Abstract
The incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are important regulators of insulin and glucagon secretion as well as lipid metabolism and appetite. These biological functions make their respective receptors (GIPR and GLP-1R) attractive targets in the treatment of both type 2 diabetes mellitus (T2DM) and obesity. The use of these native peptides in the treatment of these conditions is limited by their short half-lives. However, long-acting GLP-1R agonists and inhibitors of the enzyme that rapidly inactivates GIP and GLP-1 (dipeptidyl peptidase IV) are in clinical use. Although there is a loss of response to both hormones in T2DM, this effect appears to be more pronounced for GIP. This has made targeting GIPR less successful than GLP-1R. Furthermore, results demonstrating that GIPR knockout mice were resistant to diet-induced obesity suggested that GIPR antagonists may prove to be useful therapeutics. More recently, molecules that activate both receptors have shown promise in terms of glycemic and body weight control. This review focused on recent advances in the understanding of the signaling mechanisms and regulation of these two clinically important receptors.
Key Words: Incretin, Glucagon-like peptide-1, Glucose-dependent insulinotropic polypeptide, Internalization, Dimerization, G protein-coupled receptor
Introduction
Oral glucose load is known to elicit a much larger insulin response than glucose administered intravenously [1]. This phenomenon, termed the ‘incretin effect’, can account for more than half of the insulin secreted in response to a meal [2]. This effect is mediated by incretin hormones secreted from the gut in response to nutrient ingestion, which act to potentiate insulin secretion in a glucose-dependent manner. To date, only two incretin hormones have been identified: glucose-dependent insulinotropic polypeptide (GIP, formerly known as gastric inhibitory polypeptide) and glucagon-like peptide-1 (GLP-1) [3]. GIP is synthesized in K cells, which are found predominantly in the duodenum and jejunum. The active 42-amino acid peptide is derived from a 153-amino acid precursor by posttranslational processing by prohormone convertase 1/3 [4]. GLP-1, on the other hand, is a posttranslational product of the proglucagon gene. Tissue-specific expression and posttranslational processing of this gene result in several peptides with important physiological functions other than GLP-1 (such as glucagon and oxyntomodulin) [5]. Full-length GLP-1(1-37) is cleaved from the proglucagon precursor in intestinal L cells (found predominantly in the ileum) but must be further processed to produce GLP-1(7-37) and GLP-1(7-36) amide before the peptide becomes biologically active. GLP-1(7-37) and GLP-1(7-36) amide are equipotent; however, the majority of the circulating active peptide is GLP-1(7-36) amide [6]. For simplicity, the term GLP-1 will be used to describe GLP-1(7-36) amide through the rest of this review.
The drivers of incretin hormone secretion are complex and may include input from the nervous and endocrine systems. However, the primary stimulus for the secretion of both GIP and GLP-1 is the ingestion of glucose, although other nutrients such as lipids and amino acids also stimulate their secretion [7]. From a pharmacological perspective it is interesting to note that the commonly used antidiabetic medication metformin increases the secretion of GLP-1 when taken orally [8] and this may contribute to the antidiabetic effect of this drug.
Loss of the incretin effect is an early characteristic of type 2 diabetes mellitus (T2DM) and while large doses of GLP-1 can overcome this impairment, it is unclear whether the same is true for GIP [9,10]. The reasons for this are unclear but several studies have demonstrated that hyperglycemia negatively affects GIP receptor (GIPR) signaling to a greater extent than the GLP-1 receptor (GLP-1R) [11,12].
GIP and GLP-1 are rapidly inactivated by the enzyme dipeptidyl peptidase IV (DPP-IV), also known as CD-26, which severely limits the use of native GIP and GLP-1 in the treatment of T2DM [13,14]. To overcome this, both long-acting GLP-1R agonists and DPP-IV inhibitors have been developed and are currently used clinically to treat T2DM [15]. GIPR has received less attention than GLP-1R as a drug target. However, as GIPR knockout mice were shown to be resistant to diet-induced obesity [16], several studies suggest that the use of GIPR antagonists may be a suitable approach to treat both T2DM and obesity [17,18]. More recently, single molecules that activate both GIPR and GLP-1R have shown promise as effective antidiabetic and antiobesity drugs [19]. The biology of the incretin hormones has been extensively reviewed elsewhere [3,7,20]. Therefore, the focus of this review was on recent advances in the understanding of the signaling mechanisms and regulation of the two incretin hormone receptors.
The Incretin Receptors
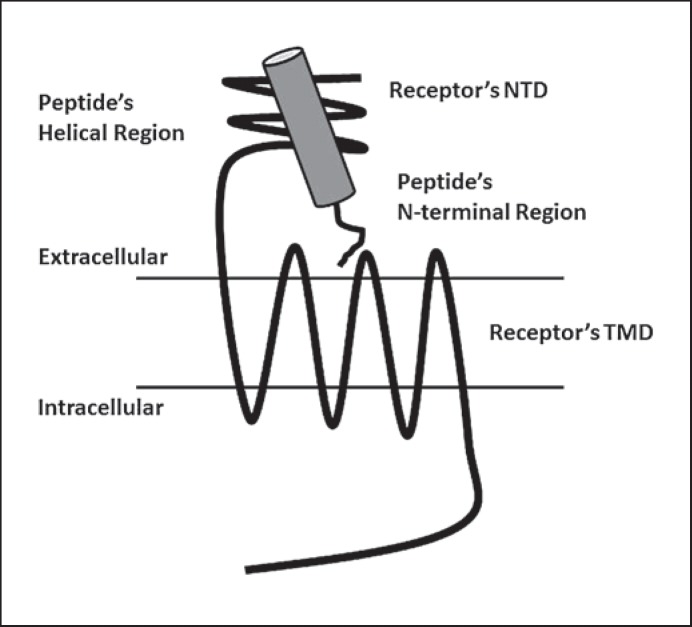
Both the receptors for GIP and GLP-1 are members of the secretin family or class B G protein-coupled receptors (GPCRs) [21]. Although GLP-1R and GIPR share considerable sequence homology (approximately 40%), they display extremely high selectivity for their respective ligands [22]. Family B GPCRs have a large extracellular N-terminal domain (NTD) linked to the 7-transmembrane helical domain that is characteristic of all GPCRs. The C-terminal region of the peptide ligand binds the NTD of the receptor, facilitating a secondary interaction between the N-terminal region of the peptide and the ‘core’ or transmembrane domain (TMD) of the receptor (fig. 1). This secondary interaction is thought to lead to activation of the receptor, allowing the TMD to interact with and activate heterotrimeric G proteins [23,24,25]. However, recent data for both GIPR and GLP-1R have shown that peptides that comprise the central region of GIP and GLP-1 are also able to activate their respective receptors, albeit with reduced potency [26,27]. Therefore, this situation requires a rethinking of the current model of ligand binding and activation for this class of receptors.
Fig. 1.
A model for peptide ligand binding to incretin receptors. The helical region of the peptide ligand binds to the NTD of the receptor, allowing the N-terminal region of the peptide to interact with the receptor's TMD.
Structures of both the NTD of GIPR and GLP-1R in complex with their respective ligands had been solved and has confirmed that the C-terminal region of the peptide binds to this domain [28,29,30]. The structure of the TMD of either GIPR or GLP-1R is yet to be determined. To date, the only members of the class B family of GPCRs which have crystal structures of their TMD published are the corticotropin-releasing factor receptor 1 and the glucagon receptor [31,32]. These structures provide valuable insight into the structure and function of this family of receptors and can be used as a template to model the structure of GIPR and GLP-1R. However, as they do not include the NTD, the orientation of this region in relation to the TMD and how peptide ligands alter this structure remain to be solved.
Receptor Expression
GIPR and GLP-1R are both expressed in pancreatic islet β-cells where they mediate the incretins' amplification of glucose-induced insulin secretion, insulin biosynthesis and inhibition of β-cell apoptosis. GIPR is also expressed in pancreatic α-cells but it is still unclear whether GLP-1R is expressed in other islet cell types [20]. Whereas GLP-1 inhibits glucagon, the actions of GIP on this counterregulatory hormone appear to be glucose dependent [33]. Based on studies using quantitative polymerase chain reaction, GLP-1R may be expressed at 10 times that of GIPR in pancreatic islets [34]. GLP-1R has been reported to be expressed in pancreatic ducts, which may contribute to the reports of pancreatitis in patients treated with GLP-1R agonists. It should be noted, however, that diabetic patients are at greater risk of developing acute pancreatitis regardless of the medication they are taking and that more recent studies did not find an association between the use of incretin-based therapies and increased risk of acute pancreatitis [35,36]. GIPR is also expressed in the adrenal cortex, adipose tissue, bone, some areas of the brain, and endothelium amongst others. Interestingly, the GIPR gene includes a PPAR-γ response element in the promoter region, and treatment with troglitazone increases GIPR expression in isolated mouse islets [37]. GLP-1R is also expressed in the central and peripheral nervous system and other tissues, including the heart, lungs, kidneys, stomach, and intestines. The broad tissue distribution of GIPR and GLP-1R accounts for the pleiotropic effects of the incretin hormones [20], which include neuro- and cardioprotective effects, regulation of adipose tissue and bone for GIP and regulation of food intake for GLP-1.
Splice Variants
GIPR exists as two isoforms: one 466 amino acids in length and the other 493 amino acids long. The additional 27 amino acids in the long form are located at the junction between transmembrane helix 7 and the C-terminal tail. Both isoforms bind GIP with similar high affinity, resulting in dose-dependent increases in intracellular cAMP [38,39]. The functional significance of the long form is currently unknown and it is unclear whether it is actually expressed in human pancreatic cells. Interestingly, a novel splice variant of GIPR which acts as dominant negative has been identified in murine pancreatic β-cells [40]. This splice variant is severely truncated (263 amino acids) due to a frame shift that introduces a stop codon to transmembrane helix 4 [40]. To date, an equivalent splice variant has not been identified in human pancreatic β-cells.
Cell Signaling
Although they have been reported to couple to other G proteins, GIPR and GLP-1R signal primarily through Gs, leading to the activation of adenylate cyclase and an increase in intracellular cAMP. In pancreatic β-cells this leads to the activation of protein kinase A, which inhibits K+ channels, resulting in membrane depolarization and the opening of voltage-gated Ca2+ channels. The increase in Ca2+ influx triggers the release of Ca2+ from intracellular stores (calcium-induced calcium release) and the subsequent fusion of insulin-containing vesicles with the plasma membrane and, ultimately, insulin release [41]. The increase in cAMP also leads to the activation of exchange protein activated by cAMP-2 (Epac2), which enhances transcription of the proinsulin gene [42].
Activation of GLP-1R has been shown to lead to transactivation of the epidermal growth factor receptor [43]. A signaling complex of arrestin2 and c-Src is thought to facilitate this transactivation [44]. The subsequent activation of phosphatidylinositol-3 kinase and Akt is thought to mediate the proliferative and prosurvival effects of GLP-1 on the pancreatic β-cell.
Constitutive Activity
GIPR has been shown to display considerable ligand-independent or basal activity, whereas GLP-1R is relatively silent in the absence of an agonist [45]. The physiological relevance of this constitutive activity is not entirely clear but a mutation resulting in a glutamic acid-to-glutamine substitution in the 6th transmembrane of the GIPR results in a receptor with lower basal activity [46]. Subjects who are homozygous for this polymorphism have been shown to have lower fasting and postoral glucose tolerance test serum C-peptide, which suggests that the high basal activity of GIPR may play an important role in normal glycemic regulation [47].
Homologous Desensitization and Internalization
Homologous desensitization refers to the loss of response to subsequent stimulation following agonist stimulation of a particular receptor [48]. For many GPCRs, such as the prototypical β2-adrenergic receptor (β2-AR), it had been established that this process involves phosphorylation of the agonist-activated receptor at serine and threonine residues in the receptor's C-terminal tail and intracellular loop regions by the family of kinases known as GPCR kinases [49]. The phosphorylated receptor can still signal through G proteins at this stage but phosphorylation also facilities the binding of another family of proteins known as arrestins to the agonist-activated receptor. Arrestin binding prevents the receptor from interacting with G proteins, desensitizing the G protein-dependent component [50]. Arrestin also mediates internalization of the receptor by targeting the receptor to clathrin-coated pits as well as activation of G protein-independent signaling pathways such as the mitogen-activated protein kinase cascade. Once internalized, the receptor may be dephosphorylated and recycled to the plasma membrane (resensitization) or targeted to lysosomes for degradation [51].
Homologous desensitization and internalization are, however, two distinct phenomena. It is also clear that not all GPCRs are desensitized or internalized by the same mechanisms [52]. Some receptors such as the β3-AR (which lacks the same phosphorylation sites in its C-terminal region as the β2-AR) may not undergo homologous desensitization, and when desensitization has been observed for this receptor it appears to be due to the downregulation of receptor mRNA or reduced Gs expression [53].
Following agonist stimulation, GLP-1R is rapidly internalized [54,55,56]. An earlier study has suggested that this was via a clathrin-coated pit-dependent process [57] but more recent work has shown that agonist-induced internalization of GLP-1R is caviolin-1 and dynamin dependent and arrestin independent [58]. Interestingly, internalization of GLP-1R is mediated by the Gαq pathway [59]. It has been known for some time that GLP-1R is capable of coupling to multiple G proteins [60]. However, the significance of this promiscuity was not completely appreciated at the time. Although the internalization of GLP-1R may contribute to the desensitization to subsequent stimulation observed in vitro, its physiological significance is unclear, as chronic exposure to the GLP-1R agonist exendin-4 does not result in an attenuation of GLP-1R-mediated glucose homeostasis or receptor downregulation in vivo [61].
Intriguingly, the inhibition of GLP-1R internalization has been shown to attenuate both cAMP production in BRIN-BD11 cells and insulin secretion in pancreatic β-cells [55,62]. This finding suggests that internalization of GLP-1R is an integral part of the signaling process. Using fluorescently labelled ligands and receptors, Kuna et al. [55] have shown that agonist-bound GLP-1R colocalizes with adenylate cyclase in endosomes. Taken together, these findings suggest that GLP-1R continues to signal following internalization. Endocytotic generation of cAMP has also been observed for other members of the secretin family of GPCRs such as the glucagon and parathyroid hormone receptors (PTHR) [63,64]. For the PTHR this phenomenon has been reported to be mediated by arrestin2 [65]. Although various groups, using a variety of techniques, have demonstrated that stimulation of GLP-1R results in the recruitment of both arrestin2 and arrestin3, both internalization and desensitization appear to be arrestin-independent processes for this particular receptor [66,67]. Furthermore, knockdown of arrestin2 in cultured pancreatic β-cells results in a reduction of both GLP-1-stimulated cAMP and insulin production [62].
Based on their interaction with arrestin, GPCRs have been classified as either class A or class B receptors. Class A receptors such as β2-AR only interact transiently with arrestin at the plasma membrane, whereas class B receptors such as PTHR cointernalize with arrestin following agonist stimulation [68]. In this regard, GLP-1R appears to be a class A receptor as, unlike PTHR, it does not cointernalize with arrestin [43]. The exact mechanism by which arrestin contributes to GLP-1R signaling still remains to be fully elucidated.
In contrast to GLP-1R, the role of internalization in signaling through GIPR has not been studied in as much detail and appears to be a somewhat controversial subject. When expressed in 3T3-L1 adipocytes, GIPR was shown to be constitutively internalized and recycled to the plasma membrane, resulting in no net change in cell surface expression [69]. This finding is compatible with the fact that GIPR displays a high level of constitutive activity in terms of cAMP production. Stimulation of this cell line with GIP causes a decrease in the amount of GIPR at the cell surface and desensitization to further stimulation with GIP. This process has been shown to be driven by a reduction in the rate of recycling of the receptor to the plasma membrane rather than an increase in internalization kinetics. However, the mechanistic details by which GIPR is sequestered are currently unknown. Furthermore, when expressed in either HEK-293 or COS-7 cells, even micromolar concentrations of GIP were insufficient to cause internalization of GIPR [70]. These findings demonstrate that GIPR behavior is cell context dependent.
Dimerization
GPCRs have been shown to function as monomers, homo- and heterodimers and also higher-order oligomers [71,72]. Resonance energy transfer studies have demonstrated that both GLP-1R and GIPR can form homodimers [73]. For GLP-1R the primary dimerization interface has been shown to be located in transmembrane helix 4. Disruption of GLP-1R dimer formation by various means abolished high-affinity GLP-1 binding and differentially affected signaling. GLP-1-mediated cAMP production and ERK1/2 phosphorylation were moderately inhibited, whereas intracellular calcium mobilization was markedly attenuated [72].
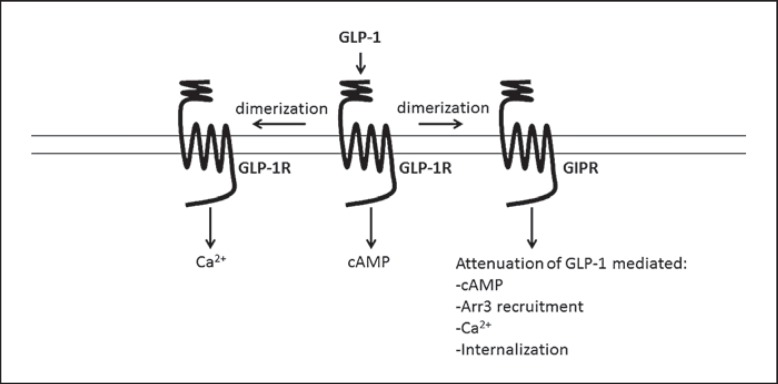
GLP-1R and GIPR also form heterodimers. The surface expression of an N-glycosylation-deficient GIPR mutant was rescued when coexpressed with GLP-1R, suggesting that dimer formation may occur during the maturation process. In the same study, GLP-R and GIPR were shown to be heterodimerized by the use of BRET (bioluminescence resonance energy transfer) [74]. BRET studies have also shown that GLP-1 promotes GLP-1R/GIPR dimer formation and that this can be reversed by treatment with GIP [67]. When coexpressed with GLP-1R in HEK-293 cells, GIPR impaired GLP-1-mediated calcium signaling and recruitment of arrestin to GLP-1R. Reversing dimer formation by treatment with GIP restored arrestin recruitment. In a more recent study coexpression of GIPR with GLP-1R in HEK-293 cells not only impaired arrestin recruitment to GLP-1R but also GLP-1-stimulated cAMP production, ERK phosphorylation, calcium signaling, and internalization of GLP-1R [70] (fig. 2). In this study, however, costimulation with GIP did not rescue GLP-1-mediated recruitment of arrestin to GLP-1R. These recent studies highlight the functional consequences of GLP-1R/GIPR dimerization and may have relevance in the development of T2DM.
Fig. 2.
Impact of homo- and heterodimerization in GLP-1R signaling. Arr3 = Arrestin3. Disruption of GLP-1R homodimer formation severely inhibits GLP-1-mediated Ca2+ mobilization, suggesting that homodimerization is involved in this signaling pathway. GLP-1 has also been shown to induce heterodimerization of GLP-1R and GIPR. Coexpression of GIPR with GLP-1R attenuates GLP-1-mediated cAMP production, arrestin3 recruitment, Ca2+ mobilization, and GLP-1R internalization. This suggests that GIPR acts as a negative regulator of GLP-1R signaling.
Conclusion
GIP and GLP-1 are important regulators of metabolism, including glycemia and pancreatic β-cell function. GLP-1R agonists such as exendin-4 (Byetta) and liraglutide (Victoza) are currently used clinically to treat T2DM and potentially have utility in the treatment of obesity. These two drugs are soon to be joined by other GLP-1R agonists with longer durations of action. Therefore, a detailed understanding of the desensitization, internalization and subsequent signaling of GLP-1R is warranted. Recent work in this area has shown that internalization appears to be an important part of the signaling process for GLP-1R, and inhibiting this process attenuates insulin release. DPP-IV inhibitors such as sitagliptin prolong the circulating half-life of both GIP and GLP-1 and are also used to treat T2DM. While GIPR agonists are not used clinically, recent reports of GIPR/GLP-1R coagonists have shown promise in terms of glucose control and weight loss. As GIPR/GLP-1R heterodimerization has been shown to regulate GLP-1R signaling, it will be important to understand how targeting both receptors with a single molecule affects this process and may highlight new opportunities to treat T2DM and obesity.
References
- 1.McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet. 1964;2:20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 2.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 3.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehmann HC, Goke R, Goke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev. 1995;16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 5.Mojsov S, Heinrich G, Wilson IB, et al. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261:11880–11889. [PubMed] [Google Scholar]
- 6.Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes. 1999;48:86–93. doi: 10.2337/diabetes.48.1.86. [DOI] [PubMed] [Google Scholar]
- 7.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Napolitano A, Miller S, Nicholls AW, et al. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One. 2014;9:e100778. doi: 10.1371/journal.pone.0100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst JJ, Knop FK, Vilsboll T, et al. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care. 2011;34((suppl 2)):S251–S257. doi: 10.2337/dc11-s227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Livak MF, Bernier M, et al. Ubiquitination is involved in glucose-mediated downregulation of GIP receptors in islets. Am J Physiol Endocrinol Metab. 2007;293:E538–E547. doi: 10.1152/ajpendo.00070.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu G, Kaneto H, Laybutt DR, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- 13.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 14.Deacon CF, Nauck MA, Meier J, et al. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85:3575–3581. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- 15.Ahren B. The future of incretin-based therapy: novel avenues - novel targets. Diabetes Obes Metab. 2011;13(suppl 1):158–166. doi: 10.1111/j.1463-1326.2011.01457.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 17.Gault VA, McClean PL, Cassidy RS, et al. Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia. 2007;50:1752–1762. doi: 10.1007/s00125-007-0710-4. [DOI] [PubMed] [Google Scholar]
- 18.Irwin N, McClean PL, O'Harte FP, et al. Early administration of the glucose-dependent insulinotropic polypeptide receptor antagonist (Pro3)GIP prevents the development of diabetes and related metabolic abnormalities associated with genetically inherited obesity in ob/ob mice. Diabetologia. 2007;50:1532–1540. doi: 10.1007/s00125-007-0692-2. [DOI] [PubMed] [Google Scholar]
- 19.Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Mayo KE, Miller LJ, Bataille D, et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 22.Al-Sabah S, Al-Fulaij M, Ahmed HA. Selectivity of peptide ligands for the human incretin receptors expressed in HEK-293 cells. Eur J Pharmacol. 2014;741:311–315. doi: 10.1016/j.ejphar.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 23.López de Maturana R, Willshaw A, Kuntzsch A, et al. The isolated N-terminal domain of the glucagon-like peptide-1 (GLP-1) receptor binds exendin peptides with much higher affinity than GLP-1. J Biol Chem. 2003;278:10195–10200. doi: 10.1074/jbc.M212147200. [DOI] [PubMed] [Google Scholar]
- 24.Al-Sabah S, Donnelly D. A model for receptor-peptide binding at the glucagon-like peptide-1 (GLP-1) receptor through the analysis of truncated ligands and receptors. Br J Pharmacol. 2003;140:339–346. doi: 10.1038/sj.bjp.0705453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol. 2012;166:27–41. doi: 10.1111/j.1476-5381.2011.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow GW, Kieffer TJ, McIntosh CH, et al. The insulinotropic region of gastric inhibitory polypeptide; fragment analysis suggests the bioactive site lies between residues 19 and 30. Can J Physiol Pharmacol. 1996;74:65–72. [PubMed] [Google Scholar]
- 27.Hinke SA, Manhart S, Pamir N, et al. Identification of a bioactive domain in the amino-terminus of glucose-dependent insulinotropic polypeptide (GIP) Biochim Biophys Acta. 2001;1547:143–155. doi: 10.1016/s0167-4838(01)00181-9. [DOI] [PubMed] [Google Scholar]
- 28.Parthier C, Kleinschmidt M, Neumann P, et al. Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc Natl Acad Sci USA. 2007;104:13942–13947. doi: 10.1073/pnas.0706404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Runge S, Thogersen H, Madsen K, et al. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem. 2008;283:11340–11347. doi: 10.1074/jbc.M708740200. [DOI] [PubMed] [Google Scholar]
- 30.Underwood CR, Garibay P, Knudsen LB, et al. Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J Biol Chem. 2010;285:723–730. doi: 10.1074/jbc.M109.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollenstein K, Kean J, Bortolato A, et al. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature. 2013;499:438–443. doi: 10.1038/nature12357. [DOI] [PubMed] [Google Scholar]
- 32.Siu FY, He M, de Graaf C, et al. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013;499:444–449. doi: 10.1038/nature12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen M, Vedtofte L, Holst JJ, et al. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. doi: 10.2337/db11-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amisten S, Salehi A, Rorsman P, et al. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther. 2013;139:359–391. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Chalmer T, Almdal TP, Vilsboll T, et al. Adverse drug reactions associated with the use of liraglutide in patients with type 2 diabetes - focus on pancreatitis and pancreas cancer. Expert Opin Drug Saf. 2015;14:171–180. doi: 10.1517/14740338.2015.975205. [DOI] [PubMed] [Google Scholar]
- 36.Consoli A, Formoso G. Potential side effects to GLP-1 agonists: understanding their safety and tolerability. Expert Opin Drug Saf. 2015;14:207–218. doi: 10.1517/14740338.2015.987122. [DOI] [PubMed] [Google Scholar]
- 37.Gupta D, Leahy AA, Monga N, et al. Peroxisome proliferator-activated receptor γ (PPARγ) and its target genes are downstream effectors of FoxO1 protein in islet β-cells: mechanism of β-cell compensation and failure. J Biol Chem. 2013;288:25440–25449. doi: 10.1074/jbc.M113.486852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volz A, Goke R, Lankat-Buttgereit B, et al. Molecular cloning, functional expression, and signal transduction of the GIP-receptor cloned from a human insulinoma. FEBS Lett. 1995;373:23–29. doi: 10.1016/0014-5793(95)01006-z. [DOI] [PubMed] [Google Scholar]
- 39.Gremlich S, Porret A, Hani EH, et al. Cloning, functional expression, and chromosomal localization of the human pancreatic islet glucose-dependent insulinotropic polypeptide receptor. Diabetes. 1995;44:1202–1208. doi: 10.2337/diab.44.10.1202. [DOI] [PubMed] [Google Scholar]
- 40.Harada N, Yamada Y, Tsukiyama K, et al. A novel GIP receptor splice variant influences GIP sensitivity of pancreatic beta-cells in obese mice. Am J Physiol Endocrinol Metab. 2008;294:E61–E68. doi: 10.1152/ajpendo.00358.2007. [DOI] [PubMed] [Google Scholar]
- 41.Yabe D, Seino Y. Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and beta cell preservation. Prog Biophys Mol Biol. 2011;107:248–256. doi: 10.1016/j.pbiomolbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buteau J, Foisy S, Joly E, et al. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 44.Talbot J, Joly E, Prentki M, et al. β-Arrestin1-mediated recruitment of c-Src underlies the proliferative action of glucagon-like peptide-1 in pancreatic β INS832/13 cells. Mol Cell Endocrinol. 2012;364:65–70. doi: 10.1016/j.mce.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Al-Sabah S, Al-Fulaij M, Shaaban G, et al. The GIP receptor displays higher basal activity than the GLP-1 receptor but does not recruit GRK2 or arrestin3 effectively. PLoS One. 2014;9:e106890. doi: 10.1371/journal.pone.0106890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fortin JP, Schroeder JC, Zhu Y, et al. Pharmacological characterization of human incretin receptor missense variants. J Pharmacol Exp Ther. 2010;332:274–280. doi: 10.1124/jpet.109.160531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almind K, Ambye L, Urhammer SA, et al. Discovery of amino acid variants in the human glucose-dependent insulinotropic polypeptide (GIP) receptor: the impact on the pancreatic beta cell responses and functional expression studies in Chinese hamster fibroblast cells. Diabetologia. 1998;41:1194–1198. doi: 10.1007/s001250051051. [DOI] [PubMed] [Google Scholar]
- 48.Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol. 2008;153((suppl 1)):S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohse MJ, Krasel C, Winstel R, et al. G-protein-coupled receptor kinases. Kidney Int. 1996;49:1047–1052. doi: 10.1038/ki.1996.153. [DOI] [PubMed] [Google Scholar]
- 50.Krasel C, Vilardaga JP, Bunemann M, et al. Kinetics of G-protein-coupled receptor signalling and desensitization. Biochem Soc Trans. 2004;32:1029–1031. doi: 10.1042/BST0321029. [DOI] [PubMed] [Google Scholar]
- 51.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mundell SJ, Kelly E. The effect of inhibitors of receptor internalization on the desensitization and resensitization of three Gs-coupled receptor responses. Br J Pharmacol. 1998;125:1594–1600. doi: 10.1038/sj.bjp.0702234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cernecka H, Sand C, Michel MC. The odd sibling: features of β3-adrenoceptor pharmacology. Mol Pharmacol. 2014;86:479–484. doi: 10.1124/mol.114.092817. [DOI] [PubMed] [Google Scholar]
- 54.Widmann C, Dolci W, Thorens B. Agonist-induced internalization and recycling of the glucagon-like peptide-1 receptor in transfected fibroblasts and in insulinomas. Biochem J. 1995;310:203–214. doi: 10.1042/bj3100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuna RS, Girada SB, Asalla S, et al. Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2013;305:E161–E170. doi: 10.1152/ajpendo.00551.2012. [DOI] [PubMed] [Google Scholar]
- 56.Roed SN, Wismann P, Underwood CR, et al. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol Cell Endocrinol. 2014;382:938–949. doi: 10.1016/j.mce.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez P, Roncero I, Blazquez E, et al. Substitution of the cysteine 438 residue in the cytoplasmic tail of the glucagon-like peptide-1 receptor alters signal transduction activity. J Endocrinol. 2005;185:35–44. doi: 10.1677/joe.1.06031. [DOI] [PubMed] [Google Scholar]
- 58.Syme CA, Zhang L, Bisello A. Caveolin-1 regulates cellular trafficking and function of the glucagon-like Peptide 1 receptor. Mol Endocrinol. 2006;20:3400–3411. doi: 10.1210/me.2006-0178. [DOI] [PubMed] [Google Scholar]
- 59.Thompson A, Kanamarlapudi V. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Gαq pathway. Biochem Pharmacol. 2015;93:72–84. doi: 10.1016/j.bcp.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Montrose-Rafizadeh C, Avdonin P, Garant MJ, et al. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999;140:1132–1140. doi: 10.1210/endo.140.3.6550. [DOI] [PubMed] [Google Scholar]
- 61.Baggio LL, Kim JG, Drucker DJ. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes. 2004;53((suppl 3)):S205–S214. doi: 10.2337/diabetes.53.suppl_3.s205. [DOI] [PubMed] [Google Scholar]
- 62.Sonoda N, Imamura T, Yoshizaki T, et al. β-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic β-cells. Proc Natl Acad Sci USA. 2008;105:6614–6619. doi: 10.1073/pnas.0710402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merlen C, Fabrega S, Desbuquois B, et al. Glucagon-mediated internalization of serine-phosphorylated glucagon receptor and Gsα in rat liver. FEBS Lett. 2006;580:5697–5704. doi: 10.1016/j.febslet.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 64.Ferrandon S, Feinstein TN, Castro M, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feinstein TN, Wehbi VL, Ardura JA, et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jorgensen R, Martini L, Schwartz TW, et al. Characterization of glucagon-like peptide-1 receptor β-arrestin 2 interaction: a high-affinity receptor phenotype. Mol Endocrinol. 2005;19:812–823. doi: 10.1210/me.2004-0312. [DOI] [PubMed] [Google Scholar]
- 67.Schelshorn D, Joly F, Mutel S, et al. Lateral allosterism in the glucagon receptor family: glucagon-like peptide 1 induces G-protein-coupled receptor heteromer formation. Mol Pharmacol. 2012;81:309–318. doi: 10.1124/mol.111.074757. [DOI] [PubMed] [Google Scholar]
- 68.Pierce KL, Lefkowitz RJ. Classical and new roles of β-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 69.Mohammad S, Patel RT, Bruno J, et al. A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin sensitivity. Mol Cell Biol. 2014;34:3618–3629. doi: 10.1128/MCB.00256-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roed SN, Nohr AC, Wismann P, et al. Functional consequences of glucagon-like peptide-1 receptor cross-talk and trafficking. J Biol Chem. 2015;290:1233–1243. doi: 10.1074/jbc.M114.592436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gurevich VV, Gurevich EV. GPCR monomers and oligomers: it takes all kinds. Trends Neurosci. 2008;31:74–81. doi: 10.1016/j.tins.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harikumar KG, Wootten D, Pinon DI, et al. Glucagon-like peptide-1 receptor dimerization differentially regulates agonist signaling but does not affect small molecule allostery. Proc Natl Acad Sci USA. 2012;109:18607–18612. doi: 10.1073/pnas.1205227109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vrecl M, Drinovec L, Elling C, et al. Opsin oligomerization in a heterologous cell system. J Recept Signal Transduct Res. 2006;26:505–526. doi: 10.1080/10799890600932253. [DOI] [PubMed] [Google Scholar]
- 74.Whitaker GM, Lynn FC, McIntosh CH, et al. Regulation of GIP and GLP1 receptor cell surface expression by N-glycosylation and receptor heteromerization. PLoS One. 2012;7:e32675. doi: 10.1371/journal.pone.0032675. [DOI] [PMC free article] [PubMed] [Google Scholar]