Abstract
Objective
The aim of this study was to report clinical and molecular findings in an Emirati child with Marinesco-Sjögren syndrome born to consanguineous parents.
Clinical Presentation and Intervention
The child presented with developmental delay, ataxia, bilateral cataracts, and dysmorphic craniofacial features, along with cerebellar atrophy. Sequencing of the SIL1 gene revealed a novel homozygous large indel mutation that was predicted to abrogate part of the 5′ untranslated region (UTR) and the first 30 amino acids of the protein.
Conclusion
This was a case of mutation in SIL1 that affected the 5′ UTR, translation initiation site and the endoplasmic reticulum-targeting signal sequence. Further studies will be needed on the functional delineation of the mutation.
Key Words: Marinesco-Sjögren syndrome, SIL1, Novel mutation, Emirati
Introduction
Marinesco-Sjögren syndrome (MSS; OMIM No. 248800) is an autosomal recessive hereditary ataxia syndrome characterized by cerebellar ataxia, hypotonia, cataracts, and intellectual disability [1]. Although these are the main symptoms, there are a range of other clinical features associated with this condition in some families, including hypogonadotropic hypogonadism, skeletal abnormalities, and microcephaly [2]. The only gene known so far to be associated with MSS, SIL1, was discovered by two independent teams simultaneously [2,3]. SIL1 plays a vital role in the translocation of proteins into the endoplasmic reticulum (ER). Studies in mouse models have shown a function for SIL1 as a nucleotide exchange factor for the ER chaperone protein BiP, as well as in ER stress-induced apoptotic signaling and the ER-associated degradation (ERAD) pathway, again via its interaction with BiP [4,5]. Hence, we report a consanguineous Emirati family affected with MSS with a novel mutation in the SIL1 gene.
Case Report
The proband was a 12-year-old male child, the youngest child of first-cousin healthy parents, who presented with developmental delay, speech delay, ataxia and bilateral cataracts. He was born at term through normal vaginal delivery following an uncomplicated pregnancy with a birth weight of 3.5 kg. There were no postnatal problems. At 12 years his weight was 33 kg (10th to 25th centile), height was 147 cm (25th centile), and head circumference was 53 cm (50th to 75th centile). The parents reported that he was an active and cooperative boy. He had subtle dysmorphic features, including bushy eyebrows and a flat mid face with a high arched palate. He had an ataxic gait, with dysmetria, intention tremor and dysdiadochokinesia. A convergent squint was present in the left eye. His muscle tone was normal and deep tone reflex was elicited. Pectus carinatum was observed, and the patient was noted to have bilateral clinodactyly of the fifth fingers as well as flat feet. A skeletal survey was normal, except for a mild kyphosis seen at the lower thoracic spine. He was able to stand with support, and walked with support with an ataxic gait. A developmental assessment at 8 years of age showed that he was able to say full sentences, but with poor articulation, and needed help with dressing and feeding. At 6 years of age a pelvic ultrasound had detected a left undescended testis, for which he had undergone orchidopexy. He had also been diagnosed with infantile bilateral cataract, which had been surgically corrected.
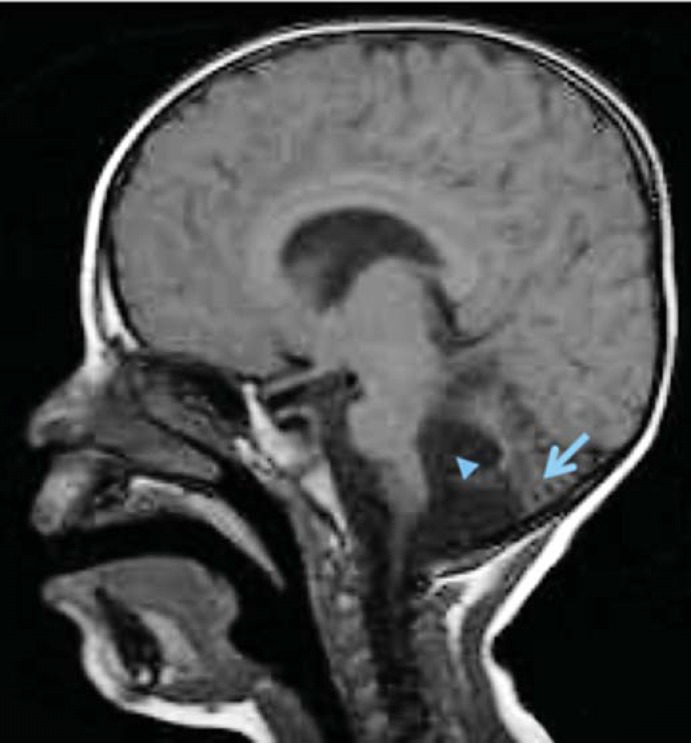
X-ray computer tomography and magnetic resonance imaging of the brain revealed cerebellar atrophy with a dilated 4th ventricle (fig. 1). All other investigations were normal and the family history was unremarkable. The patient's four living siblings were all healthy. The mother had had one spontaneous abortion. Based on the clinical features, a tentative diagnosis of MSS was made.
Fig. 1.
Magnetic resonance imaging scan of the patient. The arrow indicates cerebellar atrophy, while the arrowhead points to the dilated 4th ventricle.
Informed written consent was obtained and genomic DNA was extracted from the patient and both of his parents. Ten milliliters of venous blood was drawn from each of the individuals and sampled in EDTA tubes. The coding region of exons 2–10 and the exon-intron boundaries of the SIL1 gene were amplified by polymerase chain reaction (PCR) and sequenced directly by Bioscientia (Ingleheim, Germany). The resulting sequence data was compared with the reference sequence NM_022464. For confirmation of the results, an independent PCR product was sequenced, also by Bioscientia.
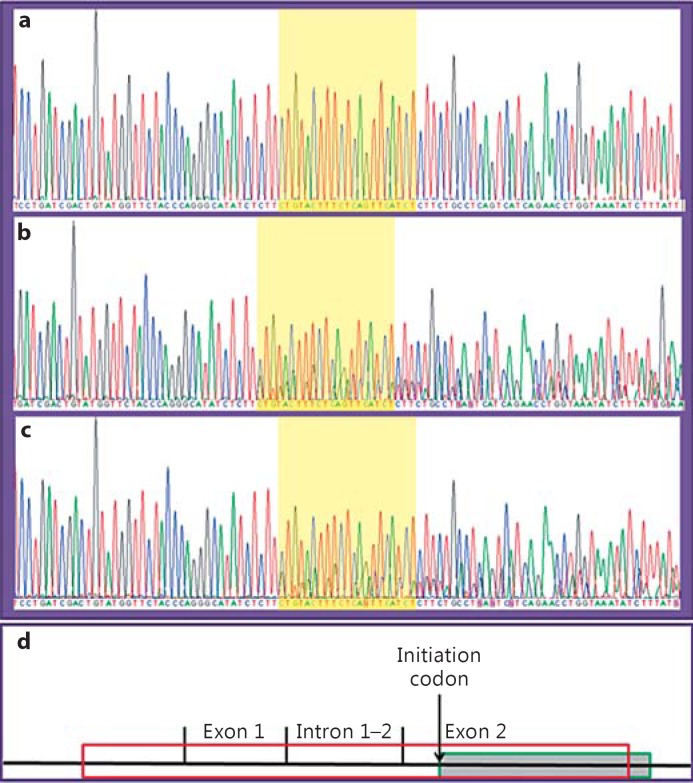
The sequencing of SIL1 revealed a homozygous deletion from the 5′ untranslated region (UTR; c.-197) to exon 1 (c.90). Instead of the deleted bases, there was a homozygous insertion of one base (C) of unclear origin and 20 bases from intron 1. Both parents turned out to be heterozygous carriers of the same c.-197_90delinsCTGTACTTTCTCAGTTCACT mutation (fig. 2). This mutation was not found in the EXAC Browser or in the GalaxC™ Allele Frequency Database, which contains >2.5 million unique Middle Eastern pathogenic mutations and variants.
Fig. 2.
Chromatograms of the patient (a), mother (b) and father (c) showing the indel mutation. d Schematic diagram showing the deletion in the context of the gene structure. The box with a red outline represents the deletion, while the shaded box with a green outline shows the ER signal sequence (colors refer to the online version only).
Discussion
This was a case of deletion in the SIL1 gene that led to MSS in an Emirati child. The mutation is expected to result in the loss of the start codon and the first 30 amino acids of the protein, as well as the entire 5′ UTR, and part of the region upstream of it. The deleted sequence included the initiation codon as well as the ER targeting sequence. Most mutations so far detected in the SIL1 gene leading up to MSS are concentrated in either intron 9 or exons 6, 9, and 10 [2,6,7]. It has been speculated that exons 6 and 9 play a pivotal role in mediating the interaction between BAP and BiP, and that exon 10 supports this interaction [2]. This view is supported by the fact that the key amino acid residues that are involved in the interaction between SIL1 and BiP are located within these regions [8]. We postulate that this mutated protein lacking the ER targeting sequence remains in the cytosol and is thereby unable to carry out the chaperoning activities required of it.
Conclusion
This was a case of a mutation in the SIL1 gene affecting the initiation codon and the ER targeting sequence that leads to a phenotype of MSS. Protein level studies would help in the further characterization of this mutation and to assess its functional impact.
Disclosure Statement
The authors have no conflicts of interest in relation to this work.
Acknowledgements
The authors wish to thank the Sheikh Hamdan Award for Medical Sciences for financial support of this work.
References
- 1.Lagier-Tourenne C, Tranebaerg L, et al. Homozygosity mapping of Marinesco-Sjögren syndrome to 5q31. Eur J Hum Genet. 2003;11:770–778. doi: 10.1038/sj.ejhg.5201068. [DOI] [PubMed] [Google Scholar]
- 2.Senderek J, Krieger M, Stendel C, et al. Mutations in SIL1 cause Marinesco-Sjogren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet. 2005;37:1312–1314. doi: 10.1038/ng1678. [DOI] [PubMed] [Google Scholar]
- 3.Anttonen AK, Mahjneh I, et al. The gene disrupted in Marinesco-Sjögren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet. 2005;37:1309–1311. doi: 10.1038/ng1677. [DOI] [PubMed] [Google Scholar]
- 4.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L, Rosales C, et al. Alteration of the unfolded protein response modifies neurodegeneration in a mouse model of Marinesco-Sjögren syndrome. Hum Mol Genet. 2010;19:25–35. doi: 10.1093/hmg/ddp464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezgu F, Krejci P, Li S, et al. Phenotype-genotype correlations in patients with Marinesco-Sjogren syndrome. Clin Genet. 2014;86:74–84. doi: 10.1111/cge.12230. [DOI] [PubMed] [Google Scholar]
- 7.Goto M, Okada M, Komaki H, et al. A nationwide survey on Marinesco-Sjogren syndrome in Japan. Orphanet J Rare Dis. 2014;9:58. doi: 10.1186/1750-1172-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan M, Li J, Sha B. Structural analysis of the Sil1-Bip complex reveals the mechanism for Sil1 to function as a nucleotide-exchange factor. Biochem J. 2011;438:447–455. doi: 10.1042/BJ20110500. [DOI] [PubMed] [Google Scholar]