Abstract
Cellular blebbing is a unique form of dynamic protrusion emanating from the plasma membrane which can be either apoptotic or nonapoptotic in nature. Blebs have been observed in a wide variety of cell types and in response to multiple mechanical and chemical stimuli. They have been linked to various physiological and pathological processes including tumor motility and invasion, as well as to various immunological disorders. They can form and retract extremely rapidly in seconds or minutes, or slowly over hours or days. This review focuses on recent evidence regarding the role of blebbing in cell locomotion with particular emphasis on its role in tumor metastasis, indicating the role of specific causative molecules. The phenomenon of blebbing has been observed in endocrine-resistant breast cancer cells in response to brief exposure to extracellular alkaline pH, which leads to enhanced invasive capacity. Genetic or pharmacological targeting of cellular blebs could serve as a potential therapeutic option to control tumor metastasis.
Key Words: Bleb, Actin, Motility, Invasion, Endocrine resistance, Breast cancer
Introduction
Cellular blebbing, first described in 1919 [1] as hyaline blisters or bubbles, was later characterized as smooth circular extensions (2-15 µm in diameter) of the plasma membrane that expand from the cytoplasm and retract to the initial site of origin [2]. These protrusions have received more attention in recent years due to their occurrence in widely differing cell types and their association with various physiological and pathological conditions [3,4,5]. This review discusses the role of the actin machinery in bleb formation, the various cell types which produce these protrusions, the heterogeneous stimuli of blebbing and its role in various functions including locomotion. In addition, recent data demonstrating the role of alkaline pH as a novel stimulus of blebbing, specifically in endocrine-resistant breast cancer cells, and its association with cell motility are discussed.
Blebs have been observed in numerous cell types including fibroblasts [6], endothelial and mesenchymal cells [4], cancer cells [7,8,9,10,11], immune cells [12,13,14], germ cells [15,16,17], ameba [18], parasites [19] and bacteria [20,21,22]. It was originally assumed that blebbing was related solely to pathological conditions in response to nonspecific cellular insults such as lipid peroxidation [23], anoxia [24] and energy depletion [24,25,26], with motility/invasion [11,27] and multidrug resistance in tumor cells [28], or as a prerequisite for necrosis. However, it is now considered to be an important physiological process which occurs during cell blastulation in Fundulus deep cells [29], cytokinesis, cell spreading, virus infection, protective mechanisms against injury (either physical or chemical stress) [30,31,32] and as a hallmark of the execution stage of apoptosis [33]. For example, rabbit renal proximal epithelial cells form extensive blebs after hypoxic injury [34].
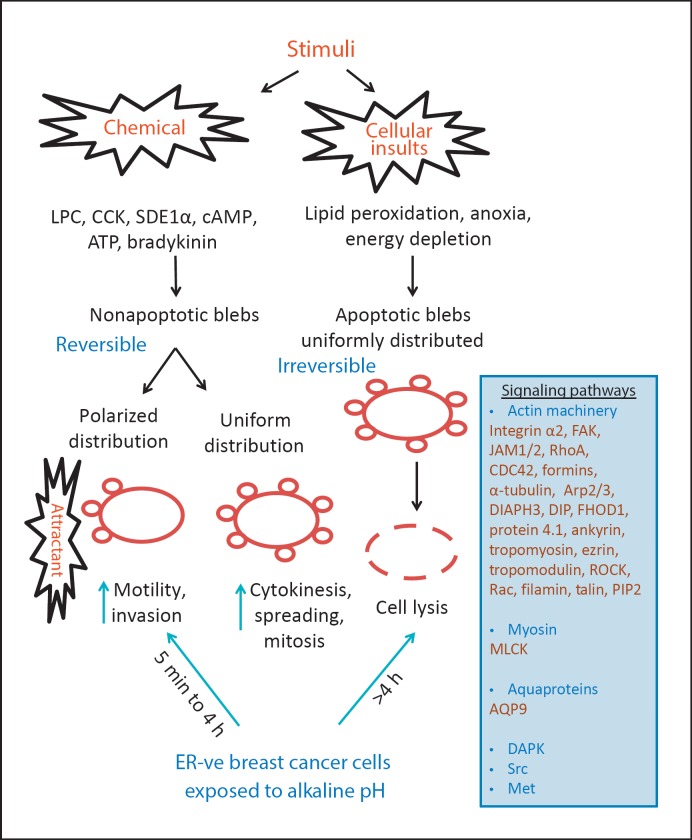
It had been suggested that bleb formation occurs at a rate faster than that of actin filament elongation and polymerization needed for the formation of other types of cellular protrusions such as lamellipodia [35,36,37]. It can be either reversible (nonapoptotic) or irreversible (apoptotic). Nonapoptotic blebbing has been seen in various cellular processes including mitosis [6,9,38], spreading [4,9,39,40,41] and migration [5,16,42,43], as well as after trypsin-mediated detachment of cultured cells [3,39]. The apoptotic form of blebbing was detected by the treatment of serum-starved cells with the caspase inhibitor Z-VAD-FMK, which enables apoptotic cells to bleb for variable periods stretching from hours to days; depolymerization of actin inhibits this phenomenon [44]. The distribution profile of newly formed blebs appears to be correlated with particular cellular processes. During cell motility, they are polarized towards the source of a stimulus, while during apoptosis/necrosis they are uniformly distributed around the entire cell surface (fig. 1). The time scale of bleb formation varies considerably. Apoptotic blebs take 24-48 h, whereas alkaline-challenged estrogen receptor (ER)-depleted breast cancer cells exhibit them in 5-10 min [11], and Dictyostelium cells form them in just 1 s [45]. In some cases, the total cell volume is preserved during blebbing [46], but in others it is significantly reduced [11,27].
Fig. 1.
Various stimuli and molecules involved in the formation of apoptotic and nonapoptotic blebs. Cellular blebbing can be induced in response to various mechanical and chemical stimuli and leads to the activation of several downstream signaling molecules depending on the stimuli and cell type. These protrusions have been shown to play an important role in various physiological and pathophysiological conditions, such as immune-related conditions and cancer pathogenesis, as discussed in the text. Apoptotic cells exhibit uniform blebbing that eventually results in cell lysis, whereas nonapoptotic blebs are reversible and involved in motile functions necessary for directional cellular migration. In the case of endocrine-resistant cancer cells, a high external pH can induce either condition depending upon the time of exposure. The panel on the right lists the molecules that have been implicated in bleb formation.
The bleb life cycle can be subdivided into three phases: (1) nucleation, (2) expansion (cytosol flowing from the cell body into the bleb [9]) and (3) retraction (driven by myosin [7]). They are formed when the plasma membrane separates from the underlying actin cortex and is pushed outwards by fluid pressure [31] exerted by contraction of the cell cortex. This leaves little F-actin beneath the bleb membrane as it expands. Various mechanisms have been suggested for the generation of these protrusions, including actin filament elongation [47,48,49], hydrostatic pressure generated by cortical contractions giving rise to pseudopod formation [50,51], gel-swelling pressure involving osmotic expansion [52], lipid or membrane flow involving endocytosis-/exocytosis-mediated recycling of membrane components [53] or cell substratum adhesiveness [54]. However, other models have suggested that their formation and extension occur as a result of an osmotic flux followed by actin polymerization [55]. Reducing intracellular hydrostatic pressure by placing cells in hypertonic media inhibits bleb and lamellipodia formation, locomotion and polarity [51,56]. Fedier and Keller [57] showed that reducing free water content inside Walker carcinoma cells by 39% inhibited bleb formation and locomotion. This leads to reduced intracellular hydrostatic pressure, increased viscoelastic resistance to passive and swelling deformation and decreased space between cytoplasmic components, without a significant increase in viscosity of the aqueous phase or any change in the amount of F-actin. Pseudopod-like blebs were also seen in U937 monocytes stimulated by permeabilization of the cellular membrane with a nanosecond-pulsed electric field; this was inhibited by partial isosmotic replacement of extracellular sodium chloride for a larger solute such as sucrose, suggesting that colloid-osmotic water uptake is the driving force for bleb formation. Pretreatment with the actin depolymerizer cytochalasin D prevented membrane blebbing, underlining the requirement of the actin cytoskeleton [58].
Other evidence has also suggested a role for aquaprotein (AQP) channels in bleb formation. The AQPs are membrane-anchored channels [59,60] defined by their permeability characteristics in water and solutes like glycerol [61]. They have been proposed to play a role in cell migration, due to their polarization towards the leading edge of migrating neutrophils [13,62,63,64]. Overexpression of AQP9 in HEK-293 cells resulted in a rapid influx of water and increased intracellular pressure, leading to the formation of filopodia bleb-like protrusions, contributing to their motile behavior [65].
Role of the Actin Machinery
The contractile cortex is a layer of cytoskeleton (50 nm to 2 µm thick) under the plasma membrane that is rich in actin filaments, myosin II and various actin-binding proteins [66]. It plays an important role in various cellular processes including cytokinesis, motility, migration, phagocytosis and tissue morphogenesis [66]. The small GTPase Ras homolog gene family member A (RhoA) regulates the contractile cortex assembly [67] and subsequent enhancement in cell polarity and locomotion [68,69]. RhoA plays an important role in the regulation of actin cortex assembly by inducing actin polymerization and subsequent interaction with myosin. Several proteins of the actin machinery, such as formins (actin-nucleating protein), actin nucleators and associated proteins (CDC42, Arp2/3, DIAPH3, DIP, FMNL1, FHOD1), have been shown to play a critical role in bleb formation in various cells [7]. It is well documented that actin is the main component of most forms of blebs [6,9,70,71,72]. Charras et al. [7] demonstrated the involvement of actin and various actin-binding proteins in the blebbing of filamin-deficient M2 cells. They found that the erythroid submembranous cytoskeleton protein 4.1 and ankyrin-β as well as tropomyosin and tropomodulin (which are involved in myosin contraction) were present inside the cytoplasmic compartment of the blebs. Microinjection of a dominant active ezrin (which stabilizes the actin membrane attachment) inhibited bleb formation, suggesting that this phenomenon occurs in areas of weak actin-membrane adhesion. The RhoA-ROCK (Rho-associated protein kinase)-myosin axis plays a central role in the formation of both apoptotic and nonapoptotic membrane blebbing [8,19,73,74]. For example, the upregulation of RhoA in the absence of the tumor suppressor genes p53 [73], Rac1 GTPase [75,76,77,78] or ROCK [79] can induce plasma membrane blebs. Furthermore, membrane blebbing can be induced in areas of weak cortex-membrane interactions caused by a lack of actin-binding protein filamin A [9,80], the membrane linker protein talin [81] or PIP2 [81,82].
The contractile activity of myosin, through its phosphorylation by myosin light chain kinase (MLCK), is also important for bleb formation [83,84]. Microinjection of catalytically active MLCK can induce blebs [85], whilst inhibitors of MLCK and Rho kinase activity, or the actin depolymerizer cytochalasin D, all inhibit bleb formation in serum-deprived Z-VAD-FMK-treated PC6-3 cells [44]. A recent study showed that shRNA-mediated knockdown of the 15-kDa selenoprotein (Sep 15) in the Chang liver cell line, as well as in T-REX-HeLa cells, resulted in cell shrinkage with rounded morphology and the formation of nonapoptotic reversible membrane blebs by remodeling of cytoskeletal proteins such as α-tubulin and F-actin [86]. Pretreatment with inhibitors of ROCK, RhoA, MLCK or blebbistatin reversed bleb morphology within 10 h, suggesting the importance of the ROCK/RhoA/MLCK pathway in membrane blebbing of Sep15-deficient cells.
Besides the well-established role of the actin machinery, various protein kinases such as death-associated protein kinase (DAPK), Src and Met have been shown to be involved in bleb formation.
Death-Associated Protein Kinase
DAPK is a calcium/calmodulin-regulated, cytoskeleton-associated serine/threonine kinase which enhances cell apoptosis in response to various stimuli [87]. Its over-expression can induce apoptotic blebs in various cell types [79,88,89], which are independent of Rho kinase but dependent on increased myosin contractility [90,91]. In marked contrast, DAPK can also inhibit membrane blebbing through the regulation of cytoskeletal proteins such as tropomyosin, which plays a role in stress fiber formation and stabilization [92,93].
Proto-Oncogene Tyrosine-Protein Kinase Src
The Src protein family plays an important role in regulating various intracellular signaling networks [94] and in controlling cell migration, proliferation and survival [95]. The Src homology 4 (SH4) domain plays a key role as an anchoring membrane-targeting domain for Src localization and activation [96,97]. Interestingly, the expression of the SH4 domains in Leishmania parasite virulence factor HASPB (hydrophilic acylated surface protein B) induced nonapoptotic ROCK-myosin-II-dependent plasma membrane blebs. Endogenous Src activity was crucial for the formation of these blebs in SH4 domain-expressing cells and led to enhanced Src-induced cell invasion [19].
Met Receptor Tyrosine Kinase
The Met receptor tyrosine kinase/hepatocyte growth factor axis plays a key role in tumor growth and metastasis [98,99,100]. Constitutively active Met expression induces membrane blebbing in the invasive Moloney sarcoma virus-transformed MDCK MSV-MDCK-INV [101,102,103], small cell lung cancer cells [104], as well as in breast cancer cell lines MDA-MB-231 and B549 [105]. This can be significantly inhibited by pretreatment with inhibitors of ROCK and actin assembly (latrunculin A and jasplakinolide). In addition, Met-induced blebbing enhances breast cancer cell motility, migration and invasion [105].
Stimuli Inducing Bleb Formation
Cell blebbing can be manipulated by mechanical or chemical treatment. It can be induced following microtubule disassembly [42,106,107], by inhibition of actin polymerization [7], increasing membrane rigidity or inactivating myosin motors [8,108], and by modulating intracellular pressure [8,10]. In addition, blebbing can be induced through alteration in cell adhesion machinery, as seen in MDA-MB-231 breast cancer cells when coated with Matrigel [109], or by detachment of rat liver epithelial cells by the stable expression of a constitutively active Q63L version of RhoA [110]. In a recent study using the Chinese hamster ovary CHO fibroblast cell line, it was shown that compression and subsequent dilation (folding and unfolding) of the plasma membrane cortical layers induced cell rounding and migration through membrane blebs [111]. Blebs can also be induced in response to various extracellular stimuli. For example, bleb formation was dependent on the presence of serum components in human conjunctiva cell culture medium [112] and on lysophosphatidic acid in the NB2a rat neuroblastoma cells [113] and zebra fish progenitor cells [18]. In addition, the neurotransmitter cholecystokinin was found to induce blebbing in rat pancreatic acinar cells [114,115], the chemoattractant cAMP (cyclic adenosine monophosphate) in Dictyostelium discoideum cells [5,43], the chemokine SDE1α in zebra fish [16], mitoxantrone in HL60 cells [116] and extracellular adenosine triphosphate (ATP) via stimulation of the ATP-gated ion channel P2X7 [117,118]. The neuropeptide bradykinin (acting through B2 receptors) induced human glioma cell migration through the generation of bleb-like structures, regulated by intracellular calcium, resulting in the contraction of the actin cytoskeleton, cytoplasmic flow and the activation of calcium-dependent K+ and Cl- channels [119]. Pretreatment with the myosin II kinase inhibitor, blebbistatin, abolished both bleb formation and motility. These data suggest the occurrence of actin-rich blebs in response to a wide range of stimuli (either mechanical or chemical).
Blebbing has been shown to play an important role in various physiological and pathophysiological conditions. Its role in enhancing cell motility and invasion, immune cell maturation and activation, and pathogenic escape is discussed in the following section. In addition, recent data are presented regarding the role of actin-rich blebs in endocrine-resistant breast cancer cells upon exposure to extracellular alkaline pH.
Role of Blebbing in Cellular Locomotion
Cell locomotion and polarity play an important role during embryonic development, wound healing and inflammation, and also in cancer metastasis [13,27,114,120]. The latter process involves directional motility/invasion towards a chemoattractant and is associated with one-sided formation of protrusions such as lamellipodia (which are flattened), microspikes (or filopodia), which are narrow and stiff rods, or blebs (also called lobopodia) [120]. Most of these actin-rich protrusions are formed preferentially at the leading edge of the crawling cells to guide them towards a site of inflammation or infection (in the case of immune cells), or to effectively enhance penetration of cancer cells into blood or lymphatic vessels. Cellular protrusions are of two main types: those driven by actin polymerization such as pseudopodia or lamellipodia, and those driven by fluid pressure such as blebs. In addition to enabling tumor invasion [121], as seen for M2 melanoma and Walker carcinoma cells [51,80] and breast cancer cells [105], blebbing is involved in the migration of primordial germ cells of zebra fish [16,17], the pathogen Entamoeba histolytica into the liver [122], Dictyostelium ameba under 2% agarose [43], Leishmania parasite [19], HEK-293 cells [65], Drosophila [123], Xenopus laevis[124] and killifish deep cells [125]. Actinomycin-rich membrane blebs are also evident in the sea urchin red spherule coelomocyte immune cells, which enhance their motility. Treatment with cytochalasin B or blebbistatin can abolish membrane blebbing and cell motility, highlighting the importance of these protrusions in immune cell motility [12]. Various reports have shown the coexistence of blebs and F-actin-driven protrusions at the leading edge of migrating cells [11,45], and blebs can give rise to pseudopods or vice versa by continued actin polymerization.
Motile cells have a variety of shapes and move at differing speeds in different environments, generally extending their outer edges by actin polymerization for forward movement [120,126,127,128]. Yoshida and Soldati [43] showed Dictyostelium cells utilizing mainly actin-driven pseudopods as well as small transient blebs, whilst another study suggested that their motility was purely bleb driven, evoked by mechanical resistance, dependent on PI3K activity and strongly chemotactic [45]. Cancer cells may switch between F-actin-driven to bleb-driven motility according to their environment [74,121]. For example, when cancer cells experience genetic impairment of F-actin polymerization, they compensate by using unbranched actin filaments or blebs for locomotion [129,130]. Furthermore, some reports have demonstrated that cancer cells could invade the extracellular matrix (ECM) through various mechanisms including mesenchymal-proteolytic degradation of ECM on a flat surface and ameboid blebbing motility in collagen gels [121] squeezing between the pores in the ECM and invading in a manner independent of proteolytic activity [74,131,132]. E. histolytica cells, the causative agents of amebiasis [133], utilize ameboid bleb-driven motility to enable them to invade various organs such as liver parenchyma [122]. E. histolytica motility using blebs is approximately two orders of magnitude faster than the average motion of mesenchymal cells. As with cancer cells, these data suggest that E. histolytica can be prompted to switch between bleb-driven and mesenchymal motility depending on the environment. Interestingly, noninvasive cells can be transformed into an invasive phenotype by the experimental induction of membrane blebs [19,73]. Sharma et al. [134] demonstrated that glioma cells produce continuous actin-rich blebs when cultured on a flat surface, while exhibiting reversible blebbing and nonblebbing phenomenon on suspended STEP (spinneret-based tunable engineered parameters) fibrous substrates. They also observed that blebs on a single cell were greater in number and longer in size when cells were in smaller and spherical morphologies. Bleb formation was significantly reduced when glioma cells were spread along the STEP nanofibers. An inverse relationship was observed between blebbing and cell migration, in contrast to previous reports using other stimuli to induce membrane blebbing. It should be noted that treatment with various growth factors, such as fibroblast growth factor, platelet-derived growth factor or epithelial growth factor, did not induce membrane blebbing in zebra fish progenitor cells [18], which is consistent with our own observations in ER-silenced endocrine-resistant breast cancer cells [11].
These data highlight the importance of blebbing in enhancing cell motility through several mechanisms. The blebs appear to induce movement through the formation of circular projections emanating from the plasma membrane. Live cell imagery of cultured cells shows a circular movement of the blebs around the cell periphery [9]. When confronted with a chemoattractant, the blebs polarize along the part of the cell facing the stimuli and thereby allow cytoplasmic streaming which propels the cell in one direction. Furthermore, actin-rich blebs could provide driving forces to enable the cell to move forward, and also allow the cell to move in a ‘swimming fashion’ due to asymmetric cell shape changes during bleb formation, particularly in a 3D environment. Blebs could provide an alternative (backup) means for cellular motility when other cellular projections are impaired or deficient.
Besides their role in cell locomotion, recent data suggest their involvement in modulating the activity of the immune system as well as in cancer pathogenesis [11,27,135].
Blebbing: A Vaccine-Based Therapy
During apoptosis, the endoplasmic reticulum, RNA and chromatin are enclosed within blebs, which become detached from the main apoptotic cell at the later stages [136,137]. This phenomenon was suggested to aid immune maturation and the formation of vaccines to enable the killing of tumor cells. It was shown that ingestion of blebs by mouse-derived dendritic cells induced their maturation and subsequent Th17 cell activation when co-cultured with splenocytes in vitro [138,139], as well as in mice in vivo [140]. Ruben et al. [116] showed that blebs were generated from mitoxantrone-treated apoptotic HL60 cells (the human HLA-A2-negative AML cell line), which contain chromatin and endoplasmic reticulum. In addition, the apoptotic blebs were ingested by monocyte-derived dendritic cells (at 48 h co-culture), leading to increased expression of the lymph node homing receptor CCR7, enhanced migration towards CCL19, increased IFNγ production and CD4+ T-cell proliferation, with higher avidity of T cells towards HL60 AML cells. It is tempting to speculate that apoptotic blebs from AML cells may serve as a source of tumor-associated antigen for dendritic cell-based vaccine therapy.
Blebbing: Recruiting Immune Cells
Spontaneous apoptosis of tonsil-derived human germinal center B cells results in the loss of various cell adhesion molecules and the formation of multiple blebs on the outer cell membrane [135]. It has been shown that blebs derived from apoptotic germinal center B cells (but not from T cells) exhibited chemotactic activity towards peripheral blood monocytes. This process might play a role in vivo in recruiting the monocytes (professional phagocytes) to eliminate apoptotic B cells and prevent inflammatory reactions.
Blebbing: Escaping the Immune System
The opportunistic pathogen Pseudomonas aeruginosa targets surface-exposed epithelial cells and can infect any part of the human body [141]. It can infect cultured epithelial cells in vitro by entering through cells which display plasma membrane blebs to allow it to be isolated from the cytoplasm and to swim rapidly within them [21,22,142]. This phenomenon has also been shown in vivo in corneal epithelial cells within excised whole mouse eyes [143]. Recent evidence suggests that P. aeruginosa-induced apoptotic epithelial cell blebbing is independent of actin contraction but dependent on the ExoS type 3 secretion system effector [22,142] and cystic fibrosis transmembrane regulator osmoregulatory function [20].
Blebbing: Disease-Causing Protrusions
Suprastimulation of pancreatic acinar cells with the cholecystokinin octapeptide results in marked basolateral membrane blebbing and ameboid shape within 2 min of the stimulation [144,145,146]. This can be quickly reversed by reducing the concentration of the stimuli. The actin machinery has been postulated to play a role in cholecystokinin-mediated blebbing of pancreatic acinar cells, accompanied by marked reorganization of cytoplasmic actin and phosphorylation of myosin II. In addition, pretreatment with the actin depolymerizer cytochalasin D, the myosin ATPase inhibitor butanedione monoxime and the MLCK inhibitor ML-9 all inhibited bleb formation. The blebs in the acinar cells were shown to be responsible for the development of acute interstitial pancreatitis in animals [147].
Blebbing in Endocrine-Resistant Breast Cancer Cells
It has recently been shown that a brief (5-min) exposure of specifically endocrine-resistant breast cancer cells to an extracellular alkaline (but not acidic) pH environment induces cell rounding and shrinkage, and the formation of actin-rich membrane blebs on the outer membrane of the cells [11,27]. Various molecules that are critical for cell motility and invasion, such as integrin α2, JAM-1 (junctional adhesion molecule-1) and FAK (focal adhesion kinase), are translocated into the cytoplasmic compartment of the newly formed blebs. Other molecules that are not important for motility, such as vimentin, do not show any cytoplasmic redistribution upon exposure to alkaline pH. Pretreatment with cytochalasin D, MLCK and Rho kinase inhibitors, as well as inhibitors of Na+/H+ and Na+/K+ channels, completely inhibits cellular blebbing. The blebs are highly dynamic, polarizing in the direction of a chemoattractant, in this case epithelial growth factor, and significantly enhancing cell invasiveness, whereas they exhibited a uniform distribution profile when exposed to a source containing the vehicle only. This phenomenon is completely reversible by returning the cells to physiological pH (pH 7.4), although prolonged exposure (beyond 4 h) induces apoptosis (evident by increased levels of various heat shock proteins). These observations are the first indication that alkaline pH can induce bleb formation and that both apoptotic and nonapoptotic blebs can form in the same cell depending on the length of exposure. Both actin and MLCK were implicated in the formation of these blebs, which is in agreement with previous reports. The loss of the epithelial marker E-cadherin may play a role in the formation of alkaline-induced blebbing in the ER-ve breast cancer cells which have undergone epithelial to mesenchymal transition. This results in weaker cell-cell contact and the cell will acquire the flexibility to modify its morphology in response to a high extracellular pH environment. Furthermore, the cytoplasmic compartment of the newly formed blebs differs from the cytoplasm in the rest of the cell as it concentrates molecules critical for cell motility and invasion. We have also generated preliminary data suggesting the involvement of secreted components from the formed blebs which aid in enhanced cell invasion. Conditioned medium from cells exposed to alkaline pH enhances the invasion of cells cultured at pH 7.4.
Conclusions
Membrane blebbing has been observed in many cell types through the activation of various signaling molecules downstream of a broad range of external mechanical and chemical stimuli. The nonapoptotic form of membrane blebs plays an important role in various cellular functions, particularly motility. Recent evidence suggests their potential usefulness in vaccine-based therapy or as possible therapeutic targets in the treatment of interstitial pancreatitis. In the case of cancer cells, these exhibit this phenomenon in order to form protrusions, which compensate in case of deformities in the F-actin machinery, to enable them to invade into the ECM and beyond or to allow them to escape from unusual hostile extracellular environments (such as exposure to alkaline pH). Targeting membrane blebs with antiblebbing agents in conjunction with anti-inflammatory or antimetastatic agents might serve as a novel treatment approach to reduce cancer cell motility and metastasis. The various stimuli and molecules involved in membrane blebbing, as well as multiple cellular functions modulated by these protrusions, are summarized in the schema illustrated in figure 1.
References
- 1.Hogue MJ. The effect of hypotonic and hypertonic solutions on fibroblasts of the embryonic chick heart in vitro. J Exp Med. 1919;30:617–648. doi: 10.1084/jem.30.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charras GT. A short history of blebbing. J Microsc. 2008;231:466–478. doi: 10.1111/j.1365-2818.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 3.Norman LL, Brugues J, Sengupta K, et al. Cell blebbing and membrane area homeostasis in spreading and retracting cells. Biophys J. 2010;99:1726–1733. doi: 10.1016/j.bpj.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norman L, Sengupta K, Aranda-Espinoza H. Blebbing dynamics during endothelial cell spreading. Eur J Cell Biol. 2011;90:37–48. doi: 10.1016/j.ejcb.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Langridge PD, Kay RR. Blebbing of Dictyostelium cells in response to chemoattractant. Exp Cell Res. 2006;312:2009–2017. doi: 10.1016/j.yexcr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Laster SM, Mackenzie JM., Jr Bleb formation and F-actin distribution during mitosis and tumor necrosis factor-induced apoptosis. Microsc Res Tech. 1996;34:272–280. doi: 10.1002/(SICI)1097-0029(19960615)34:3<272::AID-JEMT10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Charras GT, Hu CK, Coughlin M, et al. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175:477–490. doi: 10.1083/jcb.200602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charras GT, Yarrow JC, Horton MA, et al. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham CC. Actin polymerization and intracellular solvent flow in cell surface blebbing. J Cell Biol. 1995;129:1589–1599. doi: 10.1083/jcb.129.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khajah MA, Mathew PM, Alam-Eldin NS, et al. Bleb formation is induced by alkaline but not acidic pH in estrogen receptor silenced breast cancer cells. Int J Oncol. 2015;46:1685–1698. doi: 10.3892/ijo.2015.2884. [DOI] [PubMed] [Google Scholar]
- 12.D'Andrea-Winslow L, Novitski AK. Active bleb formation is abated in Lytechinus variegatus red spherule coelomocytes after disruption of acto-myosin contractility. Integr Zool. 2008;3:115–122. doi: 10.1111/j.1749-4877.2008.00086.x. [DOI] [PubMed] [Google Scholar]
- 13.Loitto VM, Forslund T, Sundqvist T, et al. Neutrophil leukocyte motility requires directed water influx. J Leukoc Biol. 2002;71:212–222. [PubMed] [Google Scholar]
- 14.Grinnell F. Migration of human neutrophils in hydrated collagen lattices. J Cell Sci. 1982;58:95–108. doi: 10.1242/jcs.58.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Dzementsei A, Schneider D, Janshoff A, et al. Migratory and adhesive properties of Xenopus laevis primordial germ cells in vitro. Biol Open. 2013;2:1279–1287. doi: 10.1242/bio.20135140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaser H, Reichman-Fried M, Castanon I, et al. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell. 2006;11:613–627. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Row RH, Maitre JL, Martin BL, et al. Completion of the epithelial to mesenchymal transition in zebrafish mesoderm requires Spadetail. Dev Biol. 2011;354:102–110. doi: 10.1016/j.ydbio.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruprecht V, Wieser S, Callan-Jones A, et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell. 2015;160:673–685. doi: 10.1016/j.cell.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tournaviti S, Hannemann S, Terjung S, et al. SH4-domain-induced plasma membrane dynamization promotes bleb-associated cell motility. J Cell Sci. 2007;120:3820–3829. doi: 10.1242/jcs.011130. [DOI] [PubMed] [Google Scholar]
- 20.Jolly AL, Takawira D, Oke OO, et al. Pseudomonas aeruginosa-induced bleb-niche formation in epithelial cells is independent of actinomyosin contraction and enhanced by loss of cystic fibrosis transmembrane-conductance regulator osmoregulatory function. MBio. 2015;6:e02533. doi: 10.1128/mBio.02533-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimer SR, Evans DJ, Stern ME, et al. Pseudomonas aeruginosa utilizes the type III secreted toxin ExoS to avoid acidified compartments within epithelial cells. PLoS One. 2013;8:e73111. doi: 10.1371/journal.pone.0073111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angus AA, Lee AA, Augustin DK, et al. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect Immun. 2008;76:1992–2001. doi: 10.1128/IAI.01221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanWinkle WB, Snuggs M, Miller JC, et al. Cytoskeletal alterations in cultured cardiomyocytes following exposure to the lipid peroxidation product, 4-hydroxynonenal. Cell Motil Cytoskeleton. 1994;28:119–134. doi: 10.1002/cm.970280204. [DOI] [PubMed] [Google Scholar]
- 24.Friedman JE, Haddad GG. Major differences in Ca2+i response to anoxia between neonatal and adult rat CA1 neurons: Role of Ca2+o and Na+o. J Neurosci. 1993;13:63–72. doi: 10.1523/JNEUROSCI.13-01-00063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson ME, Gores GJ, Uhl CB, et al. Cytosolic free calcium and cell death during metabolic inhibition in a neuronal cell line. J Neurosci. 1994;14:4040–4049. doi: 10.1523/JNEUROSCI.14-07-04040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcussen M. Induction of cell surface blebbing by increased cellular Pi concentration. Biochem J. 1996;318:955–958. doi: 10.1042/bj3180955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khajah MA, Almohri I, Mathew PM, et al. Extracellular alkaline pH leads to increased metastatic potential of estrogen receptor silenced endocrine resistant breast cancer cells. PLoS One. 2013;8:2013. doi: 10.1371/journal.pone.0076327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong J, Jaiswal R, Mathys JM, et al. Microparticles and their emerging role in cancer multidrug resistance. Cancer Treat Rev. 2012;38:226–234. doi: 10.1016/j.ctrv.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Trinkaus JP. Surface activity and locomotion of Fundulus deep cells during blastula and gastrula stages. Dev Biol. 1973;30:69–103. doi: 10.1016/0012-1606(73)90049-3. [DOI] [PubMed] [Google Scholar]
- 30.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- 32.Babiychuk EB, Monastyrskaya K, Potez S, et al. Blebbing confers resistance against cell lysis. Cell Death Differ. 2011;18:80–89. doi: 10.1038/cdd.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Dai J, Grant RL, et al. Loss of cytoskeletal support is not sufficient for anoxic plasma membrane disruption in renal cells. Am J Physiol. 1997;272:C1319–C1328. doi: 10.1152/ajpcell.1997.272.4.C1319. [DOI] [PubMed] [Google Scholar]
- 35.Wang YL. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985;101:597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theriot JA, Mitchison TJ. Actin microfilament dynamics in locomoting cells. Nature. 1991;352:126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- 37.Sheetz MP, Wayne DB, Pearlman AL. Extension of filopodia by motor-dependent actin assembly. Cell Motil Cytoskeleton. 1992;22:160–169. doi: 10.1002/cm.970220303. [DOI] [PubMed] [Google Scholar]
- 38.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci USA. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bereiter-Hahn J, Luck M, Miebach T, et al. Spreading of trypsinized cells: cytoskeletal dynamics and energy requirements. J Cell Sci. 1990;96:171–188. doi: 10.1242/jcs.96.1.171. [DOI] [PubMed] [Google Scholar]
- 40.Erickson CA, Trinkaus JP. Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp Cell Res. 1976;99:375–384. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- 41.Hoglund AS. The arrangement of microfilaments and microtubules in the periphery of spreading fibroblasts and glial cells. Tissue Cell. 1985;17:649–666. doi: 10.1016/0040-8166(85)90002-3. [DOI] [PubMed] [Google Scholar]
- 42.Keller H, Eggli P. Protrusive activity, cytoplasmic compartmentalization, and restriction rings in locomoting blebbing walker carcinosarcoma cells are related to detachment of cortical actin from the plasma membrane. Cell Motil Cytoskeleton. 1998;41:181–193. doi: 10.1002/(SICI)1097-0169(1998)41:2<181::AID-CM8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida K, Soldati T. Dissection of amoeboid movement into two mechanically distinct modes. J Cell Sci. 2006;119:3833–3844. doi: 10.1242/jcs.03152. [DOI] [PubMed] [Google Scholar]
- 44.Mills JC, Stone NL, Erhardt J, et al. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zatulovskiy E, Tyson R, Bretschneider T, et al. Bleb-driven chemotaxis of Dictyostelium cells. J Cell Biol. 2014;204:1027–1044. doi: 10.1083/jcb.201306147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skoufias DA, DeBonis S, Saoudi Y, et al. S-trityl-L-cysteine is a reversible, tight binding inhibitor of the human kinesin Eg5 that specifically blocks mitotic progression. J Biol Chem. 2006;281:17559–17569. doi: 10.1074/jbc.M511735200. [DOI] [PubMed] [Google Scholar]
- 47.Cooper JA. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- 48.Peskin CS, Odell GM, Oster GF. Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys J. 1993;65:316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cramer LP, Mitchison TJ, Theriot JA. Actin-dependent motile forces and cell motility. Curr Opin Cell Biol. 1994;6:82–86. doi: 10.1016/0955-0674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 50.Bereiter-Hahn J, Strohmeier R, Kunzenbacher I, et al. Locomotion of Xenopus epidermis cells in primary culture. J Cell Sci. 1981;52:289–311. doi: 10.1242/jcs.52.1.289. [DOI] [PubMed] [Google Scholar]
- 51.Keller HU, Bebie H. Protrusive activity quantitatively determines the rate and direction of cell locomotion. Cell Motil Cytoskeleton. 1996;33:241–251. doi: 10.1002/(SICI)1097-0169(1996)33:4<241::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 52.Oster G. Biophysics of the leading lamella. Cell Motil Cytoskeleton. 1988;10:164–171. doi: 10.1002/cm.970100121. [DOI] [PubMed] [Google Scholar]
- 53.Bretscher MS. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 1989;8:1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carter SB. Principles of cell motility: the direction of cell movement and cancer invasion. Nature. 1965;208:1183–1187. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- 55.Dong C, Aznavoorian S, Liotta LA. Two phases of pseudopod protrusion in tumor cells revealed by a micropipette. Microvasc Res. 1994;47:55–67. doi: 10.1006/mvre.1994.1005. [DOI] [PubMed] [Google Scholar]
- 56.Strohmeier R, Bereiter-Hahn J. Hydrostatic pressure in epidermal cells is dependent on Ca-mediated contractions. J Cell Sci. 1987;88:631–640. doi: 10.1242/jcs.88.5.631. [DOI] [PubMed] [Google Scholar]
- 57.Fedier A, Keller HU. Suppression of bleb formation, locomotion, and polarity of Walker carcinosarcoma cells by hypertonic media correlates with cell volume reduction but not with changes in the F-actin content. Cell Motil Cytoskeleton. 1997;37:326–337. doi: 10.1002/(SICI)1097-0169(1997)37:4<326::AID-CM4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 58.Rassokhin MA, Pakhomov AG. Electric field exposure triggers and guides formation of pseudopod-like blebs in U937 monocytes. J Membr Biol. 2012;245:521–529. doi: 10.1007/s00232-012-9433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preston GM, Carroll TP, Guggino WB, et al. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 60.Agre P, Smith BL, Baumgarten R, et al. Human red cell Aquaporin CHIP. II. Expression during normal fetal development and in a novel form of congenital dyserythropoietic anemia. J Clin Invest. 1994;94:1050–1058. doi: 10.1172/JCI117419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonen T, Walz T. The structure of aquaporins. Q Rev Biophys. 2006;39:361–396. doi: 10.1017/S0033583506004458. [DOI] [PubMed] [Google Scholar]
- 62.Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saadoun S, Papadopoulos MC, Hara-Chikuma M, et al. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 64.Loitto VM, Karlsson T, Magnusson KE. Water flux in cell motility: expanding the mechanisms of membrane protrusion. Cell Motil Cytoskeleton. 2009;66:237–247. doi: 10.1002/cm.20357. [DOI] [PubMed] [Google Scholar]
- 65.Karlsson T, Bolshakova A, Magalhaes MA, et al. Fluxes of water through aquaporin 9 weaken membrane-cytoskeleton anchorage and promote formation of membrane protrusions. PLoS One. 2013;8:3. doi: 10.1371/journal.pone.0059901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bray D, White JG. Cortical flow in animal cells. Science. 1988;239:883–888. doi: 10.1126/science.3277283. [DOI] [PubMed] [Google Scholar]
- 67.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 68.Lee JH, Katakai T, Hara T, et al. Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. J Cell Biol. 2004;167:327–337. doi: 10.1083/jcb.200403091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamijo K, Ohara N, Abe M, et al. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cotter TG, Lennon SV, Glynn JM, et al. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res. 1992;52:997–1005. [PubMed] [Google Scholar]
- 71.Pitzer F, Dantes A, Fuchs T, et al. Removal of proteasomes from the nucleus and their accumulation in apoptotic blebs during programmed cell death. FEBS Lett. 1996;394:47–50. doi: 10.1016/0014-5793(96)00920-9. [DOI] [PubMed] [Google Scholar]
- 72.Vemuri GS, Zhang J, Huang R, et al. Thrombin stimulates wortmannin-inhibitable phosphoinositide 3-kinase and membrane blebbing in CHRF-288 cells. Biochem J. 1996;314:805–810. doi: 10.1042/bj3140805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gadea G, de Toledo M, Anguille C, et al. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178:23–30. doi: 10.1083/jcb.200701120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 75.Sander EE, ten Klooster JP, van Delft S, et al. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz MA, Meredith JE, Kiosses WB. An activated Rac mutant functions as a dominant negative for membrane ruffling. Oncogene. 1998;17:625–629. doi: 10.1038/sj.onc.1201977. [DOI] [PubMed] [Google Scholar]
- 78.Lee E, Seastone DJ, Harris E, et al. RacB regulates cytoskeletal function in Dictyostelium spp. Eukaryot Cell. 2003;2:474–485. doi: 10.1128/EC.2.3.474-485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shani G, Marash L, Gozuacik D, et al. Death-associated protein kinase phosphorylates ZIP kinase, forming a unique kinase hierarchy to activate its cell death functions. Mol Cell Biol. 2004;24:8611–8626. doi: 10.1128/MCB.24.19.8611-8626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cunningham CC, Gorlin JB, Kwiatkowski DJ, et al. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, Litvinov RI, Chen X, et al. Loss of PIP5KIγ, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest. 2008;118:812–819. doi: 10.1172/JCI34239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raucher D, Stauffer T, Chen W, et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 83.Kohama K, Ye LH, Hayakawa K, et al. Myosin light chain kinase: an actin-binding protein that regulates an ATP-dependent interaction with myosin. Trends Pharmacol Sci. 1996;17:284–287. doi: 10.1016/0165-6147(96)10033-x. [DOI] [PubMed] [Google Scholar]
- 84.Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinases. J Muscle Res Cell Motil. 1997;18:1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- 85.Fishkind DJ, Cao LG, Wang YL. Microinjection of the catalytic fragment of myosin light chain kinase into dividing cells: effects on mitosis and cytokinesis. J Cell Biol. 1991;114:967–975. doi: 10.1083/jcb.114.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bang J, Jang M, Huh JH, et al. Deficiency of the 15-kDa selenoprotein led to cytoskeleton remodeling and non-apoptotic membrane blebbing through a RhoA/ROCK pathway. Biochem Biophys Res Commun. 2015;456:884–890. doi: 10.1016/j.bbrc.2014.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bialik S, Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 88.Cohen O, Feinstein E, Kimchi A. Dap-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen O, Inbal B, Kissil JL, et al. DAP-kinase participates in TNF-α- and Fas-induced apoptosis and its function requires the death domain. J Cell Biol. 1999;146:141–148. doi: 10.1083/jcb.146.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bialik S, Bresnick AR, Kimchi A. DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ. 2004;11:631–644. doi: 10.1038/sj.cdd.4401386. [DOI] [PubMed] [Google Scholar]
- 91.Kuo JC, Lin JR, Staddon JM, et al. Uncoordinated regulation of stress fibers and focal adhesions by DAP kinase. J Cell Sci. 2003;116:4777–4790. doi: 10.1242/jcs.00794. [DOI] [PubMed] [Google Scholar]
- 92.Cooper JA. Actin dynamics: tropomyosin provides stability. Curr Biol. 2002;12:R523–R525. doi: 10.1016/s0960-9822(02)01028-x. [DOI] [PubMed] [Google Scholar]
- 93.Houle F, Poirier A, Dumaresq J, et al. Dap kinase mediates the phosphorylation of tropomyosin-1 downstream of the ERK pathway, which regulates the formation of stress fibers in response to oxidative stress. J Cell Sci. 2007;120:3666–3677. doi: 10.1242/jcs.003251. [DOI] [PubMed] [Google Scholar]
- 94.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 95.Frame MC. Newest findings on the oldest oncogene; how activated Src does it. J Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- 96.Kaplan JM, Mardon G, Bishop JM, et al. The first seven amino acids encoded by the v-Src oncogene act as a myristylation signal: lysine 7 is a critical determinant. Mol Cell Biol. 1988;8:2435–2441. doi: 10.1128/mcb.8.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sigal CT, Zhou W, Buser CA, et al. Amino-terminal basic residues of Src mediate membrane binding through electrostatic interaction with acidic phospholipids. Proc Natl Acad Sci USA. 1994;91:12253–12257. doi: 10.1073/pnas.91.25.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the C-Met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 99.Zeng Q, Chen S, You Z, et al. Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NF-κB. J Biol Chem. 2002;277:25203–25208. doi: 10.1074/jbc.M201598200. [DOI] [PubMed] [Google Scholar]
- 100.Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting Met in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 101.Nguyen TN, Wang HJ, Zalzal S, et al. Purification and characterization of β-actin-rich tumor cell pseudopodia: role of glycolysis. Exp Cell Res. 2000;258:171–183. doi: 10.1006/excr.2000.4929. [DOI] [PubMed] [Google Scholar]
- 102.Vadnais J, Nault G, Daher Z, et al. Autocrine activation of the hepatocyte growth factor receptor/Met tyrosine kinase induces tumor cell motility by regulating pseudopodial protrusion. J Biol Chem. 2002;277:48342–48350. doi: 10.1074/jbc.M209481200. [DOI] [PubMed] [Google Scholar]
- 103.Jia Z, Vadnais J, Lu ML, et al. Rho/ROCK-dependent pseudopodial protrusion and cellular blebbing are regulated by p38 MAPK in tumour cells exhibiting autocrine C-Met activation. Biol Cell. 2006;98:337–351. doi: 10.1042/BC20050088. [DOI] [PubMed] [Google Scholar]
- 104.Maulik G, Kijima T, Ma PC, et al. Modulation of the C-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 2002;8:620–627. [PubMed] [Google Scholar]
- 105.Laser-Azogui A, Diamant-Levi T, Israeli S, et al. Met-induced membrane blebbing leads to amoeboid cell motility and invasion. Oncogene. 2014;33:1788–1798. doi: 10.1038/onc.2013.138. [DOI] [PubMed] [Google Scholar]
- 106.Cuvelier D, Thery M, Chu YS, et al. The universal dynamics of cell spreading. Curr Biol. 2007;17:694–699. doi: 10.1016/j.cub.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 107.Keller H, Rentsch P, Hagmann J. Differences in cortical actin structure and dynamics document that different types of blebs are formed by distinct mechanisms. Exp Cell Res. 2002;277:161–172. doi: 10.1006/excr.2002.5552. [DOI] [PubMed] [Google Scholar]
- 108.Cheung A, Dantzig JA, Hollingworth S, et al. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- 109.Kitzing TM, Sahadevan AS, Brandt DT, et al. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21:1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vasiliev JM, Omelchenko T, Gelfand IM, et al. Rho overexpression leads to mitosis-associated detachment of cells from epithelial sheets: a link to the mechanism of cancer dissemination. Proc Natl Acad Sci USA. 2004;101:12526–12530. doi: 10.1073/pnas.0404723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kapustina M, Elston TC, Jacobson K. Compression and dilation of the membrane-cortex layer generates rapid changes in cell shape. J Cell Biol. 2013;200:95–108. doi: 10.1083/jcb.201204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taylor AC. Attachment and spreading of cells in culture. Exp Cell Res. 1961;8:154–173. doi: 10.1016/0014-4827(61)90346-9. [DOI] [PubMed] [Google Scholar]
- 113.Hagmann J, Burger MM, Dagan D. Regulation of plasma membrane blebbing by the cytoskeleton. J Cell Biochem. 1999;73:488–499. [PubMed] [Google Scholar]
- 114.Torgerson RR, McNiven MA. The actin-myosin cytoskeleton mediates reversible agonist-induced membrane blebbing. J Cell Sci. 1998;111:2911–2922. doi: 10.1242/jcs.111.19.2911. [DOI] [PubMed] [Google Scholar]
- 115.Singh VP, McNiven MA. Src-mediated cortactin phosphorylation regulates actin localization and injurious blebbing in acinar cells. Mol Biol Cell. 2008;19:2339–2347. doi: 10.1091/mbc.E07-11-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruben JM, van den Ancker W, Bontkes HJ, et al. Apoptotic blebs from leukemic cells as a preferred source of tumor-associated antigen for dendritic cell-based vaccines. Cancer Immunol Immunother. 2014;63:335–345. doi: 10.1007/s00262-013-1515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MacKenzie A, Wilson HL, Kiss-Toth E, et al. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 118.Verhoef PA, Estacion M, Schilling W, et al. P2x7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1β release. J Immunol. 2003;170:5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- 119.Seifert S, Sontheimer H. Bradykinin enhances invasion of malignant glioma into the brain parenchyma by inducing cells to undergo amoeboid migration. J Physiol. 2014;592:5109–5127. doi: 10.1113/jphysiol.2014.274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 121.Wolf K, Mazo I, Leung H, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maugis B, Brugues J, Nassoy P, et al. Dynamic instability of the intracellular pressure drives bleb-based motility. J Cell Sci. 2010;123:3884–3892. doi: 10.1242/jcs.065672. [DOI] [PubMed] [Google Scholar]
- 123.Jaglarz MK, Howard KR. The active migration of Drosophila primordial germ cells. Development. 1995;121:3495–3503. doi: 10.1242/dev.121.11.3495. [DOI] [PubMed] [Google Scholar]
- 124.Wylie CC, Heasman J. The formation of the gonadal ridge in Xenopus laevis. I. A light and transmission electron microscope study. J Embryol Exp Morphol. 1976;35:125–138. [PubMed] [Google Scholar]
- 125.Fink RD, Trinkaus JP. Fundulus deep cells: directional migration in response to epithelial wounding. Dev Biol. 1988;129:179–190. doi: 10.1016/0012-1606(88)90172-8. [DOI] [PubMed] [Google Scholar]
- 126.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 127.Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21:636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 128.Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suraneni P, Rubinstein B, Unruh JR, et al. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J Cell Biol. 2012;197:239–251. doi: 10.1083/jcb.201112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Derivery E, Fink J, Martin D, et al. Free Brick1 is a trimeric precursor in the assembly of a functional wave complex. PLoS One. 2008;3:2462. doi: 10.1371/journal.pone.0002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 132.Pathak A, Kumar S. Biophysical regulation of tumor cell invasion: moving beyond matrix stiffness. Integr Biol. 2011;3:267–278. doi: 10.1039/c0ib00095g. [DOI] [PubMed] [Google Scholar]
- 133.Stanley SL., Jr Amoebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 134.Sharma P, Sheets K, Elankumaran S, et al. The mechanistic influence of aligned nanofibers on cell shape, migration and blebbing dynamics of glioma cells. Integr Biol. 2013;5:1036–1044. doi: 10.1039/c3ib40073e. [DOI] [PubMed] [Google Scholar]
- 135.Segundo C, Medina F, Rodriguez C, et al. Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: role of apoptotic blebs in monocyte chemotaxis. Blood. 1999;94:1012–1020. [PubMed] [Google Scholar]
- 136.Lane JD, Allan VJ, Woodman PG. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J Cell Sci. 2005;118:4059–4071. doi: 10.1242/jcs.02529. [DOI] [PubMed] [Google Scholar]
- 137.Fransen JH, Hilbrands LB, Ruben J, et al. Mouse dendritic cells matured by ingestion of apoptotic blebs induce T cells to produce interleukin-17. Arthritis Rheum. 2009;60:2304–2313. doi: 10.1002/art.24719. [DOI] [PubMed] [Google Scholar]
- 138.Fischer E, Kobold S, Kleber S, et al. Cryptic epitopes induce high-titer humoral immune response in patients with cancer. J Immunol. 2010;185:3095–3102. doi: 10.4049/jimmunol.0902166. [DOI] [PubMed] [Google Scholar]
- 139.Fransen JH, Hilbrands LB, Jacobs CW, et al. Both early and late apoptotic blebs are taken up by DC and induce IL-6 production. Autoimmunity. 2009;42:325–327. doi: 10.1080/08916930902828049. [DOI] [PubMed] [Google Scholar]
- 140.Verdijk P, Aarntzen EH, Lesterhuis WJ, et al. Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin Cancer Res. 2009;15:2531–2540. doi: 10.1158/1078-0432.CCR-08-2729. [DOI] [PubMed] [Google Scholar]
- 141.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 142.Angus AA, Evans DJ, Barbieri JT, et al. The ADP-ribosylation domain of Pseudomonas aeruginosa ExoS is required for membrane bleb niche formation and bacterial survival within epithelial cells. Infect Immun. 2010;78:4500–4510. doi: 10.1128/IAI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tam C, LeDue J, Mun JJ, et al. 3D quantitative imaging of unprocessed live tissue reveals epithelial defense against bacterial adhesion and subsequent traversal requires MyD88. PLoS One. 2011;6:25. doi: 10.1371/journal.pone.0024008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Adler G, Kern HF, Pan GZ, et al. Secretagogue-induced membrane alterations in dispersed acini from rat pancreas. Eur J Cell Biol. 1984;33:234–241. [PubMed] [Google Scholar]
- 145.Burnham DB, Williams JA. Effects of high concentrations of secretagogues on the morphology and secretory activity of the pancreas: a role for microfilaments. Cell Tissue Res. 1982;222:201–212. doi: 10.1007/BF00218300. [DOI] [PubMed] [Google Scholar]
- 146.O'Konski MS, Pandol SJ. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. J Clin Invest. 1990;86:1649–1657. doi: 10.1172/JCI114887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]