Abstract
The present study aimed to explore the mechanisms by which c-Myc is involved in mitotic catastrophe. HeLa-630 is a cell line stably silenced for c-Myc expression that was established in the laboratory of the School of Radiation Medicine and Protection. Multinucleated cells were observed in this line, and gene expression analysis was utilized to examine differences in gene expression in these cells compared with in the control cells transfected with the control plasmid. Gene ontology analysis was performed for differentially expressed genes. Expression profile analyses revealed that cells with silenced c-Myc exhibited abnormal expression patterns of genes involved in various functions, including the regulation of microtubule nucleation, centrosome duplication, the formation of pericentriolar material, DNA synthesis and metabolism, protein metabolism and the regulation of ion concentrations. Pathway analyses of differentially expressed genes demonstrated that these genes were primarily involved in diverse signal transduction pathways, including not only the adherens junction pathway, the transforming growth factor-β signaling pathway and the Wnt signaling pathway, among others, but also signaling pathways with roles in cytokine and immune regulation. The proportion of multinucleated cells with multipolar spindles was significantly higher in silenced c-Myc cells as compared with the control cells, and this discrepancy became more pronounced following cell irradiation. The inhibition of c-Myc in tumors may account for the radiosensitization of certain tumor cell types.
Keywords: c-Myc, mitotic catastrophe, radiosensitization
Introduction
Previous studies have established that c-Myc serves important roles in cell cycle regulation, cell growth, differentiation, transformation, apoptosis and genomic instability (1,2). The c-Myc protein also fulfills essential functions in damage repair processes; in particular, this protein is associated not only with the promoters of the double-stranded break repair genes Nijmegen breakage syndrome 1, Ku70, Rad51, breast cancer 2, early onset, Rad50, Rad54L and DNA-dependent protein kinase catalytic subunit (DNA-PKcs), but also with the expression of various mismatch repair genes (3–5). The inhibition of proper DNA-damage repair by c-Myc can lead to genomic instability, causing tumor cells to become sensitive to specific drugs and/or radiation (6). Our prior studies (7,8) revealed the existence of a novel signaling pathway for maintaining the stability of the c-Myc protein: DNA-PKcs/protein kinase B (Akt)/glycogen synthase kinase 3 (GSK3)/c-Myc. Our recent study determined that c-Myc participates in the repair process of ionizing radiation (IR)-induced DNA double-strand breaks (9). It has been established that IR can cause a variety of DNA damage and abnormal checkpoints of cell cycle; each is able to induce mitotic catastrophe (10). In 2012, Kessler et al (11) reported that a SUMOylation-dependent transcriptional sub-program is required for the carcinogenic effects of the c-Myc gene. The inactivation of SUMO-activating enzyme (SAE) 2 leads to the excessive activation of the c-Myc gene, which induces mitotic catastrophe and cell death (11). These types of SUMOylation-dependent Myc switcher genes are essential for mitotic spindle function (11). The results of this study (11) provide further evidence for the involvement of c-Myc in mitosis. However, whether c-Myc is directly involved in the process of ionizing radiation-induced mitotic catastrophe remains unclear. Therefore, the current study aimed to investigate this issue.
Materials and methods
Materials and reagents
Human cervical epithelial cells (HeLa-NC) purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences (Shanghai, China) and c-Myc silenced cells (HeLa-630 cells stably expressing myc-shRNA-630 were generated in our previous study) (9), and were maintained in the laboratory of the School of Radiation Medicine and Protection (Suzhou, China) (9). The HeLa-NC and HeLa-630 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Biological Industries, Beit Haemek, Israel) at 37°C in a humidified incubator with 5% CO2. Protease inhibitor tablets were purchased from Roche Diagnostics GmbH (Mannheim, Germany). Microarray detection was performed with assistance from Shanghai Kang-Chen Biotechnology (Shanghai, China).
mRNA microarray. RNA extraction
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to extract total RNA according to the manufacturer's instructions, and the resulting RNA solutions were stored at −70°C. The ratio of OD260/OD280 was utilized to determine the purity, and the product of OD260 multiplied by 40 µg/ml was the concentration of RNA samples. Denaturing agarose gel electrophoresis was used to detect RNA integrity (12).
The synthesis of cDNA and the fluorescent labeling of samples
Once double-stranded cDNA was synthesized, the DNA was labeled using a NimbleGen One-Color labeling kit (Roche NimbleGen, Inc., Madison, WI, USA). The samples were then labeled with Cy3-random nonamers (supplied in the kit). Based on the concentrations (determined using a NanoDrop ND-1000; Thermo Fisher Scientific, Inc.) of the labeled products, the volume of the Cy3-labeled sample in each tube was calculated (1 µg cDNA in 40 µl diluted Cy3-random nonamers). Equal quantities of samples were used for subsequent stages of the hybridization experiments.
Genome-wide microarray hybridization
NimbleGen microarray (Roche NimbleGen, Inc.) was detected by Shanghai Kang-Chen Biotechnology. A total of six microarrays were used, including three biological replicates for HeLa-NC and HeLa-630 cells. The NimbleGen Hybridization System 4 (Roche NimbleGen, Inc.) was used for hybridization. The hybridization reaction was performed using a hybridization kit (Roche NimbleGen, Inc.) in accordance with NimbleGen's instructions. Once the reaction was complete, the array was subjected to elution using a Wash Buffer kit (Roche NimbleGen, Inc.) and spun dry by centrifugation (200 × g, 25°C, 5 min).
Image acquisition and data analysis
Microarray results were analyzed using a GenePix 4000 B single-channel scanner (Axon Instruments, Inc., Union City, CA, USA). NimbleScan v.2.5 (Roche NimbleGen, Inc.) was used to read the values of the raw microarray signals (532 nm); these signal values were then corrected and normalized according to the NimbleGen's instructions. GeneSpring v11.0 software (Agilent Technologies, Inc., Santa Clara, CA, USA) was used for statistical analysis, clustering and pathway analysis and visualizations. A threshold value of 2 was established; thus, increases and decreases ≥2-fold were regarded as cases of upregulation and downregulation, respectively.
Quantification of the proportion of multinucleated cells by flow cytometry
HeLa-NC and HeLa-630 cells were seeded into 60-mm culture dishes with 5×105 cells in each dish. The dishes were then exposed to 4 Gy of 60Coγ radiation. Cells were collected 48 h post-IR. The collected cells were washed with PBS, fixed in pre-cooled 70% ethanol at −20°C for 24 h, and centrifuged at 700 × g, at 4°C for 10 min. The fixative was discarded, cells were washed twice in PBS and 10 mg/ml RNase (Sangon Biotech Co., Ltd., Shanghai, China) was added to the cell samples to a final concentration of 50 µg/ml, which were then incubated at 37°C for 30 min. Propidium iodide was then added to the cell samples to a final concentration of 50 µg/ml, and cells were analyzed by flow cytometry (CyFlow Green; PARTEC GmbH, Münster, Germany). These experiments were repeated three times.
Immunofluorescence analysis of multinucleated cells and mitotic catastrophe
HeLa-NC and HeLa-630 cells (104 cells) were seeded onto cover slips (circular; diameter, 12 mm; Thermo Fisher Scientific, Inc.). These cells were cultured overnight in a 37°C incubator and then exposed to 4 Gy of 60Coγ radiation. Cells were collected at 48 h post-IR. The collected cells were washed twice in PBS, fixed with 4% paraformaldehyde at room temperature for 30 min, washed three times with PBS for 10 min per wash, permeabilized in a 0.3% Triton X-100 solution on ice for 15 min and then blocked in a solution of PBS and 1% fetal bovine serum albumin (Biological Industries) at room temperature for 1 h. Each cell sample was then incubated overnight at 4°C with a 1:200 dilution of a primary anti-α-tubulin (cat no. 2125; Cell Signaling Technology, Inc., Danvers, MA, USA) or an anti-γ-tubulin antibody (cat no. ab16504; Abcam, Cambridge, UK). Following this incubation, samples were washed three times in PBS for 10 min per wash and then incubated with Alexa Fluor® 488 Phalloidin (green; cat no. 8878) or Alexa Fluor® 647 Phalloidin (red; cat no. 8940) labeled secondary antibody (Cell Signaling Technology, Inc.) at room temperature for 1 h (incubation began in the dark). Finally, each sample was washed three times with PBS for 10 min per wash, stained with DAPI (2 mg/ml; Cell Signaling Technology, Inc.) for 10 min at room temperature and imaged using confocal laser scanning microscopy (FV1000; FV10-ASW software; Olympus Corporation, Tokyo, Japan).
Statistical analysis
Experimental data are expressed as the mean ± standard deviation. The SPSSv.10.0 software package (SPSS, Inc., Chicago, IL, USA) was used to process and analyze the data. Differences between groups were determined using unpaired two-tailed Student's t-tests. P<0.05 was considered to indicate a statistically significant difference.
Results
An increase in multinucleated cells within the HeLa-630 cell population
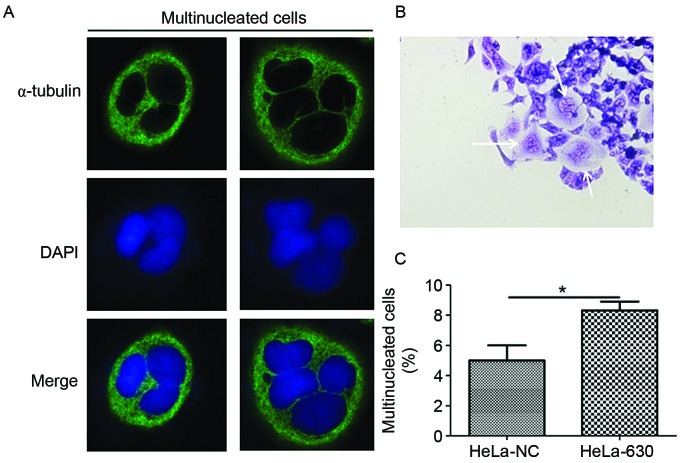
Light microscopy observation revealed that multinucleation frequently occurred in HeLa-630 cells during the G418 selection and growth process (Fig. 1A). Therefore, immunofluorescence and flow cytometry were used to further examine the HeLa-630 cells. Immunofluorescence results demonstrated that multinucleated cells, including cells with 3–4 nuclei, were identified among HeLa-630 and HeLa-NC cells (Fig. 1B). The flow cytometry results indicated that there were a greater number of multinucleated cells among the HeLa-630 cell population compared with the HeLa-NC cell population (Fig. 1C).
Figure 1.
The multinucleated cells in c-Myc-knockdown cells (HeLa-630). (A) Multinucleated cells observed under laser-scanning confocal microscopy. (B) Multinucleated cells observed under light microscopy. (C) The quantity of multinucleated cells as detected by flow cytometry. *P<0.05 compared with HeLa-NC cells.
Differentially expressed genes in HeLa-630 cells
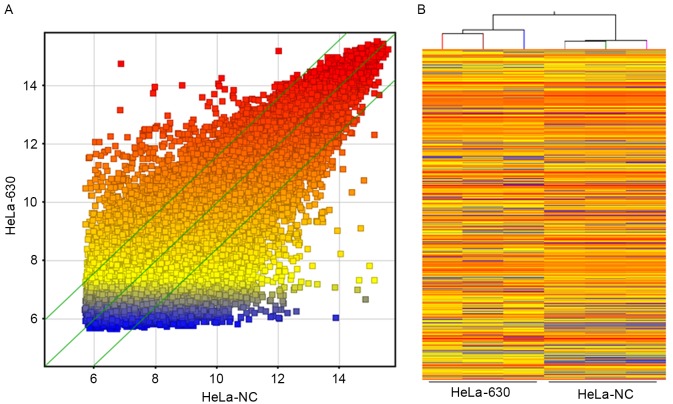
Whole-genome expression profiles for three dishes of HeLa-NC cells and three dishes of HeLa-630 cells revealed that ≤2745 genes exhibited differential expression in these two cell lines, based on the statistical significance parameters of P<0.05 and a fold change of 2; of these genes, 1,098 were upregulated in HeLa-630 cells and 1,647 were downregulated in HeLa-630 cells. These results are presented as a cluster analysis and a scatter plot in Fig. 2.
Figure 2.
(A) A scatter plot and (B) a hierarchical clustering analysis diagram of genome-wide expression microarray data.
GeneSpring v.11.0 software was used to perform GO analysis and signaling pathway analysis of differentially expressed genes from the whole-genome expression profile microarray results, in order to investigate the biological significance of these genes. In the analysis results, changes in gene expression levels are presented in terms of the three GO domains, as follows: Biological process (BP); molecular function (MF); cellular component (CC). The first 10 terms in each category for downregulated and upregulated genes are arranged from highest to lowest p-value in Tables I and II, respectively.
Table I.
GO analysis of the biological functions of downregulated mRNAs.
| GO.ID | Term | Count | Pop.Hits |
|---|---|---|---|
| BP | |||
| GO:0007020 | Microtubule nucleation | 6 | 10 |
| GO:0010718 | Positive regulation of epithelial to mesenchymal transition | 10 | 18 |
| GO:0010770 | Positive regulation of cell morphogenesis involved in differentiation | 10 | 19 |
| GO:0048820 | Hair follicle maturation | 6 | 12 |
| GO:0030206 | Chondroitin sulfate biosynthetic process | 5 | 10 |
| GO:0060325 | Face morphogenesis | 5 | 10 |
| GO:0045197 | Establishment or maintenance of epithelial cell apical/basal polarity | 5 | 11 |
| GO:0070723 | Response to cholesterol | 5 | 11 |
| GO:0010717 | Regulation of epithelial to mesenchymal transition | 12 | 27 |
| GO:0051298 | Centrosome duplication | 7 | 16 |
| CC | |||
| GO:0000242 | Pericentriolar material | 5 | 12 |
| GO:0009925 | Basal plasma membrane | 7 | 22 |
| GO:0045178 | Basal part of cell | 8 | 26 |
| GO:0005868 | Cytoplasmic dynein complex | 3 | 10 |
| GO:0005952 | cAMP-dependent protein kinase complex | 3 | 10 |
| GO:0012507 | ER to Golgi transport vesicle membrane | 5 | 17 |
| GO:0043073 | Germ cell nucleus | 5 | 17 |
| GO:0005901 | caveolae | 16 | 55 |
| GO:0030663 | COPI coated vesicle membrane | 4 | 14 |
| GO:0005801 | cis-Golgi network | 6 | 22 |
| MF | |||
| GO:0005160 | Transforming growth factor-β receptor binding | 9 | 19 |
| GO:0070411 | I-SMAD binding | 5 | 11 |
| GO:0004115 | 3′,5′-cyclic-AMP phosphodiesterase activity | 7 | 16 |
| GO:0005072 | Transforming growth factor-β receptor, cytoplasmic mediator activity | 4 | 10 |
| GO:0004675 | Transmembrane receptor protein serine/threonine kinase activity | 7 | 19 |
| GO:0051010 | Microtubule plus-end binding | 4 | 11 |
| GO:0051879 | Hsp90 protein binding | 4 | 11 |
| GO:0005024 | Transforming growth factor-β receptor activity | 6 | 17 |
| GO:0004697 | Protein kinase C activity | 5 | 15 |
| GO:0045295 | γ-catenin binding | 4 | 12 |
GO, gene ontology.
Table II.
GO analysis of the biological functions of upregulated mRNAs.
| GO.ID | Term | Count | Pop.Hits |
|---|---|---|---|
| BP | |||
| GO:0007512 | Adult heart development | 5 | 12 |
| GO:0032098 | Regulation of appetite | 5 | 12 |
| GO:0042749 | Regulation of circadian sleep/wake cycle | 6 | 15 |
| GO:0045187 | Regulation of circadian sleep/wake cycle, sleep | 6 | 15 |
| GO:0022410 | Circadian sleep/wake cycle process | 6 | 16 |
| GO:0050802 | Circadian sleep/wake cycle, sleep | 6 | 16 |
| GO:0001977 | Renal system process involved in regulation of blood volume | 4 | 11 |
| GO:0050482 | Arachidonic acid secretion | 4 | 11 |
| GO:0042745 | Circadian sleep/wake cycle | 6 | 17 |
| GO:0050879 | Multicellular organismal movement | 8 | 23 |
| CC | |||
| GO:0005865 | Striated muscle thin filament | 5 | 14 |
| GO:0000786 | Nucleosome | 18 | 66 |
| GO:0001772 | Immunological synapse | 3 | 12 |
| GO:0034362 | Low-density lipoprotein particle | 3 | 12 |
| GO:0034364 | High-density lipoprotein particle | 5 | 24 |
| GO:0034361 | Very-low-density lipoprotein particle | 4 | 20 |
| GO:0034385 | Triglyceride-rich lipoprotein particle | 4 | 20 |
| GO:0042734 | Presynaptic membrane | 7 | 39 |
| GO:0032993 | Protein-DNA complex | 18 | 102 |
| GO:0032994 | Protein-lipid complex | 6 | 35 |
| MF | |||
| GO:0051183 | Vitamin transporter activity | 5 | 15 |
| GO:0015250 | Water channel activity | 3 | 10 |
| GO:0015026 | Co-receptor activity | 6 | 21 |
| GO:0030547 | Receptor inhibitor activity | 4 | 14 |
| GO:0005372 | Water transmembrane transporter activity | 3 | 11 |
| GO:0005184 | Neuropeptide hormone activity | 6 | 23 |
| GO:0005313 | L-glutamate transmembrane transporter activity | 3 | 12 |
| GO:0015172 | Acidic amino acid transmembrane transporter activity | 3 | 12 |
| GO:0016918 | Retinal binding | 3 | 12 |
| GO:0048019 | Receptor antagonist activity | 3 | 13 |
GO, gene ontology.
GO analysis for downregulated genes in HeLa-630 cells revealed that the primary terms in the BP domain included references to microtubule nucleation, regulation of epithelial-mesenchymal transition (EMT), morphological regulation during cell differentiation, follicle maturation, chondroitin sulfate synthesis, the establishment or maintenance of basal epithelial cell polarity, response to cholesterol, centrosome duplication and various other processes. Major terms in the GO CC domain included references to pericentriolar material, the extracellular matrix, the basal region of the cell, the cytoplasmic dynein complex, the cAMP-dependent protein kinase complex, ER-Golgi transport vesicle membranes and the Golgi network, among numerous other components. Major terms in the GO MF domain included references to transforming growth factor-β (TGF-β) receptor binding, cytoplasmic mediator activity, transmembrane receptor serine/threonine kinase activity, microtubule binding, heat shock protein 90 binding, protein kinase C activity and other functions.
GO analysis for the upregulated genes in HeLa-630 cells revealed that the main terms in the BP domain included references to adult heart development, appetite regulation, circadian sleep/wake cycle regulation, renal system processes involved in blood volume regulation, arachidonic acid secretion, multicellular organismal movement and other processes. The main terms in the GO CC domain included references to striated muscle thin filaments, nucleosomes, immunological synapses, low-density lipoprotein, high-density lipoprotein, very-low-density lipoprotein, the presynaptic membrane, protein-DNA complexes and protein-lipid complexes, among various other components. Major terms in the GO MF domain included references to vitamin transport activity, water channel activity, co-receptor activity, receptor inhibitor activity, water transmembrane transporter activity, neuropeptide hormone activity, L-glutamate transmembrane transporter activity, acidic amino acid transmembrane transporter activity, retinal binding, receptor antagonist activity and numerous other functions.
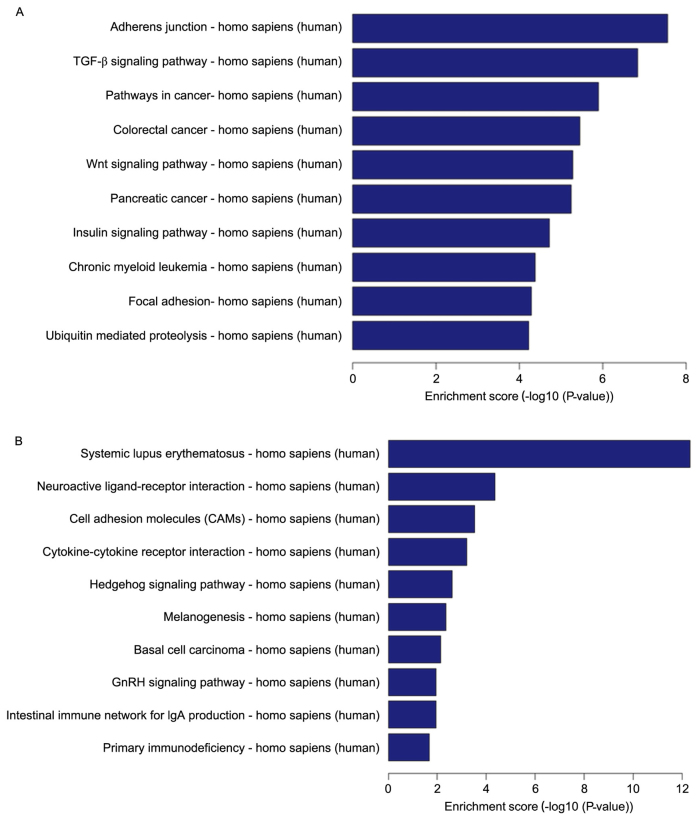
The pathway analysis for the 10 results with the highest p-values of among the downregulated differentially expressed genes and the pathway analysis of upregulated genes are presented in Fig. 3. Downregulated differentially expressed genes were mainly principally involved in adherens junctions, the TGF-β signaling pathway, pathways in cancer, the Wnt signaling pathway, the insulin signaling pathway, focal adhesions and ubiquitin-mediated proteolysis, among other diverse activities functions (Fig. 3A). Upregulated differentially expressed genes were mainly primarily involved in systematic lupus erythematosus, neuroactive ligand-receptor interactions, cell adhesion, cytokine-cytokine receptor interactions, the Hedgehog signaling pathway, melanogenesis, basal cell carcinoma, the gonadotropin-releasing hormone (GnRH) signaling pathway, the intestinal immune network for IgA production, and primary immunodeficiency, among various other activities (Fig. 3B).
Figure 3.
Gene ontology analysis of differentially expressed mRNAs involved in signaling pathways. (A) Downregulated genes. (B) Upregulated genes.
Mitotic catastrophe in HeLa-630 cells following irradiation
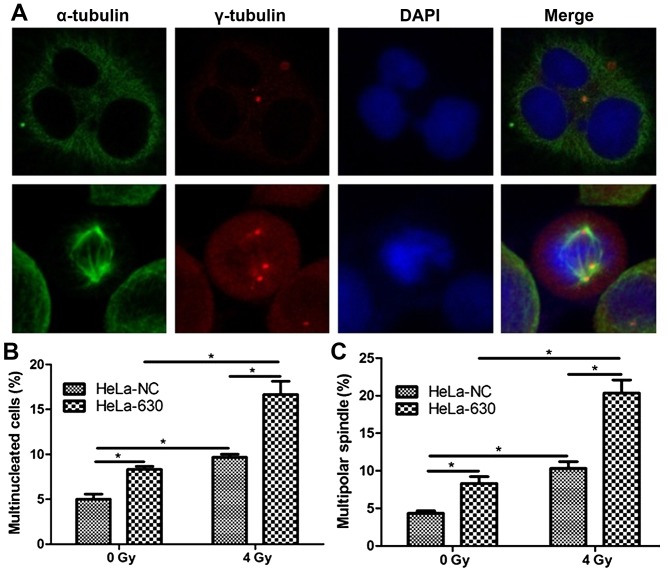
As indicated in Fig. 4, following the exposure of HeLa-630 cells to 4 Gy of 60Coγ radiation, various abnormalities were observed, including centriole abnormalities and multipolar spindles. Flow cytometry revealed that, at 48 h post radiation, the proportion of multinucleated cells was significantly greater in the HeLa-630 cell group, as compared with in the HeLa-NC cell group (P<0.05).
Figure 4.
Mitotic catastrophe at 48 h following radiation exposure in HeLa-NC and HeLa-630 cells. (A) Multinucleated cells and multipolar spindles in HeLa-630 cells. (B) Quantity of multinucleated cells as determined by flow cytometry. (C) Quantity of multipolar spindles as determined by confocal microscopy. *P<0.05.
Discussion
C-Myc was initially discovered as a proto-oncogene with activity in numerous types of human malignancies; however, c-Myc is also a transcription factor and a certain level of c-Myc protein expression is required for the growth and proliferation of normal cells (13,14). However, the overexpression of c-Myc in transgenic mice induces malignant T-cell lymphoma and acute myeloid leukemia (15); in these mice, the inhibition of c-Myc expression produces significant anti-tumor effects. In c-Myc knockout mice, normal embryonic development proceeds for 9.5–10.5 days, but will subsequently cease (16); this finding indicates that, in mammals, c-Myc serves a vital role in normal embryonic development and in overall growth and developmental processes. In addition, c-Myc promotes the self-renewal and differentiation of stem and progenitor cells in mice and contributes to maintaining embryonic stem cell characteristics (17,18). Takahashi et al (19) used a retroviral vector to co-transfect the four reprogramming factors octamer-binding transcription factor 4 (Oct4), SRY-box 2 (Sox2), c-Myc and Krüppel-like factor 4 (Klf4) into mouse/human fibroblasts and produced induced pluripotent stem cells (iPSCs). Furthermore, this generation of iPSCs does not require egg cells or embryos; instead, only the introduction of the four transcriptionally active genes Oct4, SOX2, c-Myc and Klf4 into the cells is required (20). These findings suggest that c-Myc has essential functions in cell proliferation, growth and transformation. Recent studies have demonstrated that c-Myc, its target genes and its microRNAs form a complex regulatory network that serves a vital role in the maintenance of cancer stem cells, EMT, metastasis and angiogenesis (21). The exploration of c-Myc functions may provide novel perspectives regarding the development of cancer treatments that target c-Myc.
In the laboratory of the School of Radiation Medicine and Protection, RNA interference techniques were utilized to establish a cell model in which c-Myc expression was inhibited (22). In the current study, an increased number of multinucleated cells were observed in the HeLa-630 cell group. There are two major causes for the formation of multinucleated cells (e.g., polyploidy). First, cell fusion can occur, wherein the cytoplasm of independent cells mixes, resulting in the generation of syncytia (23). Second, multinucleated cells may be produced when nuclear division occurs in mononuclear cells, but cellular division does not occur due to failed cytokinesis (24). In this second case, polyploid cells formed by the failure of cytokinesis cannot effectively undergo mitosis, thus, these cells must eventually die. This fate is regarded as a form of proliferative cell death (25). Therefore, the proportion of multinucleated cells was elevated in the HeLa-630 cell population due to a reduction in c-Myc protein expression. The results of GO analysis of the downregulated genes in these cells revealed that changes in the three BP, MF and CC domains suggested that c-Myc is involved in the regulation of cell growth and morphology. Following c-Myc silencing, abnormalities in microtubule nucleation, centrosome duplication and pericentriolar material occur; these phenomena may help to explain why the proportion of multinucleated cells is elevated in HeLa-630 cells. In our prior studies (7,8), a novel signaling pathway was proposed for maintaining the stability of the c-Myc protein: DNA-PKcs/Akt/GSK3/c-Myc. Previous findings have identified DNA-PKcs as an upstream regulator of c-Myc protein stability (26). Inhibiting the expression or activity of DNA-PKcs leads to various cellular abnormalities during mitosis, includingthe presence of extraneous centrosomes, abnormal spindle structure and microtubule damage (27). Furthermore, due to cytokinesis failure, the inhibition of DNA-PKcs induces various phenotypic changes, including an increase in the proportion of multinucleated cells (28). An elevated proportion of multinucleated cells due to DNA-PKcs inactivation may occur not only as a result of damage from ionizing radiation, but also under natural growth conditions (29). From the results of the current study, it may be hypothesized that the c-Myc protein serves an important role in the maintenance of karyotype stability by DNA-PKcs.
The results of GO analysis of the upregulated genes in HeLa-630 cells revealed that changes in the three BP, MF and CC domains suggested that the c-Myc protein is also involved in the regulation of DNA synthesis and metabolism, protein metabolism and the regulation of ion concentrations, among various other functions. It has previously been reported that c-Myc is involved in the regulation of DNA synthesis and metabolism via promoting the transcription of the carbamoyl-phosphate synthetase 2/aspartate transcarbamylase/dihydroorotasegene (30). This metabolism-regulating function may be involved in the formation of multinucleated cells.
Downregulated differentially expressed genes were primarily involved in the adherens junctions and the TGF-β signaling pathway. An increasing body of evidence indicates that alterations in the levels of cadherin-dependent adherens junctions regulate the stability of tight junction complexes (31). Reduced expression of the c-Myc gene repressed E-cadherin promoter activity, and subsequently decreased E-cadherin mRNA and protein levels (32). Blocking TGF-β upregulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells (33). Therefore, c-Myc serves a role in cancer via E-cadherin and the TGF-β pathway. Based on the comprehensive findings of the aforementioned investigations and the results of the current study, it is evident that c-Myc is involved in mitotic processes. With regard to the significance of this finding in radiotherapy, a series of previous reports have demonstrated that the overexpression of c-Myc contributes to cancer radioresistance (34,35). One study suggested that c-Myc downregulation sensitizes melanoma cells to radiotherapy by inhibiting MLH1 and MSH2 mismatch repair proteins in a p53-independent manner (4). Another study revealed that the sensitization of non-Hodgkin's lymphoma cells to ionizing radiation by rituximab is achieved through the inhibition of c-Myc expression (36). In the present study, it was observed that irradiation causes a greater amount of mitotic catastrophe in HeLa-630 cells than HeLa-NC cells. It maybe concluded that the radiosensitization of certain tumor cell types may be achieved by inducing mitotic catastrophe through the inhibition of c-Myc expression. In conclusion, the results of the current study demonstrated that inhibition of c-Myc expression accounts for an increased number of multinucleated cells in HeLa cells, and that the radiosensitization of inactivating c-Myc oncogene may be associated with an increased degree of mitotic catastrophe.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81172706) and by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- 1.Cowling VH, Cole MD. Mechanism of transcriptional activation by the Myc oncoproteins. Semin Cancer Biol. 2006;16:242–252. doi: 10.1016/j.semcancer.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6:635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 3.Chiang YC, Teng SC, Su YN, Hsieh FJ, Wu KJ. c-Myc directly regulates the transcription of the NBS1 gene involved in DNA double-strand break repair. J Biol Chem. 2003;278:19286–19291. doi: 10.1074/jbc.M212043200. [DOI] [PubMed] [Google Scholar]
- 4.Bucci B, D'Agnano I, Amendola D, Citti A, Raza GH, Miceli R, De Paula U, Marchese R, Albini S, Felsani A, et al. Myc down-regulation sensitizes melanoma cells to radiotherapy by inhibiting MLH1 and MSH2 mismatch repair proteins. Clin Cancer Res. 2005;11:2756–2767. doi: 10.1158/1078-0432.CCR-04-1582. [DOI] [PubMed] [Google Scholar]
- 5.Bindra RS, Glazer PM. Co-repression of mismatch repair gene expression by hypoxia in cancer cells: Role of the Myc/Max network. Cancer Lett. 2007;252:93–103. doi: 10.1016/j.canlet.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Bristow RG, Ozcelik H, Jalali F, Chan N, Vesprini D. Homologous recombination and prostate cancer: A model for novel DNA repair targets and therapies. Radiother Oncol. 2007;83:220–230. doi: 10.1016/j.radonc.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 7.An J, Huang YC, Xu QZ, Zhou LJ, Shang ZF, Huang B, Wang Y, Liu XD, Wu DC, Zhou PK. DNA-PKcs plays a dominant role in the regulation of H2AX phosphorylation in response to DNA damage and cell cycle progression. BMC Mol Biol. 2010;11:18. doi: 10.1186/1471-2199-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An J, Xu QZ, Sui JL, Bai B, Zhou PK. Downregulation of c-Myc protein by siRNA-mediated silencing of DNA-PKcs in HeLa cells. Int J Cancer. 2005;117:531–537. doi: 10.1002/ijc.21093. [DOI] [PubMed] [Google Scholar]
- 9.Cui F, Fan R, Chen Q, He Y, Song M, Shang Z, Zhang S, Zhu W, Cao J, Guan H, Zhou PK. The involvement of c-Myc in the DNA double-strand break repair via regulating radiation-induced phosphorylation of ATM and DNA-PKcs activity. Mol Cell Biochem. 2015;406:43–51. doi: 10.1007/s11010-015-2422-2. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson D, Löfroth PO, Johansson L, Riklund KA, Stigbrand T. Cell cycle disturbances and mitotic catastrophes in HeLa Hep2 cells following 2.5 to 10 Gy of ionizing radiation. Clin Cancer Res. 2007;13:5501s–5508s. doi: 10.1158/1078-0432.CCR-07-0980. [DOI] [PubMed] [Google Scholar]
- 11.Kessler JD, Kahle KT, Sun T, Meerbrey KL, Schlabach MR, Schmitt EM, Skinner SO, Xu Q, Li MZ, Hartman ZC, et al. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science. 2012;335:348–353. doi: 10.1126/science.1212728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masek T, Vopalensky V, Suchomelova P, Pospisek M. Denaturing RNA electrophoresis in TAE agarose gels. Anal Biochem. 2005;336:46–50. doi: 10.1016/j.ab.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/MCB.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalra N, Kumar V. c-Fos is a mediator of the c-Myc-induced apoptotic signaling in serum-deprived hepatoma cells via the p38 mitogen-activated protein kinase pathway. J Biol Chem. 2004;279:25313–25329. doi: 10.1074/jbc.M400932200. [DOI] [PubMed] [Google Scholar]
- 15.Luo H, Li Q, O'Neal J, Kreisel F, Le Beau MM, Tomasson MH. c-Myc rapidly induces acute myeloid leukemia in mice without evidence of lymphoma-associated antiapoptotic mutations. Blood. 2005;106:2452–2461. doi: 10.1182/blood-2005-02-0734. [DOI] [PubMed] [Google Scholar]
- 16.Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993;7:671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda SY, Tsuneyoshi N, Sumi T, Hasegawa K, Tada T, Nakatsuji N, Suemori H. NANOG maintains self-renewal of primate ES cells in the absence of a feeder layer. Genes Cells. 2006;11:1115–1123. doi: 10.1111/j.1365-2443.2006.01000.x. [DOI] [PubMed] [Google Scholar]
- 18.Loh YH, Nq JH, Nq HH. Molecular framework underlying pluripotency. Cell Cycle. 2008;7:885–891. doi: 10.4161/cc.7.7.5636. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Han DW, Greber B, Wu G, Tapia N, Araúzo-Bravo MJ, Ko K, Bernemann C, Stehling M, Schöler HR. Direct reprogramming of fibroblasts into epiblast stem cells. Nat Cell Biol. 2011;13:66–71. doi: 10.1038/ncb2136. [DOI] [PubMed] [Google Scholar]
- 21.Jackstadt R, Hermeking H. MicroRNAs as regulators and mediators of c-MYC function. Biochim Biophys Acta. 2015;1849:544–553. doi: 10.1016/j.bbagrm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg F, Gerber SD, Rieckmann T, Trueb B. Rapid fusion and syncytium formation of heterologous cells upon expression of the FGFRL1 receptor. J Biol Chem. 2010;285:37704–37715. doi: 10.1074/jbc.M110.140517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornik TC, Neniskyte U, Brown GC. Inflammation induces multinucleation of Microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. J Neurochem. 2014;128:650–661. doi: 10.1111/jnc.12477. [DOI] [PubMed] [Google Scholar]
- 25.Zhang HY, Gu YY, Li ZG, Jia YH, Yuan L, Li SY, An GS, Ni JH, Jia HT. Exposure of human lung cancer cells to 8-chloro-adenosine induces G2/M arrest and mitotic catastrophe. Neoplasia. 2004;6:802–812. doi: 10.1593/neo.04247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An J, Yang DY, Xu QZ, Zhang SM, Huo YY, Shang ZF, Wang Y, Wu DC, Zhou PK. DNA-dependent protein kinase catalytic subunit modulates the stability of c-Myc oncoprotein. Mol Cancer. 2008;7:32. doi: 10.1186/1476-4598-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KJ, Lin YF, Chou HY, Yajima H, Fattah KR, Lee SC, Chen BP. Involvement of DNA-dependent protein kinase in normal cell cycle progression through mitosis. J Biol Chem. 2011;286:12796–12802. doi: 10.1074/jbc.M110.212969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang B, Shang ZF, Li B, Wang Y, Liu XD, Zhang SM, Guan H, Rang WQ, Hu JA, Zhou PK. DNA-PKcs associates with PLK1 and is involved in proper chromosome segregation and cytokinesis. J Cell Biochem. 2014;115:1077–1088. doi: 10.1002/jcb.24703. [DOI] [PubMed] [Google Scholar]
- 29.Shang ZF, Huang B, Xu QZ, Zhang SM, Fan R, Liu XD, Wang Y, Zhou PK. Inactivation of DNA-dependent protein kinase leads to spindle disruption and mitotic catastrophe with attenuated checkpoint protein 2 phosphorylation in response to DNA damage. Cancer Res. 2010;70:3657–3666. doi: 10.1158/0008-5472.CAN-09-3362. [DOI] [PubMed] [Google Scholar]
- 30.Eberhardy SR, Farnham PJ. c-Myc mediates activation of the cad promoter via a post-RNA polymerase II recruitment mechanism. J Biol Chem. 2001;276:48562–48571. doi: 10.1074/jbc.M109014200. [DOI] [PubMed] [Google Scholar]
- 31.El Sayegh TY, Kapus A, McCulloch CA. Beyond the epithelium: Cadherin function in fibrous connective tissues. FEBS Lett. 2007;581:167–174. doi: 10.1016/j.febslet.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene. 2005;24:8277–8290. doi: 10.1038/sj.onc.1208991. [DOI] [PubMed] [Google Scholar]
- 33.Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557–1566. doi: 10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 34.Amatangelo MD, Goodyear S, Varma D, Stearns ME. c-Myc expression and MEK1-induced Erk2 nuclear localization are required for TGF-beta induced epithelial-mesenchymal transition and invasion in prostate cancer. Carcinogenesis. 2012;33:1965–1975. doi: 10.1093/carcin/bgs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim BY, Kwak SY, Yang JS, Han YH. Phosphorylation and stabilization of c-Myc by NEMO renders cells resistant to ionizing radiation through up-regulation of γ-GCS. Oncol Rep. 2011;26:1587–1593. doi: 10.3892/or.2011.1432. [DOI] [PubMed] [Google Scholar]
- 36.Skvortsova I, Popper BA, Skvortsov S, Saurer M, Auer T, Moser R, Kamleitner H, Zwierzina H, Lukas P. Pretreatment with rituximab enhances radiosensitivity of non-Hodgkin's lymphoma cells. J Radiat Res. 2005;46:241–248. doi: 10.1269/jrr.46.241. [DOI] [PubMed] [Google Scholar]