Abstract
Objective
To explore a possible role for activity-regulated cytoskeleton-associated (Arc/Arg3.1) protein in the clinical identification of children with autism.
Subjects and Methods
The plasma levels of Arc/Arg3.1 in 62 boys with autism and 32 healthy boys were measured using an enzyme-linked immunosorbent assay (ELISA). The Childhood Autism Rating Scale (CARS) was used to assess the severity of autism as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). The Mann-Whitney U test was used for comparisons between children with autism and healthy children. The Spearman r correlation coefficient (r) was used to determine the relationship between the CARS scores among patients with autism and different variables.
Results
The mean plasma level of Arc/Arg3.1 protein in autism was 1.689 ± 0.917 pg/ml, significantly higher than that of healthy controls, i.e. 0.792 ± 1.056 pg/ml (p < 0.001). No significant relationship was found between plasma levels of Arc/Arg3.1 protein and CARS scores (r = −0.06; p > 0.05) or age (r = −0.27; p > 0.05).
Conclusions
The mean plasma level of Arc/Arg3.1 protein was higher in children with autism than in controls, suggesting that Arc/Arg3.1 could be a potential early blood biomarker for diagnosis of autism.
Key Words: Autism spectrum disorder, Activity-regulated cytoskeleton-associated protein, Memory dysfunction
Introduction
Autism is a neurodevelopmental disorder characterized by impaired verbal and non-verbal communication and reciprocal social interaction as well as stereotyped behaviours and evidence of a developmental delay in the first 3 years of life [1]. Over the years, research findings, especially from our laboratory, have suggested the possible involvement of immune system alterations in the pathophysiology of autism spectrum disorder (ASD) [2]. Thus, research studies investigating potential clinical biomarkers that might elucidate the aetiology of ASD are encouraged [3].
The ability to remember recent events is significantly inferior in children with autism than in healthy children, and the impairment of social communication may be related to these memory deficits [4]. Molecular mechanisms of memory storage in autism are still not well understood. Activity-regulated cytoskeletal-associated (Arc/Arg3.1) protein is localized in the neuronal dendrites, and is widely considered an important protein in neurobiology as a reliable marker for plasticity changes in the brain [5,6].
Dysfunctions in the production of Arc/Arg3.1 protein have been implicated as important factors in various neurological conditions including Alzheimer's disease [7], intellectual disability [8] and autism [9]. The capacity of neurons to regulate their own excitable ‘synaptic homeostasis’ is influenced by the Arc/Arg3.1 level, and disruption of this process is one of the fundamental causes of neuronal dysfunction in ASD [7]. Additionally, memory storage in the mammalian brain is believed to depend on the plasticity of the synaptic connections. In a study using rats, Arc/Arg3.1 protein knockout animals exhibited an unusual phenotype, where short-term learning was normal but long-term memories could not be formed [10]. The defect in synaptic plasticity results from an alteration in the number of neurotransmitter receptors located on a synapse [11].
Many studies have used Arc/Arg3.1 protein as a neuronal indicator to examine the brain regions necessary for memory retrieval; however, the function of Arc/Arg3.1 in memory acquisition and reconsolidation is not fully understood [10,11]. Previous studies have reported differences in the expression patterns of proteins such as osteopontin [12] and desert hedgehog [13] in plasma samples from children with ASD compared with control samples. A recent report found that the brains of patients with Alzheimer's disease lacked Arc/Arg3.1 protein, suggestive of dysfunctions in Arc/Arg3.1 protein production and transport; this might also be a vital mechanism underlying the memory dysfunction in children with autism [8]. Based on these previous findings, it would be interesting to determine whether the level of Arc/Arg3.1 protein differs significantly in children with ASD and control children and whether it could, therefore, be a potentially useful biomarker for clinically identifying children with autism. Thus, this study explored a possible role for Arc/Arg3.1 protein in memory dysfunction, using plasma samples from subjects with ASD and healthy controls, with the purpose of a possible clinical identification of children with autism.
Subjects and Methods
Study Populations
This case-control study was conducted on 62 male children with autism and 32 healthy male children between 2 and 11 years of age. The autistic group was recruited from October 2013 to May 2014 at The Autism Research and Treatment Center, Faculty of Medicine, King Saud University, Riyadh, Saudi Arabia, where the study was done. The exclusion criteria were: associated neurological diseases (such as cerebral palsy and tuberous sclerosis), metabolic disorders (such as phenylketonuria), allergic, inflammatory or autoimmune disorders and being on any medication.
ASD was diagnosed on the basis of the ASD criteria defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) [1]. On the day of the screening test, the Childhood Autism Rating Scale (CARS) was used to further measure the severity of ASD [14]. The CARS test is a 15-item, behavioural rating scale (relating to people, emotional response, imitation, body use, object use, listening response, fear or nervousness, verbal communication, non-verbal communication, activity level, consistency of intellectual response, adaptation to change, taste, touch and smell responses, and general impressions). The test was developed to identify autism as well as to quantitatively describe the severity of the disorder. The internal consistency reliability α coefficient is 0.94, the inter-rater reliability correlation coefficient is 0.71 and the test-retest correlation coefficient is 0.88 [15]. CARS scores have a high criterion-related validity when compared to clinical ratings during the same diagnostic sessions, with a significant correlation of 0.84. A total score of 15-29.5 is considered non-autistic, a score of 30-36.5 is considered mild-to-moderate autism and a score of 37-60 is considered moderate-to-severe autism.
Informed written consent was obtained from the parents of each subject, and the study was approved by the Institutional Ethics Committee. All procedures were in accordance with the Helsinki Declaration.
The control children were recruited from the Well Baby Clinic of King Khalid University Hospital, Faculty of Medicine at King Saud University, in Riyadh, Saudi Arabia, from among patients who had come for the routine follow-up of their growth parameters. These children were not related to the children with autism and demonstrated no clinical findings suggestive of immunological or neuropsychiatric disorders.
Blood samples were taken after an overnight fast. All blood samples were collected by a paediatric nurse, and the children diagnosed with autism were supervised by a paediatric psychiatrist with special training in the field of childhood psychosis. Venous blood was collected into 3-ml heparin tubes.
Ethylene-diamine-tetra-acetic acid (EDTA) or heparin was used as an anticoagulant and the blood was centrifuged for 15 min at 1,000 g and 2-8°C within 30 min of collection. The supernatant was assayed immediately or aliquoted and stored at −80°C until further use. Repeated freeze-thaw cycles were avoided. The samples were centrifuged again after thawing before the assay.
The assay employed the quantitative sandwich enzyme-linked immunosorbent assay (ELISA) technique. Antibodies specific to Arc/Arg3.1 were pre-coated onto a microplate. Standards and samples were pipetted into the wells, and any Arc/Arg3.1 present was bound to the immobilized antibodies.
Statistical Analysis
All the data were analysed using a commercially available software package, IBM SPSS Statistics v21. Age and CARS scores are presented as means ± SD. Because of non-normally distributed continuous variables, the Mann-Whitney U test was used for comparisons of Arc/Arg3.1 protein levels between the autistic and control groups. The null hypothesis was that there would be no difference in the data distributions for Arc/Arg3.1 protein levels between participants diagnosed with ASD and control children. The Spearman correlation coefficient (r) was used to determine the relationships between different variables. For all the statistical tests employed, a two-tailed p value ≤0.05 was considered statistically significant.
Results
The demographic data of the subjects with and without autism are given in table 1. The mean age was 5.56 ± 2.3 years. Based on the CARS, 35 (56.4%) with scores of 30-36.5 had mild-to-moderate autism and 27 (41.5%) with scores of 37-60 had severe autism.
Table 1.
Plasma levels of Arc/Arg3.1 protein in children with autism and the relationship with autism severity
| Groups | Mean age, years | Arc/Arg3.1, ng/ml | p value | CARS score |
|---|---|---|---|---|
| Children with autism (n = 62) | 5.39 ± 3.6 | 1.689 ± 0.92 | <0.001 | |
| Children with mild-to-moderate autism (n = 35) | 4.58 ± 3.36 | 1.88 ± 1.04 | 0.23 | <36.5 |
| Children with severe autism (n = 27) | 9 ± 2.788 | 1.33 ± 0.68 | 0.019 | >36.5 |
| Healthy children (n = 32) | 5.56 ± 2.299 | 0.792 ± 1.056 | <0.001 |
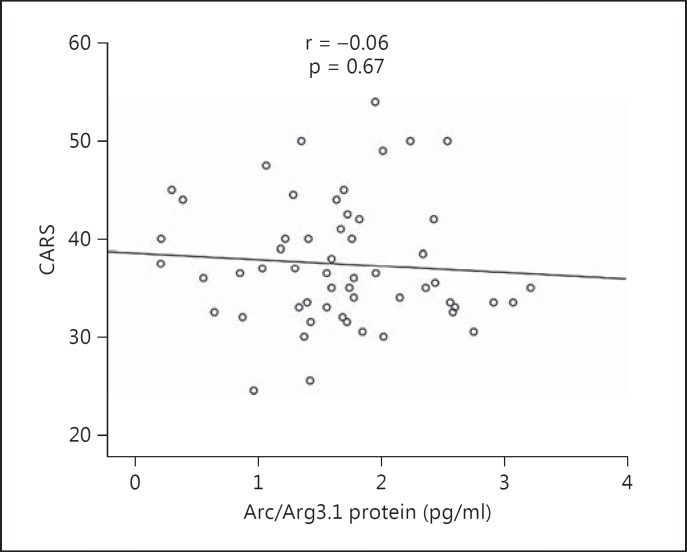
Children with autism had significantly higher plasma Arc/Arg3.1 protein levels (1.689 ± 0.917 pg/ml) than the healthy controls (0.792 ± 1.055 pg/ml, p < 0.001). Additionally, children with severe autism had significantly lower Arc/Arg3.1 protein levels (1.33 ± 0.68 pg/ml) than children with mild-to-moderate autism (1.88 ± 1.04 pg/ml, p = 0.02). Moreover, there were no correlations between Arc/Arg3.1 protein and CARS scores in children with autism (r = −0.06; p > 0.05; fig. 1). The plasma Arc/Arg3.1 protein levels of subjects with autism had no significant correlation with their age (p = 0.09; p > 0.05; fig. 2).
Fig. 1.
No significant correlation is observed between Arc/Arg3.1 levels and the severity of autism as assessed with the CARS.
Fig. 2.
No significant correlation is observed between Arc/Arg 3.1 levels and the age of children with autism.
Discussion
The children with autism had significantly higher plasma Arc/Arg3.1 protein levels than the healthy controls. This might suggest a potential role of Arc/Arg3.1 protein in the pathophysiology of ASD. However, there was clearly also a wide variation in Arc/Arg3.1 protein expression, both in the individuals with autism and between autistic children and control children. Thus, our results suggest that the higher Arc/Arg3.1 protein level could be a marker of the disease rather than being a direct pathogenic agent. However, no other study on this protein and ASD has been reported with which to compare our results.
The sharp increase in neurodevelopmental disorder reports has led to several studies on the identification of possible biological markers [16] that could be useful in future intervention strategies [17] and to discriminate between subjects with ASD and healthy controls [18]. One such possible marker is the Arc/Arg3.1 protein, which plays a significant role in synaptic plasticity dysfunction, neurotransmission and synaptic connectivity, a role which is still not fully understood [19]. Autistic children present with unusual responses to sensory stimuli, such as high sensitivity to touch, light and sounds [20,21]. Researchers have shown that Arc/Arg3.1 protein levels increase in rat brains in response to various stimuli such as cocaine, amphetamine and caffeine. Arc/Arg3.1 protein transcripts appear within 5 min of stimulation, which makes Arc an immediate early protein [22] that responds rapidly to neuronal activity [23]. Thus, in children with ASD who show hypersensitivity to environmental stimuli [24], Arc/Arg3.1 may accumulate in neuronal dendrites after the high-frequency stimulation of neurons [25]. In rats, when neurons are strongly stimulated, such as during seizure, this results in the accumulation of Arc/Arg3.1 mRNA in the corresponding region of synaptic innervation on the dentate granule cells [25]. Similarly, Arc/Arg3.1 protein is rapidly induced by the surrounding environment, such as by novel sounds or taste stimuli as well as the spatial exploration of novel environments [22].
Recent research illustrated that stimulating a child who has ASD with auditory and visual stimuli during a functional magnetic resonance imaging scan can lead to a hyper-response to mildly aversive sensory stimuli, mainly in the areas responsible for sensory processing and emotion regulation [26]. Based on this fact, increased Arc/Arg3.1 protein levels in children with ASD might be associated with the hyper-response to surrounding stimuli [26], and the interaction between genetic predisposition and the environment in which the child lives could contribute to the appearance of autism disorder [27].
ASD affects information processing in the brain by altering nerve cell development, connection and organization, although the exact mechanisms are not well understood. When stimuli reach and/or exceed the capacity of the attention- and memory-based networks in ASD, affected individuals may have more difficulty with the automatic processing of working memory [28].
In fact, learning and memory involve the modification of neuronal synaptic responses to electrical activity, and memory is classified into short- and long-term memory. Arc/Arg3.1 protein has been documented to play an important role in long-term memory [6]. In Arc/Arg3.1 protein knockout animals, short-term memory was found to be intact, but the long-term memory could not be established [10]. Therefore, the examination of Arc/Arg3.1 protein could provide insight into the cellular processes of memory consolidation [21].
Memories are thought to be encoded by the modification of synaptic strength [29] due to protein synthesis for long-term potentiation, maintenance and memory consolidation processes [10]. If decreased Arc/Arg3.1 plays a role in memory consolidation, it would thus be logical that autistic children with severe disease have significantly lower levels of Arc/Arg3.1 protein than children with mild-to-moderate autism (p = 0.019).
In this study, the higher levels of Arc/Arg3.1 protein in mild ASD could have been due to a protective mechanism because, in mild ASD, the further increase in Arc/Arg3.1 protein produced a negative feedback response on the production of Arc/Arg3.1 protein, shown by the lower level of Arc/Arg3.1 protein in severe ASD.
Arc/Arg3.1 protein has been implicated in hippocampal synaptic plasticity and spatial learning [5], and this synaptic plasticity might provide a protective effect against increased Arc/Arg3.1 protein, to avoid excessive excitation in children with severe autism by limiting calcium entry [30].
The limitations of this study were its small sample size and cross-sectional design. Because there were no correlations between Arc/Arg3.1 protein and CARS scores in children with autism, the elevation of Arc/Arg3.1 protein was not found to be linked to the severity of autism as assessed by the CARS. This was maybe related to the inadequate size of the control group (n = 32) in contrast to the group of children with autism (n = 62).
Conclusions
Arc/Arg3.1 protein levels were higher in children with autism, even though there was no significant correlation with autism severity. This finding might indicate that Arc/Arg3.1 protein levels could be used as an early blood biomarker for diagnosis of ASD.
Acknowledgements
I would like to thank the Autism Research and Treatment Center and the Al-Amoudi Chair for Autism Research at King Khalid Hospital, King Saud University, Riyadh, Saudi Arabia, King Abdul Aziz City for Science and Technology (KACST) and the National Plan for Science and Technology (NPST) at King Saud University for providing this work with the necessary funds. I also thank Shahid Bashir and Dost Muhammad Halepoto for their diagnostic assessment and technical assistance regarding ASD.
References
- 1.American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) ed 4. Washington: 2000. [Google Scholar]
- 2.Al-Ayadhi LY, Mostafa GA. A lack of association between elevated serum levels of S100B protein and autoimmunity in autistic children. J Neuroinflammation. 2012;9:54. doi: 10.1186/1742-2094-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson H. Potential biomarkers of autism. Biomark Med. 2014;8:311–312. doi: 10.2217/bmm.14.21. [DOI] [PubMed] [Google Scholar]
- 4.Boucher J. Memory for recent events in autistic children. J Autism Dev Disord. 1981;11:293–301. doi: 10.1007/BF01531512. [DOI] [PubMed] [Google Scholar]
- 5.Guzowski JF, Lyford GL, Stevenson GD, et al. Inhibition of activity-dependent ARC protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyford GL Yamagata K, Kaufmann WE, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 7.Toro R, Yamagata K, Kaufmann WE, et al. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet. 2010;26:363–372. doi: 10.1016/j.tig.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Korb E, Wilkinson CL, Delgado RN, et al. Arc in the nucleus regulates PML-dependent GluA1 transcription and homeostatic plasticity. Nat Neurosci. 2013;16:874–83. doi: 10.1038/nn.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011;34:591–598. doi: 10.1016/j.tins.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plath N, Ohana O, Dammermann B, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Gerrow K, Triller A. Synaptic stability and plasticity in a floating world. Curr Opin Neurobiol. 2010;20:631–639. doi: 10.1016/j.conb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ayadhi LY, Mostafa GA. Increased serum osteopontin levels in autistic children: relation to the disease severity. Brain Behav Immun. 2011;25:1393–1398. doi: 10.1016/j.bbi.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Bashir S, Halepoto DM, Al-Ayadhi L. Serum level of desert hedgehog protein in autism spectrum disorder: preliminary results. Med Princ Prac. 2013;23:14–17. doi: 10.1159/000354295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schopler E, Reichler RJ, DeVellis RF, et al. Toward objective classification of childhood autism: childhood autism rating scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 15.Belmonte MK, Allen G, Beckel-Mitchener A, et al. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162:1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- 17.Aman MG. Treatment planning for patients with autism spectrum disorders. J Clin Psychiatry. 2005;66:38. [PubMed] [Google Scholar]
- 18.Mizejewski GJ, Lindau-Shepard B, Pass KA, et al. Newborn screening for autism: in search of candidate biomarkers. Biomark Med. 2013;7:247–260. doi: 10.2217/bmm.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill M, Jones RS. Sensory-perceptual abnormalities in autism: a case for more research? J Autism Dev Disord. 1997;27:283–293. doi: 10.1023/a:1025850431170. [DOI] [PubMed] [Google Scholar]
- 21.Kern J. The possible role of the cerebellum in autism/PDD: disruption of a multisensory feedback loop. Med Hypotheses. 2002;59:255–260. doi: 10.1016/s0306-9877(02)00212-8. [DOI] [PubMed] [Google Scholar]
- 22.Ramírez-Amaya V, Vazdarjanova A, Mikhael D, et al. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha RN, Wissink EM, Bailey ER, et al. Rapid activity-induced transcription of Arc and other IEGs relies on poised RNA polymerase II. Nat Neurosci. 2011;14:848–856. doi: 10.1038/nn.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souders MC, Mason TB, Valladares O, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32:1566–1578. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steward O, Wallace CS, Lyford GL, et al. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 26.Green SA, Rudie JD, Colich NL, et al. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52:1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newschaffer CJ, Croen LA, Daniels J, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 28.Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003;17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- 29.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci. 2005;8:768–775. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]