivity (53–94.4s%) of malaria antibody ELISA tests confirmed those of previous studies [12,13,14]. Target antigen characteristics of P. vivax isolates in these different geographic regions could have caused the discrepancy in the sensitivities reported in these studies. Although malaria antibody tests for P. vivax are not reliable enough for screening donated blood, until now, other malaria tests, such as rapid diagnostic tests, PCR and microscopy, have not been useful mass screening methods in large blood-processing centers with huge numbers of samples. ELISA is a fast, relatively inexpensive, and reliable way to detect malaria [17,18,19,20]. While malaria antibody ELISA tests have frequently been used to investigate the spread of malaria, there are few available brand kits of the malaria antigen ELISA test [21]. Interestingly, a recent report stated that a malaria antigen ELISA test had a high sensitivity of 98.8s% for detecting P. falciparum [20]. Another malaria antigen test, the CELISA antigen test for P. falciparum, also had a higher sensitivity than the malaria antibody ELISA test (73–95.5s%; table 4). Recently, the pLDH antigen detection ELISA for Plasmodium was evaluated in Africa and reported as an interesting tool for blood donation qualification in order to ensure blood safety in malaria-endemic areas [22]. However, a malaria antigen test for P. vivax has not yet been implemented in blood centers in the ROK.
Table 4.
Comparison of various malaria antibody and antigen ELISA tests to Giemsa-stained smear microscopy results and PCR results to evaluate the sensitivity and specificity of these tests for P. falciparum and P. vivax
| Malaria ELISA |
P. vivax |
P. falciparum |
Standard method | ||
|---|---|---|---|---|---|
| sensitivity, s% | specificity, s% | sensitivity, s% | specificity, s% | ||
| Malaria CELISA antigen [24] | 56 (14/25) | 80 (581/723) | 73 (83/114) | 80 (581/723) | microscopy |
| Newmarket malarial antibody EIA [10] | – | – | 82.6 (114/138) | – | microscopy |
| ELISA malaria antibody test [12] | 75 (18/24) | 99.6 (2,145/2,152) | 95.5 (63/66) | 99.6 (2,145/2,152) | microscopy |
| ELISA malaria antibody test [13] | 53 (53/100) | 89.1 (326/366) | – | – | microscopy and PCR |
| Genedia malaria antibody ELISA 2.0 test [14] | 94.4 (236/251) | 99.0 (198/200) | – | – | microscopy and PCR |
| Malaria IgG CELISA [20] | 98.8 (77/78) | 100 (615/615) | 100 (7/7) | – | microscopy and PCR |
| Genedia malaria antigen ELISA test in this study | 94.7 (284/300) | 99.3 (724/729) | – | – | microscopy and PCR |
| Combined Genedia malaria antigen and antibody | |||||
| ELISA 2.0 test in this study | 98.3 (295/300) | 97.9 (714/729) | microscopy and PCR | ||
EIA = Enzyme immunoassay.
In this study, the Genedia malaria antibody ELISA 2.0 test showed similar sensitivities and specificities compared to a previous report [14]. However, in our study, the sensitivity and mean sample/CO values against the malaria antibody seemed to decrease over time with a still high sample/CO value, which was similar to a previous report [23]. The sensitivity of malaria antibody tests is known to be strongly influenced by the length of time after the malaria invasion [9]. In our study, we observed decreased sensitivity of the malaria antibody ELISA according to the time intervals after the exposure. The sensitivity differences of this study may come from differences of subjects with follow-up samples with or without standard treatments.
The Genedia malaria antigen ELISA test could not detect malaria antigens in 41 posttreatment patients, 25 of which were positive by the malaria antibody test. Therefore, the malaria antibody assay is still valuable. Malaria antibody and antigen tests in this study were mutually complementary for malaria detection. Combining the two tests increased the sensitivity of malaria tests and is useful in detecting P. vivax malaria risk in suspected patients. Implementing a suitable donor-screening test could help prevent TTM with minimal loss of donors.
The most efficient way to detect malaria would be blood donation screening using nucleic acid amplification testing techniques; this would be expected to reduce deferrals due to malaria. However, malaria nucleic acid amplification tests for mass screening are currently not available in both endemic and nonendemic areas. Hence, the combined use of antibody and antigen tests represents a relatively inexpensive and reliable method for screening for P. vivax in large numbers of blood bank samples.
Conclusion
A combination of antigen and antibody ELISAs improved the diagnostic sensitivity in P. vivax-confirmed cases in the ROK. A combined ELISA test may be useful in detecting acute infections prior to the formation of antibodies and in monitoring current infections.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgment
This work was supported by a grant from the National Research Foundation of Korea (2012R1A1A2006650) funded by the Korean Government.
References
- 1.Korea Centers for Disease Control and Prevention . Infectious Diseases Surveillance Yearbook 2012. Seoul: Korea Centers for Disease Control and Prevention; 2013. http://www.cdc.go.kr/CDC/info. [Google Scholar]
- 2.Cho YH, Kwon SY, Seo DH, et al. Transfusion-transmitted malaria in Korea – 10 cases during 1997–2001. Korean J Blood Transfus. 2001;12:263–270. [Google Scholar]
- 3.Lee YH, Lee HK, Choi KH, et al. Transfusion-induced malaria in a child after open heart surgery in Korea. J Korean Med Sci. 2001;16:789–791. doi: 10.3346/jkms.2001.16.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitchen AD, Chiodini PL. Malaria and blood transfusion. Vox Sang. 2006;90:77–84. doi: 10.1111/j.1423-0410.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 5.Korea Centers for Disease Control and Prevention Blood Donation Record . Donor Selection Guideline. Seoul: Korea Centers for Disease Control and Prevention; 2007. http://www.cdc.go.kr/CDC/main.jsp. [Google Scholar]
- 6.Kitchen AD, Barbara JA, Hewitt PE. Documented cases of post-transfusion malaria occurring in England: a review in relation to current and proposed donor selection guidelines. Vox Sang. 2005;89:77–80. doi: 10.1111/j.1423-0410.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 7.Kitchen AD, Lowe PH, Lalloo K, et al. Evaluation of a malarial antibody assay for use in the screening of blood and tissue products for clinical use. Vox Sang. 2004;87:150–155. doi: 10.1111/j.1423-0410.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 8.Seed CR, Kitchen A, Davis TM. The current status and potential role of laboratory testing to prevent transfusion-transmitted malaria. Transfus Med Rev. 2005;19:229–240. doi: 10.1016/j.tmrv.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Jelinek T, von Sonnenburg F, Kumlien S, et al. Retrospective immunodiagnosis of malaria in nonimmune travelers returning from the tropics. J Travel Med. 1995;2:225–228. doi: 10.1111/j.1708-8305.1995.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 10.Seed CR, Cheng A, Davis TM, et al. The efficacy of a malarial antibody enzyme immunoassay for establishing the reinstatement status of blood donors potentially exposed to malaria. Vox Sang. 2005;88:98–106. doi: 10.1111/j.1423-0410.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 11.Knappik M, Peyerl-Hoffmann G, Jelinek T. Plasmodium falciparum: use of a NANP19 antibody-test for the detection of infection in non-immune travellers. Trop Med Int Health. 2002;7:652–656. doi: 10.1046/j.1365-3156.2002.00906.x. [DOI] [PubMed] [Google Scholar]
- 12.Doderer C, Heschung A, Guntz P, et al. A new ELISA kit which uses a combination of Plasmodium falciparum extract and recombinant Plasmodium vivax antigens as an alternative to IFAT for detection of malaria antibodies. Malar J. 2007;6:19. doi: 10.1186/1475-2875-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh JS, Kim JS, Lee CH, et al. Evaluation of a malaria antibody enzyme immunoassay for use in blood screening. Mem Inst Oswaldo Cruz. 2008;103:75–78. doi: 10.1590/s0074-02762008005000008. [DOI] [PubMed] [Google Scholar]
- 14.Nam MH, Kim JS, Cho CH, et al. Evaluation of Plasmodium vivax ELISA for the blood screen. Trop Med Int Health. 2010;15:1436–1441. doi: 10.1111/j.1365-3156.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 15.Park CG, Chwae YJ, Kim JI, et al. Serologic responses of Korean soldiers serving in malaria-endemic areas during a recent outbreak of Plasmodium vivax. Am J Trop Med Hyg. 2000;62:720–725. doi: 10.4269/ajtmh.2000.62.720. [DOI] [PubMed] [Google Scholar]
- 16.Lim CS, Kim YK, Lee KN, et al. Malaria detection rate of donated blood and blood sample in risky area. Korean J Blood Transfus. 1997;8:103–111. [Google Scholar]
- 17.Purdy E, Perry E, Gorlin J, et al. Transfusion-transmitted malaria: unpreventable by current donor exclusion guidelines? Transfusion. 2004;44:464. doi: 10.1111/j.1537-2995.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- 18.Razakandrainibe R, Thonier V, Ratsimbasoa A, et al. Epidemiological situation of malaria in Madagascar: baseline data for monitoring the impact of malaria control programmes using serological markers. Acta Trop. 2009;111:160–167. doi: 10.1016/j.actatropica.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Lee KN, Suh IB, Chang EA, et al. Prevalence of antibodies to the circumsporozite protein of Plasmodium vivax in five different regions of Korea. Trop Med Int Health. 2003;8:1062–1067. doi: 10.1046/j.1360-2276.2003.01136.x. [DOI] [PubMed] [Google Scholar]
- 20.Lim CS, Yoon JK, Chang EA, et al. Seroprevalence to the circumsporozoite protein peptide antigen of Plasmodium vivax in Korean children. Microbiol Immunol. 2005;49:521–527. doi: 10.1111/j.1348-0421.2005.tb03757.x. [DOI] [PubMed] [Google Scholar]
- 21.Noedl H, Yingyuen K, Laoboonchai A, et al. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg. 2006;75:1205–1208. [PubMed] [Google Scholar]
- 22.Atchade PS, Doderer-Lang C, Chabi N, et al. Is a Plasmodium lactate dehydrogenase (pLDH) enzyme-linked immunosorbent (ELISA)-based assay a valid tool for detecting risky malaria blood donations in Africa? Malar J. 2013;8:279. doi: 10.1186/1475-2875-12-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber B, Berger A, Rabenau H, et al. Evaluation of a new combined antigen and antibody human immunodeficiency virus screening assay, VIDAS HIV DUO Ultra. J Clin Microbiol. 2002;40:1420–1426. doi: 10.1128/JCM.40.4.1420-1426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiodini PL, Hartley S, Hewitt PE, et al. Evaluation of a malaria antibody ELISA and its value in reducing potential wastage of red cell donations from blood donors exposed to malaria, with a note on a case of transfusion transmitted malaria. Vox Sang. 1997;73:143–148. doi: 10.1046/j.1423-0410.1997.7330143.x. [DOI] [PubMed] [Google Scholar]