Abstract
Objective
The aim of this study was to assess the concordance between the Rachmilewitz endoscopic activity index (EAI) and the Harpaz histopathological activity scoring system (HSS), which are used for evaluating the disease activity of ulcerative colitis (UC).
Subjects and Methods
This study included 109 patients with UC. Based on the disease extent, patients were divided into two groups as left-sided colitis and pancolitis. Patients were grouped as inactive, mild, moderate and severe depending on the Rachmilewitz EAI and Harpaz HSS. Kendal's tau and kappa (κ) statistics were used to assess the agreement between endoscopic and histopathological scores. A receiver operating characteristic (ROC) curve was also analyzed to evaluate the sensitivity and specificity of endoscopic scores to predict inactive histopathological disease.
Results
In the left-sided colitis group, there were slight and poor agreements in the inactive endoscopic subscores (ESS) with inactive Harpaz HSS (κ: 0.598, p < 0.001) and moderate ESS with moderate Harpaz HSS (κ: 0.236, p = 0.046). There was no agreement between mild ESS and mild Harpaz HSS and between severe ESS and severe Harpaz HSS (κ: 0.071, p = 0.573 and κ: 0.160, p = 0.151, respectively). In the pancolitis group, there was no significant agreement between inactive, mild, moderate and severe ESS and the equivalent Harpaz HSS grades (κ: −0.194, p = 0.187; κ: 0.125, p = 0.397; κ: 0.148, p = 0.175 and κ: 0.174, p = 0.153, respectively). The ROC curve showed that the ESS indicating inactive disease had a low sensitivity to predict histologically inactive disease.
Conclusion
The concordance between the endoscopic and histopathological indices was poor. Using both scores in the follow-up of patients with UC is necessary for treatment planning.
Key Words: Harpaz histopathological scoring, Rachmilewitz endoscopic activity index, Ulcerative colitis
Introduction
Ulcerative colitis (UC) is an inflammatory bowel disease that is characterized by a chronic colonic mucosal inflammation process with relapse and remission episodes [1]. It is a chronic disease that requires long-term treatment with anti-inflammatory agents, such as 5-aminosalicylic acid (5-ASA), immunosuppressives and anti-tumor necrosis factor medications [2,3]. A proper evaluation of the disease activity and prevalence is critical in determining an optimal treatment strategy. The diagnosis and evaluation of disease activity is done depending on the patient symptoms, as well as colonoscopic and histopathological findings [1].
The prognosis of the disease, treatment choice and treatment response differ across UC patients [4]. An evaluation of mucosal healing is one of the most important criteria in selecting an anti-inflammatory agent [4]. Histopathological and endoscopic activity assessments play important roles in deciding the most convenient treatment option and evaluating a patient's response to a treatment [4]. There is no standard investigative method to determine mucosal healing, and using endoscopic or histological examinations alone has not been commonly accepted [4].
There are different scoring systems that are used to evaluate endoscopic findings in UC patients [5,6]. One of them is the Rachmilewitz endoscopic activity index (EAI), which is frequently used in a patient's treatment process [7]. The Rachmilewitz EAI uses scores that vary between 0 and 12 and is dependent on the mucosal appearance, vascular pattern, ulceration and fragility criteria [7].
Many scoring indices have been developed to evaluate the histopathological activity in UC patients [8]. Of these indices, the Harpaz histological scoring system (HSS) is one of the easiest to use and is the most reliable method [9]. In this scoring system, neutrophil infiltration, epithelial cells, cryptitis, crypt abscesses, ulceration and erosion are evaluated [9].
In cases of overwhelming endoscopic findings, pathological findings are expected to also be overwhelming. In cases with few endoscopic findings, pathological findings are also expected to be minimal. However, contradictory findings among clinical, endoscopic and pathological findings can be encountered when assessing the disease activity [10].
In UC cases, determining equivalent endoscopic findings on a histopathological examination is thought to play an important role in making decisions about changing or stopping treatment. After the administration of an effective treatment and during normal disease progression, histological disease severity is altered [11]. In addition to beginning a biological therapy, mucosal healing is currently used as a significant endpoint in clinical trials [2]. In addition, mucosal healing has recently been shown to be associated with better patient outcomes, which consist mostly of lower colectomy rates and a lower hospitalization risk [12,13]. Active microscopic inflammation increases the risk of neoplasia [12,13]. Therefore, the aim of this study was to evaluate the correlation between the Rachmilewitz EAI and Harpaz HSS scales, which are used to determine the degree of disease activity in UC cases receiving treatment.
Subjects and Method
Patients
One hundred and nine patients (aged 17-80 years) who were treated in the Department of Gastroenterology of our hospital were included in the study. A diagnosis of UC was made based on clinical, radiological, endoscopic and histopathological findings. Patient demographic data, routine biochemical serum inflammatory marker levels and medical histories were obtained from the medical records. The phone numbers of patients included in the study were gathered from the hospital records and the history of each patient's medication was compiled through phone calls to each patient. Mesalazine treatment, immunosuppressive therapy and histories of previous steroid therapy were evaluated using a questionnaire. Patients were evaluated on the presence of steroid resistance and steroid addiction. Patients whose disease severity was determined with a colonoscopic examination were divided into two groups as left-sided colitis and pancolitis.
Evaluation of Disease Activity
An endoscopic evaluation was made by a trained gastroenterology specialist with at least 5 years of experience (M.K.). Colonoscopy findings were rated according to the Rachmilewitz EAI [4]. Rachmilewitz endoscopic subscores (ESS) were defined based on the Rachmilewitz EAI: inactive, 0-3; mild, 4-6; moderate, 7-9, and severe, 10-12. The criteria used in the endoscopic evaluations are summarized in table 1.
Table 1.
Endoscopic index
| Endoscopic finding | Score |
|---|---|
| Granulation scattering reflected light | |
| Yes | 0 |
| No | 2 |
| Vascular pattern | |
| Normal | 0 |
| Faded/disturbed | 1 |
| Completely absent | 2 |
| Vulnerability of mucosa | |
| None | 0 |
| Slightly increased (contact bleeding) | 2 |
| Greatly increased (spontaneous bleeding) | 4 |
| Mucosal damage (mucus, fibrin, exudate, erosions, ulcer) | |
| None | 0 |
| Slight | 2 |
| Pronounced | 4 |
Biopsies obtained during each colonoscopic examination were evaluated by a trained pathologist (G.G.S.) with at least 5 years of experience who was blinded to the patient endoscopic activity scores or other clinical and medical data. The Harpaz HSS scoring was used in the histopathological evaluation and graded based on epithelial neutrophil infiltration, cryptitis, crypt abscesses, ulceration and erosion. The Harpaz HSS scoring was made according to the parameters shown in table 2, categorized as: inactive, 0; mild, 1; moderate, 2, and severe, 3.
Table 2.
Harpaz HSS
| Inflammatory activity | Score | Defining histopathological characteristics |
|---|---|---|
| Inactive/ quiescent/normal | 0 | No epithelial infiltration by neutrophils |
| Mildly active | 1 | Neutrophil infiltration of <50% of sampled crypts or cross-sections, no ulcers or erosions |
| Moderately active | 2 | Neutrophil infiltration of ≥50% of sampled crypts or cross-sections, no ulcers or erosions |
| Severely active | 3 | Erosion or ulceration, irrespective of other features |
Statistical Analysis
Statistical analyses were performed using SPSS 15.0 (Chicago, Ill., USA). Intergroup comparisons of categorical variables were performed using a χ2 test. Concordance between the endoscopic activity and histological activity scores were investigated for all cohorts and separately for the left-sided colitis and pancolitis groups using Kendall's tau correlation analysis and kappa (κ) statistics. The degree of agreement between the scores was measured as described by Altman [14]: very poor agreement, 0.2; slight agreement, 0.2-0.4; moderate agreement, 0.4-0.6; substantial (good, high) agreement, 0.6-0.8, and excellent (almost perfect) agreement, >0.8. Receiver operating characteristic (ROC) curves were used to analyze the specificity and sensitivity of low Rachmilewitz endoscopic scores in predicting histopathologically inactive disease.
Results
The mean age of the patients was 46.2 ± 15.6 years. Sixty-three of the patients were male. Sixty-three patients were diagnosed with left-sided colitis and 46 had pancolitis.
In the left sided-sided colitis cohort (n = 63), Rachmilewitz ESS revealed that 16 (25.4%) patients had inactive ESS, 10 (15.8%) had mild ESS, 19 (30.2%) had moderate ESS and 18 (28.6%) had severe ESS. By comparison, Harpaz HSS revealed that 18 (28.6%) patients had inactive colitis, 9 (14.4%) had mild colitis, 28 (44.4%) had moderate colitis and 18 (28.6%) had severe colitis (table 3). The concordance of the Harpaz HSS with the Rachmilewitz ESS is shown in figure 1. In the left-sided colitis cohort, although there was a slight and poor agreement in the inactive and moderate scores, there was no agreement in the mild and severe scores: 4 (25%) of the 16 cases with inactive Rachmilewitz ESS were misclassified as mild, moderate or severe Harpaz HSS; 8 (80%) of the 10 cases with mild Rachmilewitz ESS were misclassified as inactive or moderate Harpaz HSS; 7 (20%) of the 19 cases with moderate Rachmilewitz ESS were misclassified as inactive, mild or severe Harpaz HSS, and 14 (77.8%) of the 18 cases with severe Rachmilewitz ESS were misclassified as inactive, mild or moderate Harpaz HSS (κ: 0.598, 95% CI 0.375 to 0.821, p < 0.001; κ: 0.071, 95% CI −0.203 to 0.345, p = 0.573; κ: 0.236, 95% CI 0.013 to 0.459, p = 0.046; κ: 0.160, 95% CI: −0.087 to 0.407, p = 0.151, respectively; table 3). The concordance between the Rachmilewitz ESS and Harpaz HSS was highest in the left-sided colitis group.
Table 3.
Distribution of cases according to and correlations between endoscopic and histopathological scores in the overall cohort and subgroups
| Rachmilewitz | Harpaz pathological scores, n (%) | Kendal's tau | p | |||
|---|---|---|---|---|---|---|
| endoscopic scores | inactive | mild | moderate | severe | correlation coefficient | value |
| Left colitis (n = 63) | 0.445 | <0.001 | ||||
| Inactive (n = 16) | 12 (75) | 2 (12.5) | 2 (12.5) | 0 | ||
| Mild (n = 10) | 3 (30) | 2 (20) | 5 (50) | 0 | ||
| Moderate (n = 19) | 1 (5.3) | 2 (10.5) | 12 (63) | 4 (21.1) | ||
| Severe (n = 18) | 2 (11.1) | 3 (16.7) | 9 (50) | 4 (22.2) | ||
| Pancolitis (n = 46) | 0.039 | 0.706 | ||||
| Inactive (n = 8) | 0 | 1 (12.5) | 7 (87.5) | 0 | ||
| Mild (n = 8) | 2 (25) | 2 (25) | 2 (25) | 2 (25) | ||
| Moderate (n = 7) | 1 (14.3) | 0 | 5 (71.4) | 1 (14.3) | ||
| Severe (n = 23) | 4 (17.4) | 4 (17.4) | 8 (34.8) | 7 (30.4) | ||
| Overall cohort (n = 109) | 0.272 | <0.001 | ||||
| Inactive (n = 24) | 12 (48) | 3 (18.8) | 9 (18) | 0 | ||
| Mild (n = 18) | 5 (20) | 4 (25) | 7 (14) | 2 (11.1) | ||
| Moderate (n = 26) | 2 (8) | 2 (12.5) | 17 (34) | 5 (27.8) | ||
| Severe (n = 41) | 6 (24) | 7 (43.8) | 17 (34) | 11 (61.1) |
Fig. 1.
Concordance of Harpaz histological scores with Rachmilewitz endoscopic scores.
In the pancolitis cohort, Rachmilewitz ESS revealed that 8 patients had inactive ESS, 8 had mild ESS, 7 had moderate ESS and 23 had severe ESS. By contrast, HSS revealed that 7 patients had inactive colitis, 7 had mild colitis, 22 had moderate colitis and 10 had severe colitis (table 3). There was no agreement between the Harpaz HSS and Rachmilewitz ESS in the pancolitis cohort: all 8 cases (100%) with inactive Rachmilewitz ESS were misclassified as mild or moderate Harpaz HSS; 6 (75%) of the 8 cases with mild Rachmilewitz ESS were misclassified as inactive, moderate or severe Harpaz HSS; 2 (28.6%) of the 7 cases with moderate Rachmilewitz ESS were misclassified as inactive or severe Harpaz HSS, and 16 (69.6%) of the 23 cases with severe Rachmilewitz ESS were misclassified as inactive, mild or moderate Harpaz HSS (κ: −0.194, 95% CI −0.292 to 0.096, p = 0.187; κ: 0.125, 95% CI −0.206 to 0.456, p = 0.397; κ: 0.148, 95% CI −0.066 to 0.362, p = 0.175; κ: 0.174, 95% CI −0.061 to 0.409, p = 0.153, respectively).
In the overall patient cohort, Rachmilewitz ESS revealed that 24 patients had inactive ESS, 18 had mild ESS, 26 had moderate ESS and 41 had severe ESS. By contrast, HSS revealed that 25 patients had inactive colitis, 16 (17.4%) had mild colitis, 50 (54.5%) had moderate colitis and 18 (19.6%) had severe colitis (table 3). In the overall cohort there was a slight agreement between the endoscopic and histological activity scores in inactive cases: 12 (50%) of the 24 cases with inactive Rachmilewitz ESS were misclassified as mild or moderate Harpaz HSS; 14 (77.8%) of the 18 cases with mild Rachmilewitz ESS were misclassified as inactive, moderate or severe Harpaz HSS; 9 (34.6%) of the 26 cases with moderate Rachmilewitz ESS were misclassified as inactive, mild or severe Harpaz HSS, and 30 (73.2%) of the 41 cases with severe Rachmilewitz ESS were misclassified as inactive, mild or moderate based on the Harpaz HSS (κ: 0.342, 95% CI 0.136 to 0.548, p < 0.001; κ: 0.095, 95% CI −0.117 to 0.306, p = 0.322; κ: 0.195, 95% CI 0.028 to 0.362, p = 0.022; κ: 0.186, 95% CI 0.016 to 0.357, p = 0.024; table 3).
The ROC curve showed that Rachmilewitz EAI scores ≤3, which indicated inactive disease, predicted an inactive Harpaz HSS with a sensitivity of 67% and specificity of 91% in the left-sided colitis cohort. However, in the pancolitis cohort, the ROC curve showed that Rachmilewitz EAI scores ≤3 predicted an inactive Harpaz HSS with a sensitivity below 1% and specificity of 80% (fig. 2).
Fig. 2.
ROC curves for Rachmilewitz endoscopic scores with the Harpaz histological scores as a state variable.
Discussion
In this study, there was a very poor and slight agreement between the endoscopic and histopathological scores in cases with inactive and moderately active disease in the left-sided colitis group, and there was no significant agreement between the endoscopic and histological activity scores in the pancolitis group.In the overall cohort, there was a slight agreement between the endoscopic and histological activity scores in the inactive cases. Furthermore, the concordance between the histopathological and endoscopic activity indices was poor. The low endoscopic activity scores predicted inactive histopathological disease with a low sensitivity.
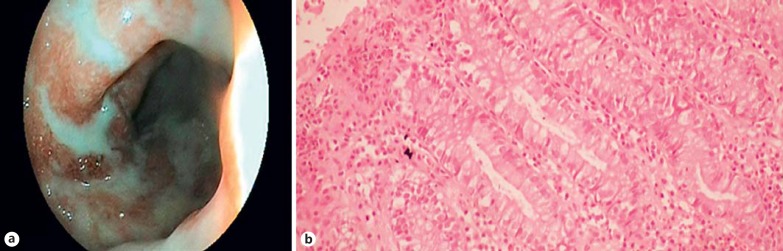
There was a poor agreement between the Rachmilewitz EAI and Harpaz HSS. Although it may be expected that pathological findings would correlate with the endoscopic and clinical findings, disparities among the clinical, endoscopic and pathological data were revealed [10]. While endoscopic studies can appear to be normal, histopathological activity can be high (fig. 3). Additionally, when an endoscopic approach is indicative of severe disease, the histopathological findings can be normal (fig. 4). In their research, Truelove and Richards [15] showed that although endoscopic findings were normal after successful induction therapy, there were frequent histological findings of active inflammation. Similarly, in clinically and endoscopically inactive UC cases, pathological signs of inflammation have been shown to increase the likelihood of a relapse occurring [16,17]. Our study is similar to previous studies in UC patients conducted with other scoring systems [16,17]. The poor concordance between the endoscopic and histopathological scoring systems could be due to the result of a delayed reflection of mucosal changes in the macroscopic appearance. Although endoscopic findings indicate normal mucosa, microscopic inflammatory processes may occur. Furthermore, in experimental animal models, Bou-Fersen et al. [18] reported that ultrastructural changes occur in the Golgi apparatus, mitochondria and endoplasmic reticulum in the mucosal and muscle layers of both the inflamed colon and uninflamed ileum of colitis.
Fig. 3.
a Mucosa appearing endoscopically normal and the lumen of the colon. b Harpaz grade 2: cryptitis, crypt abscess and neutrophils in the lamina propria. Original magnification ×200.
Fig. 4.
a Rachmilewitz EAI 10: absent vascular pattern, mucosal friability, marked erosions and ulceration. b Harpaz grade 0 with no epithelial infiltration by neutrophils. Original magnification ×200.
Another plausible explanation for the poor concordance might be an endoscopist's preference for selecting a mucosal area for biopsy during endoscopy. Whereas some endoscopists prefer not to take biopsies from intensely active areas because of the risk of complications, such as perforation, others may prefer to take biopsies in more active areas to determine the highest disease activity. Therefore, it is of critical importance to evaluate multiple biopsies during diagnosis and follow-up [19]. For cases in routine daily practice, it is necessary to rule out more severe inflammation with serial biopsy sections.
Regardless of the reason for the poor concordance, this study indicates that evaluating both endoscopic and pathological findings could provide the most benefit to the patient. Lemmens et al. [1] reported a significant correlation between an endoscopic Mayo score and Geboes histological score for UC patients, but they suggested that for clinical follow-up and treatment decisions, histological scores along with an endoscopic examination should also be used. Furthermore, they suggested that a microscopic examination would be better than an endoscopic evaluation, especially to identify severe disease. The nature of mucosal inflammation and its degree is important in UC follow-up and treatment. Especially when there is no distinctive endoscopic mucosal disease, the existence of histopathologically active disease can guide clinicians to properly treat the disease [20].
Conclusion
In this study, the concordance between histopathological and endoscopic activity indices was poor. Because neither the histopathological nor endoscopic score was a better technique for evaluating disease activity, we recommend that the follow-up of patients with UC and determination of a treatment plan be decided multidimensionally by considering both histopathological and endoscopic results.
References
- 1.Lemmens B, Arijs I, van Assche G, et al. Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm Bowel Dis. 2013;19:1194–1201. doi: 10.1097/MIB.0b013e318280e75f. [DOI] [PubMed] [Google Scholar]
- 2.Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease: is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8:1582–1597. doi: 10.1016/j.crohns.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Dave M, Loftus EV., Jr Mucosal healing in inflammatory bowel disease - a true paradigm of success? Gastroenterol Hepatol. 2012;8:29–38. [PMC free article] [PubMed] [Google Scholar]
- 4.Ha C, Kornbluth A. Mucosal healing in inflammatory bowel disease: where do we stand? Curr Gastroenterol Rep. 2010;12:471–478. doi: 10.1007/s11894-010-0146-8. [DOI] [PubMed] [Google Scholar]
- 5.Cooney RM, Warren BF, Altman DG, et al. Outcome measurement in clinical trials for ulcerative colitis: towards standardisation. Trials. 2007;8:17–26. doi: 10.1186/1745-6215-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosli MH, Feagan BG, Sandborn WJ, et al. Histologic evaluation of ulcerative colitis: a systematic review of disease activity indices. Inflamm Bowel Dis. 2014;20:564–575. doi: 10.1097/01.MIB.0000437986.00190.71. [DOI] [PubMed] [Google Scholar]
- 9.Hefti MM, Chessin DB, Harpaz NH, et al. Severity of inflammation as a predictor of colectomy in patients with chronic ulcerative colitis. Dis Colon Rectum. 2009;52:193–197. doi: 10.1007/DCR.0b013e31819ad456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoepfer AM, Beglinger C, Straumann A, et al. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851–1858. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 11.Geboes K, Dalle I. Influence of treatment on morphological features of mucosal inflammation. Gut. 2002;50:37–42. doi: 10.1136/gut.50.suppl_3.iii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lichtenstein GR, Yan S, Bala M, et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology. 2005;128:862–869. doi: 10.1053/j.gastro.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 13.Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 14.Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall/CRC; 1991. [Google Scholar]
- 15.Truelove SC, Richards WC. Biopsy studies in ulcerative colitis. Br Med J. 1956;1:1315–1318. doi: 10.1136/bmj.1.4979.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley SA, Mani V, Goodman MJ, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–178. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitton A, Peppercorn MA, Antonioli DA, et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13–20. doi: 10.1053/gast.2001.20912. [DOI] [PubMed] [Google Scholar]
- 18.Bou-Fersen AM, Anim JT, Khan I. Experimental colitis is associated with ultrastructural changes in inflamed and uninflamed regions of the gastrointestinal tract. Med Princ Pract. 2008;17:190–196. doi: 10.1159/000117791. [DOI] [PubMed] [Google Scholar]
- 19.Feakins RM. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013;66:1005–1026. doi: 10.1136/jclinpath-2013-201885. [DOI] [PubMed] [Google Scholar]
- 20.Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:483–489. doi: 10.1016/j.cgh.2010.12.028. [DOI] [PubMed] [Google Scholar]