Abstract
Objectives
The aim of this study was to explore whether or not the antidepressant actions of fluoxetine (FLX) are correlated with extracellular signal-regulated kinase 1 and 2 (ERK1/2) and nuclear factor κ-light chain enhancer of activated B cells (NF-κB) in the hippocampus (HC) and prefrontal cortex (PFC) of rats.
Materials and Methods
A total of 108 male Sprague-Dawley rats were randomly divided into 6 groups of 18 rats each. Group 1 was the control group, while group 2 comprised the depressed model in which rats were subjected to 28 days of forced-swimming stress (FST); groups 3–6 were also subjected to 28 days of FST and treated with FLX once a day for 1 day (group 3; F1d), 1 week (group 4; F1w), 2 weeks (group 5; F2w), or 4 weeks (group 6; F4w). The control group was not subjected to FST or treated with FLX. Behavior tests that included the Morris water maze (MWM) and saccharin preference were performed, and ERK1/2 and NF-κB proteins were assayed using Western blot.
Results
The rats in the control group and in groups 5 and 6 (F2w and F4w, respectively) had a significantly shorter average escape latency, needed more attempts in order to successfully cross the platform, and had a greater saccharin preference than those in the depressed group (p < 0.05). In the depressed group, the phosphorylated ERK1/2 (p-ERK1/2) and phosphorylated NF-κB (p-NF-κB) expression in the HC and PFC were lower than in the control group (p < 0.05). Treatment with FLX reversed the changes in the expression of p-ERK1/2 and p-NF-κB in rats in the F2w and F4w groups.
Conclusions
In this study, FLX treatment for 2 weeks or longer reversed the impaired spatial learning, memory, and anhedonia observed in the depressed model rats and upregulated the activities of the ERK1/2-NF-κB signaling pathway.
Key Words: Major depression, Fluoxetine, ERK1/2, NF-κB, Signaling pathway
Introduction
Major depressive disorder (MDD) is a common, recurrent, and etiologically complex mental disease; approximately 3–5% of the population worldwide is afflicted with MDD [1], but the etiology and pathophysiological mechanisms of depression have not been precisely defined.
Current antidepressant treatments that modulate the serotonergic or noradrenergic brain activity are generally effective [2]. However, the onset of therapeutic effects requires 2–4 weeks, and a first course of therapy provides symptom relief in only 60–65% of patients [3]. Because of the lag period between the initiation of antidepressant treatment and the onset of clinical effects, researchers have inferred that a presynaptic and postsynaptic adaptive process including a signaling pathway might be involved in depression [4]. Extracellular signal-regulated kinase (ERK) and nuclear factor κ-light chain enhancer of activated B cells (NF-κB) have emerged as critical points of convergence in the signal transduction pathways that regulate neuronal plasticity [5]. Increasing evidence implicates signal transduction pathways, especially the ERK1/2-NF-κB signaling pathway, as contributors to the mechanisms of antidepressant action and the pathophysiology of depression [6,7].
ERK1/2 is a member of the mitogen-activated protein kinase (MAPK) family and an important intracellular signaling molecule, and it is extensively distributed throughout the central nervous system, most prominently in the hippocampus (HC) and the prefrontal cortex (PFC) [8]. Overexpression of the ERK protein kinase family has been reported to affect the regulation of synaptic plasticity, memory formation, and the long-term potentiation process [9]. ERK has been reported to be differentially regulated by stress between the limbic [7] and reward-related brain regions [10]. To complicate things further, the stress-induced alterations in ERK signaling within the hippocampal formation are not consistent among studies [11,12,13].
NF-κB is a transcription factor and a downstream target of the ERK pathway that plays a pivotal role in the regulation of multigene signaling pathways. The activation of NF-κB causes a delay in neutrophil apoptosis, extends the cell cycle length, increases the number of neutrophils, and activates and produces many inflammatory mediators and free radicals [14]. However, little is known regarding the role of NF-κB in emotional disorders such as depression and especially depression induced by stress.
In addition, there are plenty of published studies examining the association between ERK [6,15,16] or NF-κB [17] and depression-related behavior; however, it is not known whether the onset of the therapeutic effects of antidepressants is associated with ERK1/2 and NF-κB signal transduction. Fluoxetine (FLX) is a widely used selective serotonin reuptake inhibitor antidepressant that acts by inhibiting the 5-HT reuptake in the synaptic cleft. We hypothesized that both ERK and NF-κB could be decreased in the HC and PFC in rats subjected to forced-swimming stress (FST), and that FLX treatment could increase the levels of ERK and NF-κB. Thus, the purpose of the present study was to investigate the effects of staggered treatment with FLX on the levels and activities of ERK1/2 and NF-κB in the HC and PFC in the model of rats with depression-like behavior subjected to FST.
Materials and Methods
Animals
Male Sprague-Dawley rats (n = 108; 250–300 g, housed 4–5 per cage; Animal Laboratory Center at the Institute of Radiation of the Chinese Academy of Medical Sciences, Tianjin, China) were used in this study. The animals had free access to water and food at the animal center, with a 12-hour light cycle (lights on at 07:00 h) and a thermoregulated environment at 23 ± 1°C. They were acclimated for 1 week before being used in the experiments. All experimental procedures were performed in accordance with the Institutional Animal Ethics Committee of Tianjin Medical University.
The male Sprague-Dawley rats were randomly divided into the following 6 groups (18 rats per group): group 1 (control), group 2 (depressed), group 3 (F1d), group 4 (F1w), group 5 (F2w), and group 6 (F4w). The rats in groups 2–6 were subjected to 28 days of FST; after the 28-day FST, the other rats in groups 3–6 were treated with FLX once a day for 1 day (group 3; F1d), 1 week (group 4; F1w), 2 weeks (group 5; F2w), or 4 weeks (group 6; F4w). The rats in group 1 (control) were neither subjected to the 28-day FST nor treated with FLX. The chronic FST was conducted according to Qi et al. [13] with slight modifications. Briefly, rats were placed into an ineluctable transparent cylinder-like organic glass container (65 cm high with a 25-cm diameter) filled with water (at 24 ± 1°C) at a depth of 40 cm between 08:00 and 10:00 h. Each rat in all of the groups except the control group was forced to swim individually for 15 min, once a day, for 28 consecutive days.
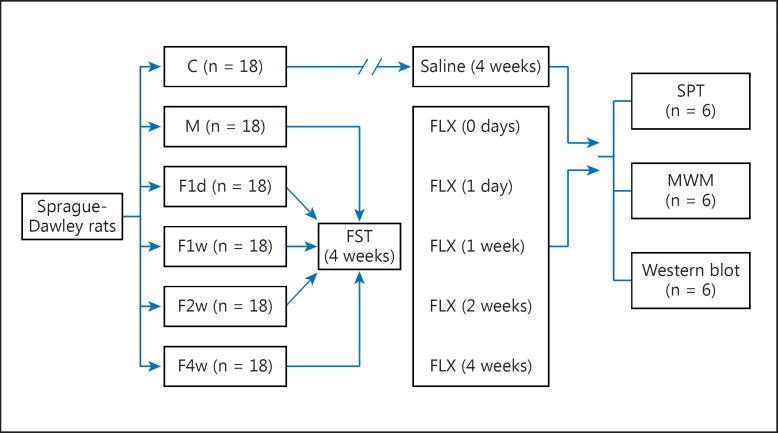
After depressive-like models were established based on the 28-day FST, 1 ml/kg saline (0.9% NaCl) was administered intragastrically daily to the control, depressed, F1d, F1w, and F2w groups, with a treatment duration of 28, 27, 21, and 14 days, respectively. The FLX, purchased from Nanjing DeBioChem Co., Ltd. (Nanjing, China), was dissolved in saline, prepared fresh before each experiment, and administered intragastrically (10 mg/kg) daily to the F1d, F1w, F2w, and F4w groups, with a treatment duration of 1 day and 1, 2, and 4 weeks, respectively. Six rats in each group were taken for the saccharine preference test, the Morris water maze (MWM) test, and Western blotting, respectively (fig. 1).
Fig. 1.
Scheme of the experiment model. C = Control; M = depressed; SPT = saccharin preference test.
Behavioral Tests
Saccharine Preference Test
The reinforcing properties of the saccharin solution were used as an index of hedonic alterations, and the reduced consumption of the sweet solutions was a measure of anhedonia. On the last day of FLX treatment, 6 rats in each group were deprived of water for 18 h (from 20:00 to 14:00 h of the next day). From the next day on, rats were given a 3-hour window for the saccharin preference test (14:00–17:00 h) once a day for 4 days. The rats were given 2 bottles of liquid, i.e. one containing water and the other containing a 1% sodium saccharin solution. At the end of the each preference test (17:00 h), the rats were given free access to water. The position of the bottles in the cages was changed every day. The amount of liquid intake was determined by weighing the bottles before and after the 3–hour window. The saccharin solution intake, the water intake, and the total fluid intake during the 4 days were calculated. The reduced consumption of sweet solutions (sucrose and saccharin) due to chronic mild stress in rats was a measure of anhedonia. Saccharin preference was calculated as the ratio of the saccharin solution intake to the total liquid intake.
MWM Test
A pool with a 150-cm diameter was utilized, with an escape platform (20-cm diameter, 30 cm high) placed 1.5 cm below the water surface (24 ± 1°C), and the water was made opaque with black ink. After 30 min of habituation in the training room, the rats were placed in the pool and allowed to search for the platform for 120 s. If the rats did not locate the platform within 120 s, they were guided to it. They were left to sit on the platform for 30 s before being returned to their cages. Six rats in each group were trained 4 times a day with a 30-min intertrial interval for 5 days. On day 6, the probe trial was performed by removing the platform and putting the rats in the water at any chosen point. The rats were allowed to search for the site of the removed platform over a time span of 2 min. The behavior of the rats was monitored via a video camera placed above the pool. A yellow drape with a white circle marker was hung over the pool. The cueing of objects outside the maze remained unvaried during training. The consistency of the experimental environment, including the tester, the testing time of the behavior of the rats, and the setting of the location of desks, chairs, and curtains in the room, was maintained. The following variables were assessed: escape latency (i.e. the time it took to find the platform in the training trials), time spent in the target quadrant, number of attempts needed to cross the platform successfully, distance traveled in the target quadrant in the pool, and heading angle (which indicates the correctness of the swim path towards the platform).
Western Blotting
Materials
The following primary antibodies were used for Western blotting: phosphorylated ERK1/2 (p-ERK1/2, #9102; Cell Signaling), total ERK1/2 (t-ERK1/2, #9103; Cell Signaling), phosphorylated NF-κB p65 (p-NF-κB, #3031; Cell Signaling), total NF-κB p65 (#3034; Cell Signaling), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary antibody (Lianke Bio Company, Hangzhou, China); polyvinylidene fluoride was purchased from Millipore (Bedford, Mass., USA). The bicinchoninic acid assay kit and enhanced chemiluminescence reagent were purchased from Pierce (Rockford, Ill., USA).
Tissue Dissection and Processing
Six rats in each group were decapitated at the end of the experiments, and their brains were rapidly removed and put on ice. The brains were placed in a stainless steel brain matrix, and the PFC was punched based on the newer (2005) edition of the Rat Brain Atlas by Paxinos and Watson [18]. The whole HC was dissected from the brain. All tissues were frozen in liquid nitrogen. Tissues were homogenized in 20 volumes of buffer (pH 7.5, containing 50 mM Tris-Cl, 2 mM EDTA, 2 mM EGTA, 1 M sodium vanadate, 5 μg/ml pepstatin A, and 0.5% nonidet P-40). The protein content of the lysates was determined using bicinchoninic acid assay kits. The lysates were mixed with 5× sodium dodecyl sulfate (SDS) to prepare the samples. All of the samples were stored at −80°C until use.
Protein Separation and Immunoblot
Proteins were separated using SDS-PAGE on 10% polyacrylamide gels. Then, the proteins were moved to polyvinylidene fluoride via an electrophoretic transfer. Blots were incubated in blocking buffer (10% nonfat dry milk powder in Tris-buffered saline containing 0.5% Tween-20, TBS) for 1 h at room temperature and washed 3× for 10 min in TBS. Blots were incubated with p-ERK1/2, ERK1/2, p-NF-κB, and NF-κB primary antibodies, respectively, overnight at 4°C and then washed 3× for 10 min in TBS. Blots were incubated with horseradish peroxidase-labeled goat anti-mouse secondary antibody IgG for 1 h at room temperature, washed 3× for 10 min in TBS, treated with enhanced chemiluminescence reagents, and exposed to film. GAPDH was also visualized using a GAPDH primary antibody and a secondary antibody (horseradish peroxidase-labeled goat anti-mouse secondary antibody IgG). Immunoblots were analyzed using Quantity One® 1D analysis software.
Statistical Analysis
The statistical analysis was performed using SPSS software (version 11.5; Beijing, China). The saccharin preference, the average escape latency in the training trials, the behavior parameters in the spatial probe trial, and the levels of ERK1/2, p-ERK1/2, p-NF-κB P65, and NF-κB P65 were analyzed in all groups by one-way ANOVA; when the data were distributed nonnormally, a nonparametric test was used. p < 0.05 was considered statistically significant.
Results
Comparison of Ratios of Saccharin
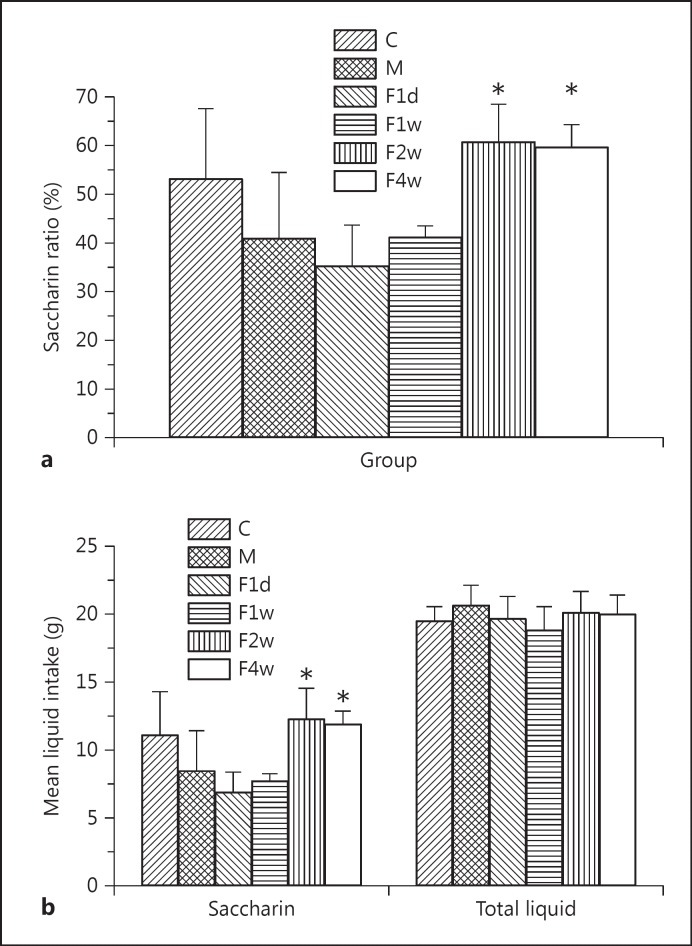
In this study, the saccharin ratios significantly differed among groups using one-way ANOVA [F(5, 30) = 7.777, p < 0.01], mostly in the F2w group and least in the F1d group. Post hoc tests revealed that the rats in the control, F2w, and F4w groups exhibited a greater saccharin preference than depressed rats (control vs. depressed, F2w vs. depressed, and F4w vs. depressed, p < 0.01; fig. 2a). There was no significant difference in saccharin intake (depressed vs. F1d and depressed vs. F1w, p > 0.05; fig. 2b). The total liquid intake did not differ among groups [F(5, 30) = 1.049, p > 0.05; fig. 2b].
Fig. 2.
a The ratio of saccharin significantly differed between groups using one-way ANOVA [F(5, 30) = 7.777, p < 0.01]. b No significant difference in saccharin intake was found between the depressed and F1d groups or between the depressed and F1w groups. C = Control; M = depressed. * p < 0.01.
Comparison of the Average Latencies to Escape in the Training Trials
The average latency to escape from water showed a lower tendency from the 1st to the 5th day, with increased test frequencies (table 1). Because the escape latency gradually stabilized after 3 days of the MWM, the escape latencies of the 3rd, 4th, and 5th days were chosen and analyzed. The one-way ANOVA on the average escape latency [F(5, 30) = 49.210, p < 0.05] indicated that there was a significant difference in the averaged escape latency of the rats in all groups. The values for rats in the depressed group and the F1d group appeared to gradually slowly decrease, with no significant difference between the 3rd and 4th days or the 4th and 5th days. The average escape latency of rats on the 5th day in the control (23.4 ± 2.8), F2w (16.4 ± 7.4), and F4w (13.9 ± 2.9) groups was significantly shorter than that in the depressed group (42.6 ± 11.6). The results revealed no significant difference in the averaged escape latency of the rats in the F2w and F4w groups compared to the C group (p > 0.05).
Table 1.
Comparison of the average escape latencies of all groups in the training trials
| Group | 1st day | 2nd day | 3rd day | 4th day | 5th day |
|---|---|---|---|---|---|
| Control | 70.9 ± 15.2 | 42.3 ± 14.6 | 36.9 ± 11.1* | 32.5 ± 11.7* | 23.4 ± 2.8* |
| Depressed | 89.8 ± 12.9 | 59.5 ± 16.1 | 50.5 ± 14.7 | 48.2 ± 14.5 | 42.6 ± 11.6 |
| F1d | 73.9 ± 12.5 | 56.1 ± 14.4 | 37.9 ± 11.3 | 36.8 ± 11.4 | 28.6 ± 7.3 |
| F1w | 80.7 ± 6.3 | 43.0 ± 6.2 | 30.7 ± 8.6 | 23.1 ± 4.9 | 19.3 ± 6.2 |
| F2w | 77.6 ± 11.9 | 25.1 ± 4.4 | 19.2 ± 7.6* | 18.2 ± 4.8* | 16.4 ± 7.4* |
| F 4w | 73.5 ± 8.8 | 23.2 ± 7.3 | 17.9 ± 7.7* | 17.9 ± 2.7* | 13.9 ± 2.9* |
Values are presented as means ± SE. n = 6 for each group.
p < 0.05.
Comparison of the Behavior Parameters of Rats in All Groups in the Spatial Probe Trial
Table 2 shows that the number of attempts (mean rank: 25.83, 12.83, 10.42, 15.25, 21.0, and 25.67 for the control, depressed, F1d, F1w, F2w, and F4w groups) the rats needed to make before crossing the platform successfully was found to be significantly different (χ2 test = 13.713, p < 0.05) by the Kruskal-Wallis test (nonparametric test). There were significant differences in the heading angle in the depressed group compared to the other groups [F(5, 30) = 5.159, p < 0.01].
Table 2.
Comparison of the behavior parameters of rats in all groups in the spatial probe trials
| Group | Attempts needed to cross the platform (mean rank), n | Heading angle, degrees | Time spent in the target quadrant/total time, % | Distance traveled in the target quadrant/total distance, % |
|---|---|---|---|---|
| Control | 25.83* | 21.49 ± 6.83a | 18.0 ± 2.6 | 22.0 ± 3.1 |
| Depressed | 12.83 | 58.31 ± 5.66 | 24.0 ± 5.5 | 27.0 ± 4.1 |
| F1d | 10.42 | 36.77 ± 11.95a | 25 ± 1.5 | 25.0 ± 1.7 |
| F1w | 15.25 | 30.86 ± 2.82a | 21.0 ± 1.0 | 22.0 ± 1.1 |
| F2w | 21.00* | 21.86 ± 4.87a | 2.01 ± 0.7 | 24.0 ± 0.9 |
| F4w | 25.67* | 13.11 ± 6.52a | 22.0 ± 1.5 | 24.0 ± 1.2 |
Values are presented as means ± SE.
p < 0.05.
p < 0.01.
Expression of ERK1/2 and p-ERK1/2 in the HC and the PFC
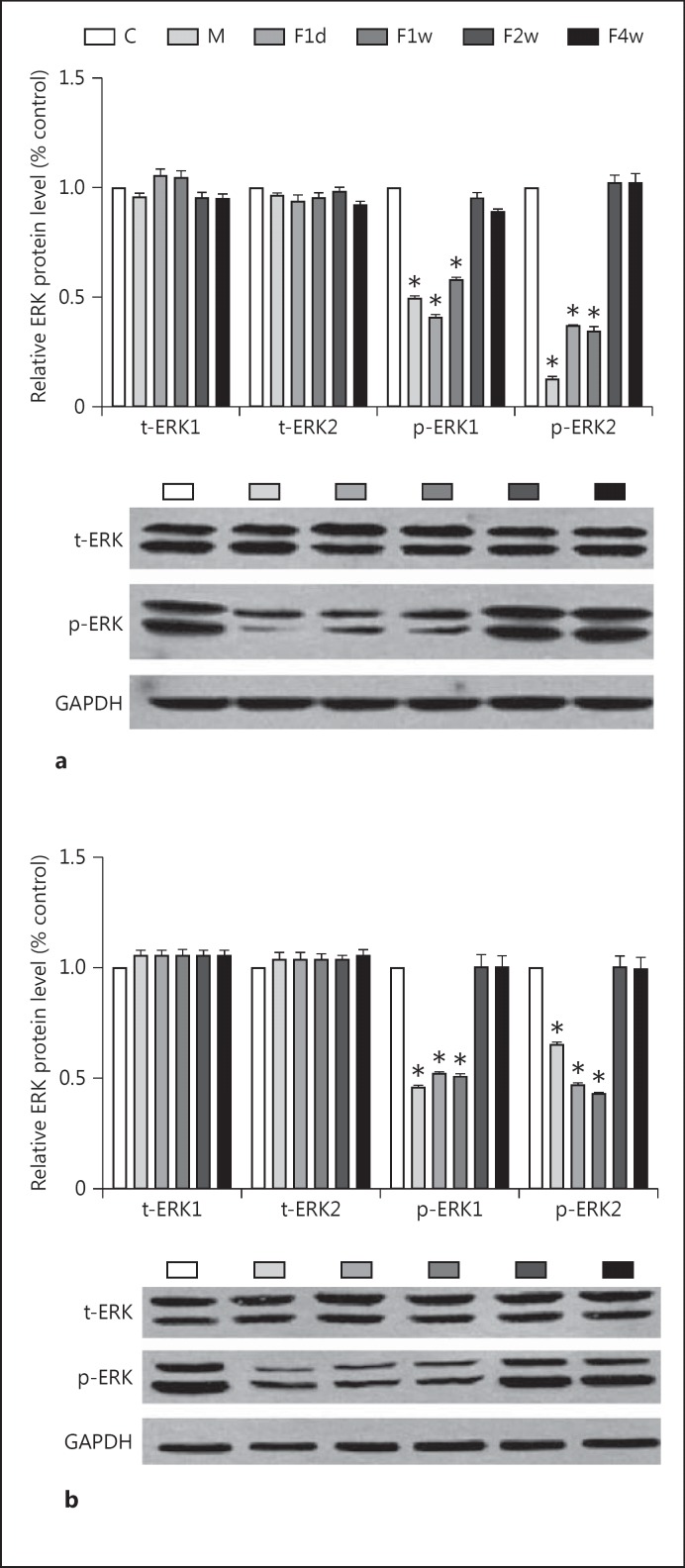
For each blot of p-ERK1/2 and ERK1/2, the relative protein level was calculated based on the ratio of absorbance of each protein in the treated groups and the control group to correct for small differences in protein loading. The one-way ANOVA showed that the p-ERK1/2 level significantly differed among groups, both in the HC and in the PFC [F(5, 30) = 278.925, p < 0.01, and F(5, 30) = 54.699, p < 0.01, respectively] (fig. 3). There was no significant difference in the level of p-ERK1/2 in the HC and the PFC in F2w and F4w groups compared to the control group (p > 0.05). No significant differences in the level of t-ERK1/2 in the HC and the PFC were noted in any of the groups (p > 0.05).
Fig. 3.
Levels of p-ERK1/2, t-ERK1/2, and GAPDH in the HC (a) and the PFC (b) in the control, depressed, F1d, F1w, and F2w groups. Bars represent the mean ± SE for each condition (n = 6 in the control, depressed, F1d, F1w, F2w, and F4w groups). * p < 0.01. C = Control; M = depressed.
Expression of p-NF-κB P65 and NF-κB P65 in the HC and the PFC
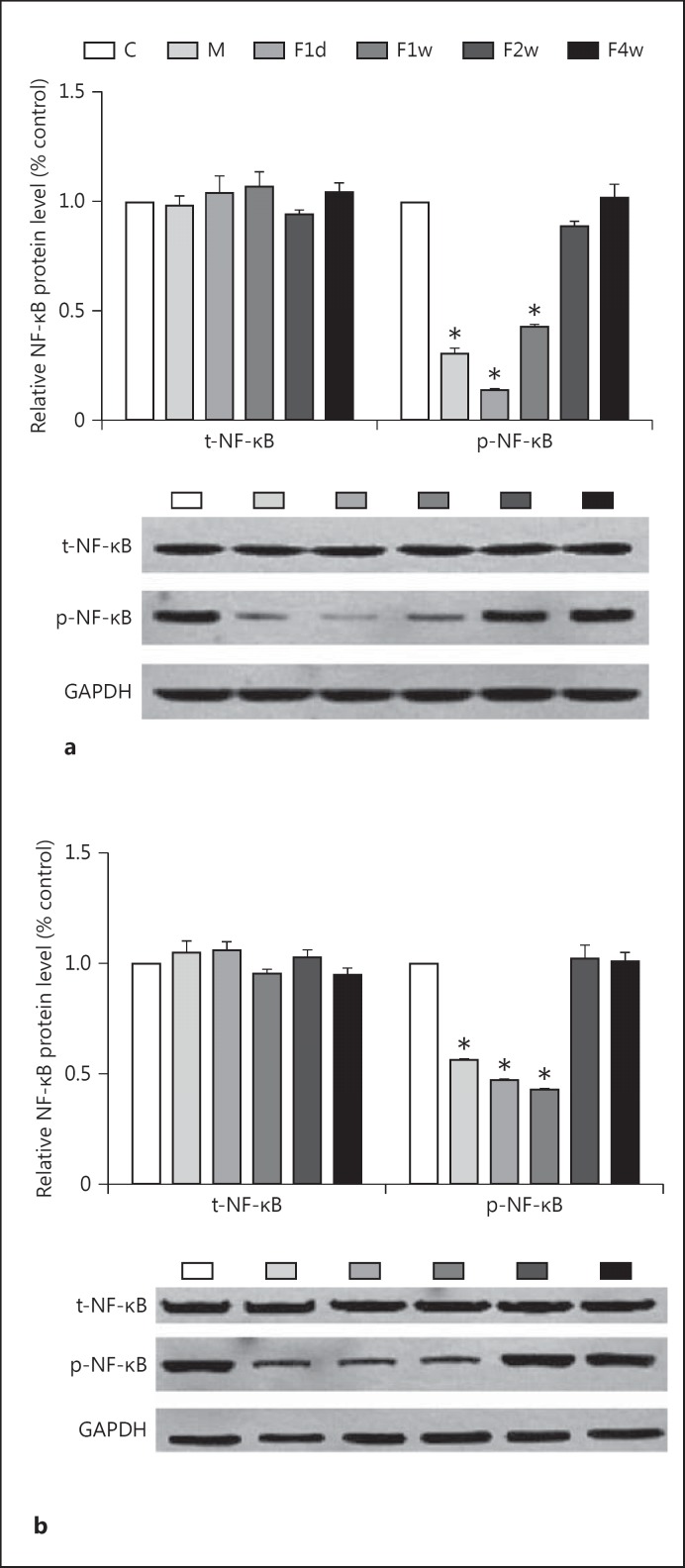
For each blot of p-NF-κB and NF-κB, the relative protein level was calculated based on the ratio of absorbance of each protein in the treated groups and the control group to correct for small differences in protein loading. The one-way ANOVA indicated that the p-NF-κB level significantly differed among groups both in the HC and in the PFC [F(5, 30) = 631.38, p < 0.01, and F(5, 30) = 557.44, p < 0.01, respectively] (fig. 4). Post hoc tests revealed that the level of p-NF-κB in the HC and PFC was significantly reduced in rats in the depressed, F1d, and F1w groups compared to the control group (p < 0.01). The greatest and smallest expression in the HC occurred in the F4w and F1d groups, respectively. The greatest and smallest expression in the PFC occurred in the F2w and F1w groups, respectively.
Fig. 4.
Levels of p-NF-κB P65, NF-κB P65, and GAPDH in the HC (a) and the PFC (b) of rats in the control, depressed, F1d, F1w, and F2w groups. Bars represent the mean ± SE for each condition (n = 6 in the control, depressed, F1d, F1w, F2w, and F4w groups). * p < 0.01. C = Control; M = depressed.
Discussion
The present study showed that the rats in the depressed, F1d, and F1w groups had a decreased ratio of saccharin to water, while the rats in the control, F2w, and F4w groups had a higher ratio of saccharin to water. The rats in the F2w and F4w groups exhibited a shorter average latency to escape, with more attempts needed to cross the platform successfully and a smaller heading angle after 14 days of FLX treatment compared to the rats in the depressed group. The results showed decreased levels of p-ERK1/2 and p-NF-κB in the HC and PFC of depressed-like rats. Moreover, the present study confirmed that 2 and 4 weeks of FLX treatment normalized the level of p-ERK1/2 and p-NF-κB.
It is known that the HC and the PFC are 2 brain regions that are very vulnerable to stress and other harmful stimuli. Moreover, clinical brain imaging studies have demonstrated that the volume of the HC is reduced in patients with depression [19]. Alterations in blood flow, metabolism, and the volume of the PFC have also been reported in depressed patients [20]. Thus, the 2 brain regions were chosen as a subsequent focus in order to explore the neuronal mechanisms of depression.
FST is a putative animal model of depression which emulates the behavioral despair paradigm of depression. Currently, it is one of the most frequently used behavioral tests to investigate antidepressant potential. Because most human depression disorders are induced by chronic stress, but not acute stress, chronic FST was chosen as our animal model of depression. In the present study, the rats with more than 2 weeks of FLX treatment showed a higher ratio of saccharin than depressed rats. This suggested that the chronic FST stress in rats induced anhedonia, which is a specific feature of a depressive-like phenotype, and 2 and 4 weeks of FLX treatment reversed the depressive-like behaviors.
The results of the MWM test suggested that the stress induced by chronic FST influenced the learning and memory of rats and that FLX improved the learning and memory of depressed-like rats. Our results are in line with those of other studies which have demonstrated that chronic FLX treatment improves depressive symptoms in depressive rats [13].
The present study demonstrated that chronic FST decreased the expression of p-ERK1/2 in the HC and PFC of rats, while FLX reversed the stress-induced disruption of p-ERK1/2 in the HC and PFC but had no effect on the total protein level of ERK1/2. Protein phosphorylation is generally considered to be a mechanism by which modulation of neuronal function is achieved through positive and negative regulation of many factors [21], and it has close relations with the cell model long-term potentiation process in the regulation of learning and memory [22]. p-ERK1/2 could be a biological marker for the induction of new functions of the neuron during different brain activities [23]. Our results are in accordance with previous reports indicating decreased p-ERK1/2 in stressed animals [13,23]. While our results showing unchanged t-ERK1/2 levels in all of the rats are similar to those reported by Yuan et al. [24], they are not in line with previous reports suggesting decreased ERK1/2 levels in depressed rats [13]. Conflicting results suggest that different effects may depend on different experimental paradigms and differences in experimental animals. It has been reported that FLX significantly increases p-ERK2 levels in the HC but has no effect on the levels of ERK1/2 either in the HC or in the PFC of naive rats [13]. The present study indicates that 2 and 4 weeks of FLX treatment could reverse the decreased level of p-ERK1/2 in the HC and the PFC in depressed rats. However, this discrepancy calls for further investigation.
NF-κB is a protein complex that controls the transcription of DNA. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, and free radicals. NF-κB has also been implicated in processes of synaptic plasticity and memory [25]. Koo et al. [17] investigated the role of NF-κB in cellular and behavioral responses to acute and chronic stress and found that inhibition of neurogenesis by stress occurs via the activation of NF-κB in neural stem-like cells and that stress-induced anhedonia, a core symptom of depression, is dependent on NF-κB. They also demonstrated that acute stress activates NF-κB signaling in GFAP+ neural stem-like cells in the adult HC, which is not in line with our research, possibly because they applied acute, not chronic, stress but also because acute stress promotes neurogenesis of the HC and in turn activates NF-κB signaling in GFAP+ neural stem-like cells in the adult HC [17]. When ERK activation is depressed, NF-κB-dependent plasticity will be disrupted. Recent research indicates that the Trier social stress test-induced increases in NF-κB DNA binding in peripheral-blood mononuclear cells were greater in major depressive patients with increased early-life stress and correlated with depression severity [26]. This suggests that NF-κB might play an important role in major depression. In order to further confirm the role of the ERK1/2-NF-κB signaling system in the molecular mechanism of depression and explore the potential target of the FLX antidepressant and the time of action, we assessed the effect of FLX on NF-κB P65 in the rat brain. The present study demonstrated that chronic FST decreased the expression of p-NF-κB P65 in the HC and PFC in rats. FLX reversed the stress-induced disruption of p-NF-κB P65 in the HC and PFC, but it had no effect on the total protein level of NF-κB P65. Thus, 2 and 4 weeks of FLX treatment can reverse the decreased level of p-NF-κB P65 in the HC and PFC of depressed rats.
It has been reported that it takes several weeks for FLX to exert antidepressant effects in patients with MDD [27]. The time needed for FLX to improve memory issues was in line with the length of time in which the antidepressant produced therapeutic effects. Impaired cognitive function, especially a decrease in memory, is an important symptom in most depressed patients, and it might be correlated with problems in the HC induced by stress. The results demonstrated that chronic FLX treatment improved the impairment of spatial learning and memory induced by stress, which suggests that the protective role of FLX was not direct for the increase in 5-HT in the synaptic cleft. Our results were in accordance with previous reports indicating decreased p-ERK2 levels in depressed human beings [28] and unchanged t-ERK1/2 levels in patients with MDD [29]. Altogether, this suggests that ERK1/2 activation might be involved in the mechanism of stress-induced major depression. While our results showing unchanged t-ERK1/2 levels in all of the rats were similar to those reported by Yuan et al. [30], they were not in line with previous reports suggesting increased ERK1/2 levels in depressed human beings [29]. Conflicting results suggest that different effects may depend on different experimental paradigms and differences in experimental animals. The effect of FLX on the ERK signaling system in the brain has been documented. There are a few studies evaluating this issue. Researchers have demonstrated that FLX markedly increases the protein levels of ERK1/2 in the PFC but not in the HC, and it has no effect on the level of p-ERK2 in naive rats [31].
In addition, the present study revealed that the reduced levels of p-NF-κB P65 in the HC and PFC of rats were accompanied by a decreased phosphorylation of ERK1/2. FLX regulated p-ERK1/2 on a time course that paralleled increases in NF-κB phosphorylation. An important consideration concerns activation of the proteins in the ERK pathway. NF-κB is a downstream target of the ERK pathway. Because their activation depends on their phosphorylation state, it can be argued that changes in the total protein level may not be crucial for their activation, or that the changes observed in several members of the pathway could account for a final outcome of reduced p-NF-κB, which then leads to a reduced translation of the regulated proteins.
In a word, we speculate that the ERK1/2 and NF-κB signal pathways could be involved in the mechanism of the antidepressant action of FLX. Further studies are needed in order to explore the role of the ERK1/2 and NF-κB pathways in patients with major depression. Antidepressants targeting the ERK1/2 and NF-κB signaling pathways will likely be a focus in a further investigation.
Conclusions
In this study, FLX treatment for 2 weeks or longer reversed the impaired spatial learning, memory, and anhedonia observed in depressed rats and upregulated the activities of the ERK1/2-NF-κB signaling pathway.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30900484 and 81371494) and the Science Foundation of the Tianjin Bureau of Public Health (2010KR10) and in part by the Science Foundation of Key Item of the Tianjin Bureau of Public Health (13KG118).
References
- 1.Lépine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(suppl 1):3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Leary OF, Dinan TG, Cryan JF. Faster, better, stronger: towards new antidepressant therapeutic strategies. Eur J Pharmacol. 2015;753:32–50. doi: 10.1016/j.ejphar.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 3.Skolnick P. Triple uptake inhibitors: therapeutic potential in depression and beyond. Expert Opin Investig Drugs. 2007;16:1365–1377. doi: 10.1517/13543784.16.9.1365. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Yu X, Yang K, et al. The relationship between SNPs in 5-HT2A signal transduction-related genes and the response efficacy to SSRIs treatments in Chinese patients with major depressive disorder. Genet Test Mol Biomarkers. 2012;16:667–671. doi: 10.1089/gtmb.2011.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Gourley SL, Wu FJ, Kiraly DD, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duman CH, Schlesinger L, Kodama M, et al. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Di Benedetto B, Radecke J, Schmidt MV, et al. Acute antidepressant treatment differently modulates ERK/MAPK activation in neurons and astrocytes of the adult mouse prefrontal cortex. Neuroscience. 2013;232:161–168. doi: 10.1016/j.neuroscience.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Iñiguez SD, Vialou V, Warren BL, et al. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fumagalli F, Molteni R, Calabrese F, et al. Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J Neurochem. 2005;93:1551–1560. doi: 10.1111/j.1471-4159.2005.03149.x. [DOI] [PubMed] [Google Scholar]
- 12.First M, Gil-Ad I, Taler M, et al. The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and ERK expression. J Mol Neurosci. 2011;45:246–255. doi: 10.1007/s12031-011-9515-5. [DOI] [PubMed] [Google Scholar]
- 13.Qi X, Lin W, Li J, et al. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31:278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- 15.Duric V, Banasr M, Licznerski P, et al. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iñiguez SD, Alcantara LF, Warren BL, et al. Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. J Neurosci. 2014;34:1007–1021. doi: 10.1523/JNEUROSCI.5725-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo JW, Russo SJ, Ferguson D, et al. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: The New Coronal Set. ed 5. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- 19.Bremner JD. Structural changes in the brain in depression and relationship to symptom recurrence. CNS Spectr. 2002;7:135–139. doi: 10.1017/s1092852900017442. [DOI] [PubMed] [Google Scholar]
- 20.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 21.Amato LC. Chronic activation of ERK and neurodegenerative diseases. Bioessays. 2003;25:1085–1095. doi: 10.1002/bies.10355. [DOI] [PubMed] [Google Scholar]
- 22.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 23.Meller E, Shen C, Nikolao TA, et al. Region-specific effects of acute and repeated restraint stress on the phosphorylation of mitogen-activated protein kinases. Brain Res. 2003;979:57–64. doi: 10.1016/s0006-8993(03)02866-x. [DOI] [PubMed] [Google Scholar]
- 24.Yuan P, Zhou R, Wang Y, et al. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124:164–169. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meffert MK, Chang JM, Wiltgen BJ, et al. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 26.Pace TW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 27.Frazer A, Benmansour S. Delayed pharmacological effects of antidepressants. Mol Psychiatry. 2002;7:S23–S28. doi: 10.1038/sj.mp.4001015. [DOI] [PubMed] [Google Scholar]
- 28.Dwivedi Y, Rizavi HS, Zhang H, et al. Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain: role of ERK kinase 1 (MEK1) Int J Neuropsychopharmacol. 2009;12:1337–1354. doi: 10.1017/S1461145709990575. [DOI] [PubMed] [Google Scholar]
- 29.Chandran A, Iyo AH, Jernigan CS, et al. Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog Neuropsychopharmacol Biol Psychiatry. 2012;40:240–245. doi: 10.1016/j.pnpbp.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan P, Zhou R, Wang Y, et al. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124:164–169. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiraboschi E, Tardito D, Kasahara J, et al. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29:1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]