Abstract
This is a critical review on research conducted in the field of pulmonary delivery of liposomes. Issues relating to the mechanism of nebulisation and liposome composition were appraised and correlated with literature reports of liposome formulations used in clinical trials to understand the role of liposome size and composition on therapeutic outcome. A major highlight was liposome inhalation for the treatment of lung cancers. Many in vivo studies that explored the potential of liposomes as anticancer carrier systems were evaluated, including animal studies and clinical trials. Liposomes can entrap anticancer drugs and localise their action in the lung following pulmonary delivery. The safety of inhaled liposomes incorporating anticancer drugs depends on the anticancer agent used and the amount of drug delivered to the target cancer in the lung. The difficulty of efficient targeting of liposomal anticancer aerosols to the cancerous tissues within the lung may result in low doses reaching the target site. Overall, following the success of liposomes as inhalable carriers in the treatment of lung infections, it is expected that more focus from research and development will be given to designing inhalable liposome carriers for the treatment of other lung diseases, including pulmonary cancers. The successful development of anticancer liposomes for inhalation may depend on the future development of effective aerosolisation devices and better targeted liposomes to maximise the benefit of therapy and reduce the potential for local and systemic adverse effects.
Key Words: Aerosol, Arikace, Formulation, Inhalation, Liposome, Lung
Introduction
In 2015, the American Cancer Society estimated that in the United States 221,200 new cases of lung cancer were diagnosed, and that 158,040 individuals could die of the condition [1]. Sihoe and Yim [2] reported that about 85% of lung cancer patients had non-small cell lung cancer, while the remaining 15% had small cell lung cancer. The survival rate in lung cancer patients is dependent on early diagnosis of the cancer, and surgical resection of the tumorous tissue is often the preferred remedy. In the majority of lung cancer patients, chemotherapy is given in order to minimise the chance of metastases, which is one of the significant problems in lung cancer sufferers [3]. The extensive distribution of lymphoid tissue in the lungs allows cancer cells to metastasise to organs distant from the pulmonary system [2]. Approximately 70% of patients with non-small cell lung cancer suffer from advanced distant metastasis at the time of diagnosis, meaning surgery is not curative at that stage of the disease [4]. Therapeutic strategies for targeting lung cancer are of great importance not only because of possible remission, but also for reducing the potential of lung cancer metastases, and thereby increasing the chance of success with surgical intervention or radiotherapy [4].
A serious limitation of pulmonary delivery of anticancer drugs is the poor water solubility of many chemotherapeutic agents (e.g. paclitaxel; PTX) [5]. In addition, the thinness of the pulmonary epithelium results in short residence of the inhaled drug in the lung and potential for systemic adverse effects. Liposomes possess unique properties that make them suitable drug carriers in the treatment of cancer. Due to the enhanced permeability and retention effect resulting from their small size (100 nm), liposomes can pass through leaky tumour blood vessels and accumulate in the cancerous tissue to release the encapsulated drug at the target site [6]. The pulmonary system offers unique targeting options due to the large surface area of the lung, evasion of first-pass metabolism, and high permeability of the pulmonary epithelium. Unlike parenteral delivery, inhalation of controlled release systems may localise the drug action in the lung for prolonged periods; it is a non-invasive route and may reduce systemic adverse effects. It is established that liposomes can localise the action of inhaled drugs in the lung, improving the therapeutic outcome of the medication and reducing systemic adverse effects [7,8,9]. Dipalmitoylphosphatidylcholine liposomes given intratracheally in mice have been reported to be taken up by pulmonary cells, and more than 50% of the phospholipid administered remained in the lung after 24 h of administration [10]. Arikace® (a liposomal formulation of amikacin) is a liposome formulation designed for the treatment of lung infection in cystic fibrosis patients through inhalation [9]; this product is currently in phase III of development [9]. The success of this formulation is expected to motivate the pharmaceutical industry to invest further in the development of more inhalable liposome formulations in other fields such as cancer. In the view of the authors of this report, gene therapy in combination with liposomal antibiotics may constitute the future research directions in the treatment of cystic fibrosis [11,12,13,14,15].
In pulmonary drug delivery, there are three main barriers against the deposition of the aerosol in the deep lung (i.e. respiratory bronchioles and the alveolar region), and these are: (a) the anatomic barrier - the tracheobronchial tree structure of the pulmonary system is the main protection mechanism against the deposition of deleterious particles and pollutants; (b) the pathological barrier - the disease status may affect the viscoelastic properties of the mucous covering the respiratory tract epithelium, hence affecting the clearance of deposited material and absorption profile, and (c) the immunological barrier - alveolar macrophages are involved in the defence mechanism and hence particles depositing in the alveolar region might be engulfed and transported to the upper respiratory tract where the mucociliary escalator can eradicate the particles because there is always competition between clearance and absorption.
The above barriers are discussed further in this review; however, it is important to bear in mind that for inhaled drug particles to be regarded ‘therapeutically useful’ they should be in ‘fine particle fraction’ (FPF; i.e. capable of reaching the bronchioles and alveoli). For this to happen, the aerodynamic size of inhaled particles should be smaller than 5 or 6 µm, with particles smaller than 2 µm being the most suitable for deposition in the alveolar region [16]. Hence, the size of inhaled particles is the prime essential factor to consider for overcoming the anatomical barrier of the lung. Accordingly, liposome formulations should be aerosolised into particles that have a high FPF, and these liposomes should encapsulate a therapeutically feasible concentration of the drug which can then exhibit prolonged release from the liposome vesicles to the desired target within the lung [9]. In this review, the studies of inhalable anticancer liposome formulations for treating cancers in the lung and those located in distant organs are described and evaluated. Furthermore, the future of this field of drug delivery was appraised in light of the recent findings of Arikace®, a nebulisable liposome formulation which has passed phase II clinical trials [9,17].
Classification of Liposomes
Liposomes can be classified according to their size and morphology into multilamellar vesicles (MLVs; 0.1-20 µm), large unilamellar vesicles (LUVs; 0.1-1 µm) and small unilamellar vesicles (SUVs; 25-100 nm). MLVs are traditionally made using a thin-film hydration technique by dissolving the phospholipid ingredients with or without cholesterol in chloroform and methanol in a round-bottomed flask, followed by the removal of the organic solvents using a rotary evaporator, which results in the casting of a thin film of lipid on the inner walls of the flask. The addition of an aqueous phase above the phase transition temperature of the lipid mixture with shaking results in the formation of the MLVs [18,19]. By contrast, LUVs are produced by extrusion of the MLVs through polycarbonate membrane filters (100-nm pore diameter) [20]. Moreover, in some investigations oligolamellar vesicles (0.1-1 µm) have been described, which are liposomes made of two or three bilayers that are generated using a reverse phase evaporation method [21] or ethanol-based proliposome technology [22]. In most applications for the pulmonary delivery of liposomes, MLVs are prepared using the traditional thin-film hydration method, as described above. This could be followed by either sonication to gain SUVs, or membrane extrusion to obtain smaller MLVs or LUVs.
Safety of Liposome Formulations for Pulmonary Drug Delivery
Liposomes for pulmonary delivery have attracted a marked interest owing to the ability of liposome vesicles to entrap therapeutic molecules and, following inhalation, localise the drug effect in the pulmonary system for a prolonged duration. This had been reported to enhance the therapeutic benefit of the drug and reduce the potential of systemic adverse effects [7,8,9]. Liposomes are prepared using phospholipids with or without cholesterol; these components are highly similar to pulmonary surfactants in mammals [8,9]. Many studies have established the high biocompatibility and biodegradability of liposomes as drug carriers in inhaled formulations. Historically, liposomes in this field were suggested as surfactant replacement therapy in patients with respiratory distress syndrome. Recently, lung surfactants based on mixtures of phospholipids have been commercialised (e.g. Survanta®) for prophylaxis against respiratory distress syndrome in neonates [23].
Many early studies have demonstrated the safety of liposomes for pulmonary administration. For example, Myers et al. [24] have shown using animal models that inhalation of HSPC (hydrogenated soy phosphatidylcholine) liposomes caused no pathological effects on alveolar macrophages [24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Prolonged nebulisation (e.g. for 30 min) of SPC or HSPC liposome concentrations (up to 150 mg/ml) has been reported not to cause any physiological abnormalities in the lungs of sheep [25]. This dose, however, should be considered taking into account that dose-related toxicity can be different for different phospholipids. Many studies using human volunteers have established the safety of liposomes for inhalation [17,25,26,27]. Arikace® is a novel anti-pseudomonal liposome formulation that has shown safety and suitability for inhalation by human cystic fibrosis subjects in phase II clinical trials [17]. In the context of pulmonary drug delivery, safety and controlled release are ideal for liposomes incorporating cytotoxic agents such as anticancer drugs. This is because no toxic effects will be elicited by the carrier (i.e. liposomes) and the action is aimed to be confined to the lungs.
Many studies have also shown that drugs entrapped in liposomes are safe for pulmonary delivery since liposomes can control the mode of drug release, hence reducing the drug amount available to exert adverse effects [7,8,9]. The safety of drugs in liposome formulations given via inhalation is not confined to anticancer agents. Genes, antimicrobial agents and antidiabetic drugs are also safe when administered in liposome formulations. For instance, no adverse effects on the histology or function of the lungs were reported when liposome-pDNA complexes were delivered to the respiratory tract [26]. Steroids are commonly and widely used as anti-inflammatory agents in prophylaxis against asthma. Investigations have demonstrated that inhaled liposome-entrapped beclometasone was well tolerated when given in therapeutic doses to humans [27]. Furthermore, nebulisation of dilauroylphosphatidylcholine-ciclosporin A (CA) did not produce tracheal irritation or abnormality in human lung functions [28]. Studies have also shown that inhaled interleukin-2 (IL-2) liposomes were non-toxic to the pulmonary system of dogs [29] and humans [30,31]. In addition, phase II clinical trials have shown that the delivery of the anticancer 9-nitrocamptothecin (9-NC) via a medical nebuliser was safe in lung cancer patients [32]. Using experimental animals, liposomal insulin formulations delivered to the lung via nebulisation have been reported to be safe, and no histological changes in the respiratory tract were observed [33]. Furthermore, the nebulisation of all-trans-retinoic acid-liposomes resulted in no toxicity in the lungs of mice [34]. Also, antimicrobial agents in liposomes given via inhalation have been shown to be safe in humans [35]. For example, the inhalation of Abelcet (lipid complex amphotericin B) is safe and efficacious as prophylaxis against fungal infections that may occur after lung transplantation [36,37].
Devices Used for the Pulmonary Delivery of Liposomes
There are four types of inhalation device: pressurised metered-dose inhalers (pMDIs), dry powder inhalers (DPIs), soft mist inhalers (SMIs) and medical nebulisers. All these devices have been investigated for the delivery of liposomes.
Pressurised Metered-Dose Inhalers
pMDIs are robust canisters enclosing a drug dissolved or dispersed in liquefied propellants. Actuation of the device with co-ordinated inspiration results in the release of a precise dose [11,16]. The propellant rapidly evaporates owing to its high vapour pressure, leaving an accurate dose of the aerosolised drug particles to be inhaled by the patient. pMDI devices have traditionally been used in the treatment of asthma since the 1950s, but serious concerns have been raised about using them both clinically, because of the limited dose reaching the deep lung, and environmentally, because propellants like chlorofluorocarbons (CFCs) have been reported to be depleting the ozone layer [11,38]. An approach to delivering liposomes using pMDIs was reported by dissolving the phospholipid in CFC propellant in which drugs like salbutamol and cosolvents like ethanol are included. Actuation of the device in front of an impinger resulted in the deposition of a drug and lipid mixture and subsequent hydration and formation of liposomes within the impinger [38,39]. The ozone-depleting effect of CFCs necessitated the introduction of the safe alternative propellant family, namely hydrofluoroalkanes, in which phospholipids have very limited solubility. Thus, pMDI formulations were made by dispersing phospholipids in PEG-phospholipids followed by the delivery of the subsequent in situ formation of liposomes in the aqueous environment of the impinger [40]. Issues of complicated formulation, poor FPF of the aerosolised dose and stability are all major limitations in the development of liposomal formulations for delivery via pMDIs. The inclusion of cosolvents in phospholipid formulations may compromise deposition in FPF [39,40].
Dry Powder Inhalers
DPIs are breath actuated, thus the problem of co-ordinated inspiration with actuation, as in the case of pMDIs, is avoided. The delivery of liposomes using DPIs has been investigated using a range of drying technologies such as spray drying, freeze drying, spray freeze drying or air jet micronisation. For example, the spray drying of drugs in liposome formulations has been shown to be appropriate for manufacturing particles with a small aerodynamic size (i.e. high FPF), and it was presumed that the rehydration of liposomes may take place following the deposition of the powder in the aqueous environment of the lung [41,42,43]. An approach to gene therapy was introduced by spray drying a lactose solution incorporating lipid-polycation-pDNA, resulting in an enhanced transfection compared to formulation prior to spray drying [44]. More recently, proliposomes have been studied for delivery via DPIs. In this context, proliposomes are powdered phospholipid formulations that can generate liposomes when they come into contact with an aqueous environment [45,46]. Inhalable proliposome formulations have been made by spray drying an ethanolic solution of phospholipid [47,48]. The FPF was reported to reach up to 35% of the formulation and it is presumed that hydration of the powdered lipid particles would happen in the aqueous milieu of the lung following inhalation of the proliposome powder [48].
Soft Mist Inhalers
SMIs are hand-held propellant-free metered dose inhalation devices that generate slow-moving aqueous aerosols for deep-lung deposition [49,50,51]. An example is the AERx® (Aradigm Corp., Novo Nordisk, Hayward, Calif., USA), an SMI that is able to deliver liposome-DNA complexes in respirable aerosols [52,53]. Large doses are needed for the treatment of many diseases in the lung (e.g. cancers, infectious diseases, etc.); however, all the aforementioned devices (i.e. pMDIs, DPIs and SMIs) can deliver only small amounts of aerosol, and thus are more appropriate for treating diseases that require small doses of the therapeutic agent (e.g. asthma).
Medical Nebulisers
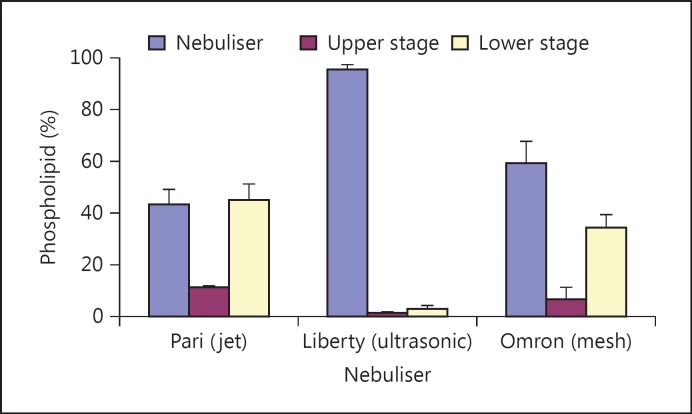
Compared to other inhalation devices, nebulisers can generate large volumes of ‘respirable’ aerosol, with no need to perform drying procedures, as in the case of DPIs, or involve propellants, as in case of pMDIs [16,50,54]. Nebulisers are the most commonly used inhalation devices for the delivery of liposomes [7,8,9,55,56]. There are three types of nebuliser: air jet, ultrasonic and vibrating mesh. Using many types of formulations, the air jet type is the best established nebuliser for the delivery of liposomes [7,8,32,57,58,59,60]. Whilst the ultrasonic nebuliser has generally been shown to be the least suitable for delivering liposomes [58,60,61], the vibrating mesh nebuliser demonstrated an excellent suitability of delivering vesicles in FPF [58,60,62,63], including large liposomes and liposome aggregates (median size around 50 µm; fig. 1) [58]. This suggests that the mesh nebuliser was capable of breaking the aggregates into discrete vesicles suitable for aerosolisation and subsequent inhalation.
Fig. 1.
Nebulisation of liposomes in front of a two-stage impinger using Pari LC Plus (air jet), Liberty (ultrasonic) and Omron MicroAir (vibrating mesh) nebulisers. The liposomes were generated in situ within nebulisers upon the hydration of particulate-based proliposomes made by coating sucrose particles with an equimolar ratio of SPC and cholesterol. The proportion of phospholipid deposited in the lower stage of the impinger was higher using the jet and mesh nebulisers compared to the ultrasonic device, which accumulated around 96% of the lipid into the nebuliser reservoir as part of the residual volume. Adapted from Elhissi and Taylor [58].
The air jet nebuliser employs compressed gas passing through a narrow ‘venturi’ nozzle at the bottom of the device to convert the liquid medication into ‘respirable’ aerosol droplets [16,50]. By contrast, the ultrasonic nebuliser utilises ultrasound waves generated via a piezoelectric crystal vibrating at a high frequency to convert the liquid into aerosols [50,64]. However, the vibrating mesh nebuliser operates using a different principle, by utilising a vibrational element that transmits the vibrations to a perforated plate with multiple micro-sized apertures to push the medication fluid through and generate slow-moving aerosol droplets with a narrow size distribution [50,63,65].
Stability of Liposomes during Nebulisation
A major issue in nebulising liposomes is the stability of vesicles during nebulisation. In principle, shearing provided by a nebuliser to convert liposome dispersions into fine aerosol droplets may result in vesicle fragmentation, with concomitant loss of the originally entrapped hydrophilic material. To minimise the instability of liposomes and loss of the entrapped hydrophilic drug, a range of strategies have been used. It was shown that vibrating mesh nebulisers customised with large mesh apertures are less disruptive to liposomes than jet nebulisers, especially when the vesicle size was optimised to be small (e.g. around 1 µm) prior to nebulisation [62]. Furthermore, the inclusion of cholesterol [9,62,66] or high-phase transition phospholipids [9,67] in liposome formulations has improved the stability of vesicles during nebulisation. Arikace® is a liposomal amikacin currently at an advanced development stage for the treatment of Pseudomonas aeruginosa biofilms in the lung [15]. This formulation is made using cholesterol-enriched dipalmitoylphosphatidylcholine (high-phase transition phospholipid) liposomes with a size of around 300 nm for inhalation using a Pari e-Flow vibrating mesh nebuliser [9], agreeing with the aforementioned investigations relating to the formulation composition, vesicle size and aerosolisation mechanism for the provision of stable liposomes with controlled release properties in the lung.
Unlike hydrophilic drugs, many studies have demonstrated that the issue of the physical stability of liposomes is less significant when the entrapped drug is hydrophobic [8,32,68,69,70,71]. Liposome-entrapped beclomethasone dipropionate showed a prolonged residence in the lung of human volunteers, although vesicles underwent a marked size reduction during jet nebulisation [8], thereby suggesting that no marked leakage of the drug had occurred. Pulmaquin® is a liposomal ciprofloxacin formulation that is currently in an advanced stage of development.
The success of PEGylated liposomes (e.g. Doxil®) at evading macrophages after parenteral injection leads to a prolonged circulation in the blood [72,73], which opened a means of investigating the effect of liposome surface coating on vesicle retention by the lung following pulmonary administration. It has been reported that budesonide encapsulated in PEGylated liposomes has a prolonged therapeutic effect in the lung of experimental animals, meaning it can be equivalent to the standard daily dose of the conventional budesonide formulation [74,75]. However, in a recent investigation it has been shown that the polymer coating of liposomes may reduce vesicle bilayer stability during nebulisation and promote drug leakage [60].
The potential of liposomes for pulmonary inhalation has been explored in various therapeutic applications, such as the treatment of asthma, lung injuries, lung infections, cystic fibrosis, diabetes and cancers, etc. In this review, focus is given to the potential of inhalable liposome formulations in the treatment of lung cancers, and we report our critical views on the current status and possible future research directions in this field.
Liposomal Drug Delivery Systems for the Treatment of Lung Cancers
More than 80% of lung cancers do not fully respond to chemotherapy [76]. One of the limitations is tumour resistance to cytotoxic agents currently used in the treatment of lung cancer (table 1) [77]. An approach to overcome the acquired resistance is to give chemotherapeutic drugs in high doses, thus causing toxicity to healthy organs. The limited clinical outcomes and high adverse effect profile of current lung cancer treatments made the development of alternative drug delivery systems of a high priority [78].
Table 1.
Treatment plan for lung cancer patients; adapted from NICE, 2011 [77]
| Lung cancer | Stage | Treatment | Type of chemotherapy | Regimen |
|---|---|---|---|---|
| NSCLC | Stage 1 | Lobectomy or pneumonectomy Radiotderapy1 |
– | – |
| Stage 2 | Lobectomy or pneumonectomy Radiotderapy1 Chemotderapy2 |
Cisplatin-based combination chemotderapy |
Cisplatin plus single tdird-generation drug (DOX, gemcitabine, PTX or vinorelbine) | |
| Stage 3 | Pneumonectomy Chemotderapy2, 3 Radiotderapy4 |
Platinum-based combination chemotderapy |
Platinum drug (carboplatin or cisplatin) plus single tdird-generation drug (DOX, gemcitabine, PTX or vinorelbine) | |
| Stage 4 | Chemotderapy | Platinum-based combination chemotderapy | Platinum drug (carboplatin or cisplatin) plus single tdird-generation drug (DOX, gemcitabine, PTX or vinorelbine) | |
| SCLC | Limited stage disease | Lobectomy followed by radiotderapy and chemotderapy | Cisplatin-based combination chemotderapy | Cisplatin plus single tdird-generation drug (DOX, gemcitabine, PTX or vinorelbine) |
| Extensive stage disease | Chemotderapy Radiotderapy to tde brain5 | Platinum-based combination chemotderapy | Platinum drug (carboplatin or cisplatin) plus single tdird-generation drug (DOX, gemcitabine, PTX or vinorelbine) | |
NSCLC = Non-small cell lung cancer; SCLC = small cell lung cancer.
Radiotherapy can be offered instead of surgery if any health problems exist.
Cisplatin-based combination chemotherapy can be offered if cancer is completely removed in order to lower the risk of the cancer coming back.
If any cancer cells are found in the lymph nodes during surgery, chemotherapy can be offered.
Radiotherapy or combined radiotherapy and chemotherapy can be offered instead of surgery if any health problems exist.
Cancer has usually spread to the brain and radiotherapy can be offered for people whose lung cancer shrinks with chemotherapy treatment.
Liposomal systems may demonstrate a promising approach for the delivery of anticancer medication via inhalation and could significantly improve the chemotherapy efficacy in lung cancer patients. Many studies have explored the potential of pulmonary delivery of anticancer drugs using liposomes; these studies are summarised in table 2.
Table 2.
In vivo studies of aerosolised liposomal formulations and their parameters in animals and humans for treating lung cancer
| Therapeutic agent | Delivery device | Type of liposome used | Subject | Study phase | Adverse effects | Dose and regiment | Monitoring of tumour | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cisplatin | PARI LC Star jet nebuliser | DPPC | Human | Phase I | Nausea, vomiting, dyspnoea, fatigue and hoarseness No DLT reached |
Escalation from 1.5 mg/m2 until DLT for 1 – 4 consecutive days every 1 – 3 weeks | Clinical examination, standard blood and urinary tests, PFT, CXR and CT of the thorax | 86 |
| 9-NC | AeroMist nebuliser | DLPC | Human | Phase I/II | Nausea, vomiting, cough, bronchial irritation, fatigue, anaemia, neutropenia DLT reversible grade 3 or 4 haematological toxicity, grade 2 neurotoxicity and grade 3 non-myelosuppressive toxicity |
0.25 – 1 mg/m2/day, 5 days per week for 8 weeks | Pulse oximeter readings daily, weekly CBC, monthly blood chemistry tests, and urine analysis. Tumour markers and a computer-assisted tomography scan of the chest were obtained at baseline and before each course. Simple spirometry, DLCO and lung volumes before and after first aerosol exposure | 32, 108 |
| IL-2 | Puritan Bennett twin jet nebuliser | DMPC | Human | Phase I | No significant adverse effects | 1.5, 3.0 and 6.0 × 106 IU of IL-2 three times a day for 8 – 84 days | Physical examination, CXR, CBC, electrolytes, BUN, creatinine, AST, ALP, bilirubin, LDH, DLCO and PFT | 31 |
| IL-2 | Puritan Bennet Twin Jet Nebuliser | DMPC | Animal (dogs) | – | Mild cough immediately after aerosolisation treatments | 1 × 106 IU of IL-2 twice daily for 15 days and then 1 × 106 IU of IL-2 three times daily for 15 days or 1 × 106 IU of IL-2 twice daily for 30 days | Physical examination, CBC, serum biochemistries (including concentration of albumin, total protein, ALT, ALP, AST, total bilirubin, BUN, creatinine, electrolytes) urinalyses, biopsy | 84 |
| 9-NC | AeroTech II nebuliser flowing to mice in a nose-only exposure chamber | DLPC | Animal (mice) | – | Skin lesions, weight loss | 0.1 – 1.0 mg/kg daily, 5 days per week for 36 – 49 days | Tumour size or volume measured by calipers | 90 |
| 9-NC and polyethyleneimine-p53 DNA (PEI-p53) | AeroTech II nebuliser | DLPC | Animal (mice) | – | Not recorded | 0.5 mg/1 ml twice a week for 2 weeks and 2 mg plasmid/10 ml once a week for 2 weeks | Lung weight | 76 |
| 9-NC | AeroTech II nebuliser | DLPC | Animal (mice) | – | Not recorded | 1- to 2-hour aerosol exposure 5 times weekly for 16 – 17 days Total deposited dose 2.3 – 3.7 mg/kg | Lung weight | 89 |
| 9-NC | AeroMist nebuliser | DLPC | Animal (mice) | – | Not recorded | 1- to 2-hour aerosol exposure 5 times weekly for 16 – 21 days Total deposited dose 2.3 – 3.7 mg/kg | Lung weight | 91 |
| Vitamin E analogue (a-TEA) and 9-NC | AeroTech II nebuliser | DLPC | Animal (mice) | – | Not recorded | Treatment course was 7 days per week for 3 weeks | Tumours were measured using calipers every other day | 93 |
| PTX | AeroMist nebuliser | DLPC | Animal (mice) | – | Not recorded | Total of 1.4 – 7.8 mg/kg of PTX were deposited in the lungs (dose regiment: 3 times per week for 3 weeks) | Lungs were resected and weighed | 102 |
| PTX together with cyclosporine A | AeroMist nebuliser | DLPC | Animal (mice) | – | Weight loss | Total of 1.4 – 7.8 mg/kg of PTX and 1.1 – 6.1 mg/kg of CA were deposited in the lungs (dose regimen: 3 times per week for 3 weeks) | Lungs were resected and weighed | 102 |
| PTX | AeroMist nebuliser | DLPC | Animal (mice) | – | Aggressiveness | Total of 5 mg/kg during a 30-min period were administered | Lungs were resected and weighed | 80, 104 |
| DOX | Collison nebuliser connected to four-port, nose-only exposure chambers | DLPC | Animal (mice) | – | Alterations of normal pulmonary parenchyma characterised by alveolar haemorrhage | 2.5 mg/kg for single inhalation every third day for 24 days | Tumour growth was monitored by bioluminescent IVIS (Xenogen) and ultrasound Vevo 2100 (VisualSonics) imaging systems | 104 |
| DOX combined with antisense oligonucleotides | Collison nebuliser connected to four-port, nose-only exposure chambers | DLPC | Animal (mice) | – | None | 2.5 mg/kg for single inhalation of DOX with 0.125 mg/kg antisense oligonucleotides every third day for 24 days | Tumour growth was monitored by bioluminescent IVIS (Xenogen) and ultrasound Vevo 2100 (VisualSonics) imaging systems | 104 |
| Camptothecin | Aerotech II nebuliser | DLPC | Animal (mice) | – | Not recorded | 81 µg/kg inhalation for 30 min only | Lungs were resected and weighted | 109 |
| DOX-liposomes | Collison jet nebuliser | EPC-Chol, DSPE-PEG | Animal (mice) | – | Very limited compared to free drug formulation | 14 µg/kg inhalation in combination with 2.5 mg/kg i.v. injection. This was compared with intravenous injection alone | Apoptosis induction in different organs (the lungs with tumour, liver, kidney, spleen, heart and brain) was measured using Cell Death Plus ELISA kit | 106 |
| DOX encapsulation in transferrin conjugated PEG liposomes | Intracorporeal nebulising catheter | Animal (athymic Rowett nude rat) | – | Not recorded | 0.2 – 0.4 mg/kg | Animal survival rate | 107 | |
5-FU = 5-Fluorouracil; CXR = chest X-ray; PFT = pulmonary function test; CBC = complete blood count; BUN = blood urea nitrogen; AST = aspartate transaminase; ALP = alka-line phosphatase; ALT = alanine transaminase; LDH = lactate dehydrogenase; DLCO = diffusing capacity of the lung for carbon monoxide; LNP = lipid-coated nanoparticles; DPPC = dipalmitoylphosphatidylcholine; DLPC = dilauroylphosphatidylcholine; DMPC = dimyristoylphosphatidylcholine; EPC-Chol = egg phosphatidylcholine with cholesterol; DSPE-PEG = pegylated distearoyl phosphatidylethanolamine.
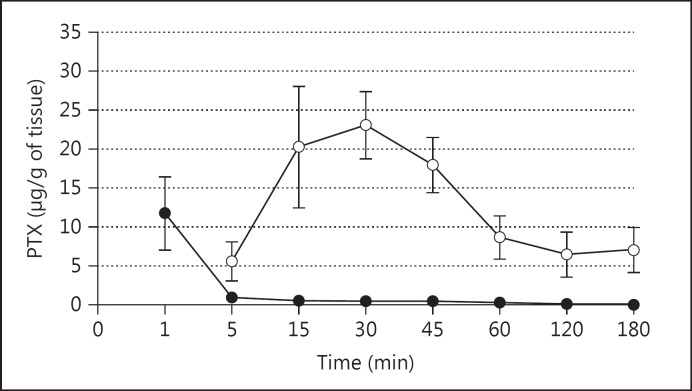
For anticancer therapy to be regarded as beneficial, the drug concentration reaching the tumour in the lung should be high in order to elicit a therapeutic effect. Many studies have indicated that drug concentrations at the tumour site have been found to be low after systemic chemotherapy [79,80,81,82]. This highlights the importance of local drug delivery at the cancer site by directly delivering the drug to the lung in case of pulmonary cancers. Koshkina et al. [80] evaluated the pharmacokinetics and therapeutic efficacy of liposomal PTX after intravenous and aerosol administration at comparative doses in mice [80]. They reported that PTX had a slower clearance and higher concentrations in the lungs following pulmonary delivery compared to administration via the intravenous route (fig. 2). The greater therapeutic effect shown with aerosol therapy can be attributed to the high concentrations of the drug reaching the tumour site owing to the greater pulmonary depositing of the anticancer molecules following aerosolisation [80].
Fig. 2.
Pulmonary pharmacokinetics of PTX administered by aerosol (⚪) or intravenously (⚫). Mice inhaled the drug for 30 min, starting at time 0 (total deposited dose 5 mg PTX/kg), or a bolus intravenous injection with 5 mg of PTX/kg was given into the tail vein at time 0 [80].
Garbuzenko et al. [81] investigated the effectiveness of the pulmonary liposomal delivery of doxorubicin (DOX) in mice after intratracheal administration compared to intravenous delivery. The intratracheal administration of DOX was much more efficient at limiting the growth of lung cancer and had limited side effects on healthy organs when compared to the systemic route. In terms of the comparison between aerosol and intratracheal delivery, Hitzman et al. [82] found that the release profiles of liposomal 5-fluorouracil reaching the lower respiratory tract of hamsters were almost identical when the same liposome formulation was used.
IL-2 has demonstrated an antitumour activity and has been investigated as a potential future therapy of cancer [83]. A few studies of IL-2 incorporation into liposomes have been performed, demonstrating promising results [31,84]. Liposomal inhalation of IL-2 resulted in a 5-fold increase in bronchoalveolar lavage leukocytes in the lungs compared to free IL-2 given in traditional solutions [84]. This suggests significant activation of the immune system in the lungs with the potential for improving disease control. Pulmonary administration of liposomes has also been proven to be effective and safe in dogs with primary lung carcinomas [29]. Phase I studies have shown that IL-2 liposomes are well tolerated and antitumour activity has been reported [31]. No published reports about IL-2 liposomes have progressed to phase II trials. Unlike many other diseases, cancer is treated with a combination of different strategies such as chemotherapy (systemic or oral), radiotherapy and surgical intervention. Thus, it is possible, in the view of the authors of this report, that the efficacy of IL-2 liposomes did not warrant further development when compared to the benefit gained by other established anticancer treatments. In a recent work, it has been reported that aerosolised IL-2 may offer prophylaxis against cancer recurrence in patients who had pulmonary melanoma metastasectomy [85]. Thus, taking into account these positive findings, in addition to the advances in inhalation device technology which maximises drug retention in liposomes during aerosol generation, it is anticipated that clinical trials of IL-2 may take new directions towards the use of liposome formulations of this drug in prophylaxis against the recurrence of cancer following surgical removal of the tumour.
Another chemotherapeutic agent, cisplatin, is one of the most commonly used drugs in current lung cancer treatments (table 1). However, its dose-limiting toxicity (DLT) in systemic administration is associated with nephrotoxicity, peripheral neuropathy and ototoxicity. Phase I studies using liposomes have shown that pharmacokinetics, safety and efficacy at the maximum tolerated dose of aerosolised liposomal cisplatin in primary or metastatic lung carcinoma were possible to achieve [86]. The side effects were mild, and no DLT was observed. The maximum delivered dose and results showed the stability of the disease in 12 out of 16 patients. Treatment was considered to be feasible and safe compared to intravenous delivery of the drug. However, the main limitation of the study was the low deposition of the drug in the target area of the respiratory system. This might be attributed to the fact that aerosol delivery of cisplatin was not performed using a 5% concentration of CO2 in the nebuliser as this was reported in another study to increase pulmonary deposition [87]. Liposomal cisplatin (SLIT Cisplatin) was developed by Transave Inc. (they also developed the liposomal amikacin formulation known as Arikace). To the best knowledge of the authors of this report, when Transave Inc. was acquired by Insmed in 2010, Arikace was taken for further advanced stages of development while SLIT Cisplatin was ‘handed over’ to Eleison Pharmaceuticals LLC for further clinical investigations.
Physiochemical properties of 9-NC and its analogue camptothecin indicate poor bioavailability, poor solubility and DLT, which all influence the formulation design [88]. These limiting factors may affect the clinical use of 9-NC via parenteral administration. Therefore, the use of aerosolised liposomal 9-NC formulations has been explored in a range of studies using animal models and clinical trials, demonstrating improved efficacy with reduced toxicity. On the basis of previous studies in animal models with lung tumour xenografts, a number of publications have reported aerosolised liposomal 9-NC to be clinically effective in reducing tumour size, with decreased toxicity profiles [89,90,91]. This offers beneficial effects of local aerosol administration with concentrations similar to parenteral dosing while decreasing the side effect profile compared to the systemic administration of 9-NC. Phase I clinical trials of aerosolised liposomal 9-NC was performed in 6 patients with primary lung cancer, where 1 patient had partial remission and 3 patients had stabilisation of their tumours [32]. A starting dose of 13.3 µg/kg/day was well tolerated by all patients and was recommended for phase II trials. The study also concluded rapid systemic absorption and a low side effect profile. DLT was reported to be relatively mild such as pharyngitis with other side effects, including nausea, vomiting, fatigue and cough. Additionally, no haematological toxicity was reported in contrast to systemic 9-NC delivery according to the studies conducted by Tedesco et al. [92]. Liposomal aerosols caused 9-NC to deposit in high concentrations which were found to be 4-10 times greater in bronchoalveolar lavage fluid than in plasma, suggesting a localised drug dose in the lung following pulmonary administration.
Overall, the antitumour effect of liposomal 9-NC has demonstrated promising results. Additionally, other studies were performed by incorporating 9-NC with the vitamin E analogue in liposome formulations to inhibit lung cancer growth [93]. This combination has increased drug bioavailability and inhibited the multidrug-resistant transporter P-glycoprotein [93]. Vitamin E co-administration reduced metastases in the sites that were not directly targeted by the aerosol. Thus, drug combinations in inhaled liposome formulations can significantly increase tumour cell death by apoptosis, and may elicit fewer side effects compared to single drug treatments. Studies have hypothesised that, in the context of the pulmonary inhalation of liposomes, lower doses of agents given in combination therapy had fewer side effects, which might be attributed to different mechanisms of actions for different anticancer drugs [76,93]. Phase I studies of 9-NC liposomes demonstrated promising findings [92]; however, further development depends on an interplay of factors, including formulation toxicity compared to other established treatment strategies.
Due to the unique anticancer mechanism of action of PTX, liposomal formulation has been studied in mice. One study proved that bioavailability (assessed by the area under the curve) in the aerosol group was 26-fold higher than that using the intravenous route while PTX clearance was slower [80]. The increased deposition of the inhaled drug might be attributed to the addition of 5% CO2 as a composition of the compressed gas used for jet nebulisation. However, studies have reported increased aggressiveness in mice behaviour as a result of therapy which can be explained by PTX-induced neurological toxicity [94]. Although high drug concentrations in the lung were reached, complete tumour growth arrest was not achieved in animal models, possibly suggesting that treatment can be improved with combination therapy or designing aerosol-generating mechanisms that are more efficient in the future. Inhalation device customisation and formulation property manipulation to generate droplets with optimum size and aerodynamic properties can be the next step to deliver targeted aerosols to certain regions of the lung [95,96]. The pathological status of the lung can be an important barrier against the proper deposition of liposomal aerosols in the human respiratory tract and may promote dose clearance. For example, it has been reported that in severely asthmatic patients, liposomes may have a limited deposition in the peripheral airways. Thus, inhalation of long-acting B2 agonists (e.g. formeterol) may enhance the deposition of subsequently inhaled liposomes [97,98]. Thus, deposition and clearance profile between lung cancer patients and healthy humans might be different and it is worth to investigate whether the cancerous tissue in the lung may constitute a barrier towards the effective deposition of the inhaled anticancer liposomes. Furthermore, during storage, many hydrophobic drugs (e.g. steroids) tend to leak and form crystals on the surface of the liposome, due to their poor steric fit into the bilayers [99,100]. We have found this to be the case also for PTX [unpubl. data]. Stress induced by freeze drying may promote the leakage of PTX from liposomes [101]. Thus, further studies should evaluate whether nebulisation-induced stress on PTX liposomes was responsible for leakage of the drug and subsequent adverse effects, and limited the therapeutic benefit of PTX following pulmonary delivery to animals.
Koshkina et al. [102] proposed the potential of co-administered PTX and CA in one liposomal formulation. CA has a high affinity for P-glycoprotein and can prevent the active elimination of other drugs from tumour cells [103]. Similar effects were seen earlier in studies with vitamin E analogues. The results proved that CA inhalation before PTX administration and continuous inhalation during PTX treatment significantly reduced the number and size of tumour lesions when compared to groups receiving CA and PTX simultaneously or PTX alone. Garbuzenko et al. [104] evaluated the liposomal incorporation of DOX and antisense oligonucleotides targeting MRP1 and BCL2. MRP1 and BCL2 protein expression is related to the cellular resistance of tumour cells [105]. That study concluded that combination therapy induced apoptotic effect and significantly suppressed the growth of lung cancer when compared to individual components applied separately. This can be attributed to the downregulation of proteins caused by antisense oligonucleotides. Recently, it has been concluded that lipid-based nanocarriers such as liposomes demonstrate a higher accumulation and longer retention time than non-lipid-based nanocarriers following inhalation using DOX as a model anticancer drug [106]. Thus, liposomes given via inhalation in combination with intravenous doses may offer an enhanced therapeutic outcome and reduced toxicity to non-cancerous cells, as demonstrated using animal models [106]. Interestingly, it has been reported that even empty liposomes may enhance the survival of animals following nebulisation therapy, which was attributed to a secondary cytotoxicity through the stimulation of lung macrophages [107].
Conclusion
Liposomal drug delivery systems have been shown/reported to improve the administration of chemotherapeutic agents in the treatment of lung cancer and prevention of metastases when compared to parenteral administration. The major side effect profile and toxicity of cytotoxic agents were reduced by delivering therapeutic concentrations locally to the lung. Although further studies are required to improve the efficacy of liposomal aerosol chemotherapeutics, the unique targeting options to the lungs clearly shows potential for the combination of active ingredients over the single drug treatment approach. In the opinion of the authors of this report the success of liposome inhalation for the treatment of cancer will be dependent on the successful design of aerosol delivery devices and targeted formulations that can reach the affected lung areas efficiently to deposit therapeutic drug doses with minimal exposure of healthy tissues to the anticancer agent. Furthermore, the successful development of inhalable anticancer formulations using liposome carriers will depend on the balance of benefit and risk compared to the other established treatment strategies. The formulation stability, nebulisation mechanism, targetability of aerosol to the cancer cells and minimised deposition in the oropharyngeal region are all factors that should be considered in the development of inhalable anticancer liposomes.
Disclosure Statement
The authors declare that no conflicts of interest exist.
References
- 1.American Cancer Society Cancer facts and figures 2013. Atlanta. http://www.cancer.org/acs/groups/content/@epidemiologysurveillance/documents/document/acspc-036845.pdf (accessed May 31, 2015).
- 2.Sihoe AD, Yim AP. Lung cancer staging. J Surg Res. 2004;117:92–106. doi: 10.1016/j.jss.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Senan S, Paul MA, Lagerwaard FJ. Treatment of early-stage lung cancer detected by screening: surgery or stereotactic ablative radiotherapy? Lancet Oncol. 2013;14:270–274. doi: 10.1016/S1470-2045(12)70592-2. [DOI] [PubMed] [Google Scholar]
- 4.Hu L, Liang G, Yuliang W, et al. Assessing the effectiveness and safety of liposomal paclitaxel in combination with cisplatin as first-line chemotherapy for patients with advanced NSCLC with regional lymph-node metastasis: study protocol for a randomized controlled trial (PLC-GC trial) Trials. 2013;14:45. doi: 10.1186/1745-6215-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadam AN, Najlah M, Wan KW, et al. Stability of parenteral nanoemulsions loaded with paclitaxel: the influence of lipid phase composition, drug concentration and storage temperature. Pharm Dev Technol. 2014;19:999–1004. doi: 10.3109/10837450.2013.840845. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin V. Passive and active drug targeting: drug delivery to tumors as an example. In: Schäfer-Korting M, editor. Drug Delivery. Vol. 197. Berlin: Springer; 2010. pp. 3–53. [DOI] [PubMed] [Google Scholar]
- 7.Taylor KM, Taylor G, Kellaway IW, et al. The influence of liposomal encapsulation on sodium cromoglycate pharmacokinetics in man. Pharm Res. 1989;6:633–636. doi: 10.1023/a:1015917918130. [DOI] [PubMed] [Google Scholar]
- 8.Saari M, Vidgren MT, Koskinen MO, et al. Pulmonary distribution and clearance of two beclomethasone liposome formulations in healthy volunteers. Int J Pharm. 1999;181:1–9. doi: 10.1016/s0378-5173(98)00398-6. [DOI] [PubMed] [Google Scholar]
- 9.Clancy JP, Dupont L, Konstan MW, et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax. 2013;68:818–825. doi: 10.1136/thoraxjnl-2012-202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto Y, Adachi Y. Pulmonary uptake of liposomal phosphatidylcholine upon intratracheal administration to rats. Chem Pharm Bull (Tokyo) 1982;30:2248–2251. doi: 10.1248/cpb.30.2248. [DOI] [PubMed] [Google Scholar]
- 11.Elhissi A, Ul Hassan I, Papapanou A, et al. Cationic liposomes as model nonviral vectors for pulmonary delivery of DNA. In: Aleš I, Chandrashekhar VK, editors. Advances in Planar Lipid Bilayers and Liposomes. Vol. 19. Cambridge: Academic Press; 2014. pp. 53–66. [Google Scholar]
- 12.Jennings MT, Riekert KA, Boyle MP. Update on key emerging challenges in cystic fibrosis. Med Princ Pract. 2014;23:393–402. doi: 10.1159/000357646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannon A, Zhang SD, Schock BC, et al. Cystic fibrosis from laboratory to bedside: the role of a20 in NF-κB-mediated inflammation. Med Princ Pract. 2015;24:301–310. doi: 10.1159/000381423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alton EWFW, Boyd AC, Porteous DJ, et al. A phase I/IIa safety and efficacy study of nebulized liposome-mediated gene therapy for cystic fibrosis supports a multidose trial. Am J Resp Crit Care Med. 2015;192:1389–1392. doi: 10.1164/rccm.201506-1193LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehsan Z, Clancy JP. Management of Pseudomonas aeruginosa infection in cystic fibrosis patients using inhaled antibiotics with a focus on nebulized liposomal amikacin. Future Microbiol. 2015;10:1901–1912. doi: 10.2217/fmb.15.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax. 1997;52((suppl 2)):S31–S44. doi: 10.1136/thx.52.2008.s31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elhissi AMA, Dennison SR, Ahmed W, et al. New delivery systems - liposomes for pulmonary delivery of antibacterial drugs. In: Phoenix DA, Frederick H, Dennison SR, editors. Novel Antimicrobial Agents and Strategies. Weinheim: Wiley-VCH; 2014. pp. 387–406. [Google Scholar]
- 18.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 19.Kirby CJ, Gregoriadis G. Liposomes. In: Mathiowitz E, editor. Encyclopaedia of Controlled Drug Delivery. New York: Wiley; 1999. pp. 461–492. [Google Scholar]
- 20.Hope MJ, Bally MB, Webb G, et al. Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim Biophys Acta. 1985;812:55–65. doi: 10.1016/0005-2736(85)90521-8. [DOI] [PubMed] [Google Scholar]
- 21.Szoka F, Jr, Papahadjopoulos D. Procedure for the preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA. 1978;75:4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrett S, Golding M, Williams WP. A simple method for the preparation of liposomes for pharmaceutical applications: characterization of the liposomes. J Pharm Pharmacol. 1991;43:154–161. doi: 10.1111/j.2042-7158.1991.tb06657.x. [DOI] [PubMed] [Google Scholar]
- 23.Paul S, Rao S, Kohan R, et al. Poractant alfa versus beractant for respiratory distress syndrome in preterm infants: a retrospective cohort study. J Paediatr Child Health. 2013;49:839–844. doi: 10.1111/jpc.12300. [DOI] [PubMed] [Google Scholar]
- 24.Myers MA, Thomas DA, Straub L, et al. Pulmonary effects of chronic exposure to liposome aerosols in mice. Exp Lung Res. 1993;19:1–19. doi: 10.3109/01902149309071077. [DOI] [PubMed] [Google Scholar]
- 25.Schreier H, McNicol KJ, Ausborn M, et al. Pulmonary delivery of amikacin liposomes and acute liposome toxicity in the sheep. Int J Pharm. 1992;87:183–193. [Google Scholar]
- 26.Canonico AE, Plitman JD, Conary JT, et al. No lung toxicity after repeated aerosol or intravenous delivery of plasmid-cationic liposome complexes. J Appl Physiol (1985) 1994;77:415–419. doi: 10.1152/jappl.1994.77.1.415. [DOI] [PubMed] [Google Scholar]
- 27.Waldrep JC, Scherer PW, Hess GD, et al. Nebulized glucocortecoids in liposomes: aerosol characteristics and human dose estimates. J Aerosol Med. 1994;7:135–145. [Google Scholar]
- 28.Gilbert BE, Knight C, Alvarez FG, et al. Tolerance of volunteers to cyclosporine a-dilauroylphosphatidylcholine liposome aerosol. Am J Respir Crit Care Med. 1997;156:1789–1793. doi: 10.1164/ajrccm.156.6.9702101. [DOI] [PubMed] [Google Scholar]
- 29.Khanna C, Anderson PM, Hasz DE, et al. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer. 1997;79:1409–1421. doi: 10.1002/(sici)1097-0142(19970401)79:7<1409::aid-cncr19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Ten RM, Anderson PM, Zein NN, et al. Interleukin-2 liposomes for primary immune deficiency using the aerosol route. Int Immunopharmacol. 2002;2:333–344. doi: 10.1016/s1567-5769(01)00143-6. [DOI] [PubMed] [Google Scholar]
- 31.Skubitz KM, Anderson PM. Inhalational interleukin-2 liposomes for pulmonary metastases: a phase I clinical trial. Anticancer Drug. 2000;11:555–563. doi: 10.1097/00001813-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Verschraegen CF, Gilbert BE, Loyer E, et al. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 2004;10:2319–2326. doi: 10.1158/1078-0432.ccr-0929-3. [DOI] [PubMed] [Google Scholar]
- 33.Jendle J, Karlberg BE, Persliden J, et al. Delivery and retention of an insulin aerosol produced by a new jet nebulizer. J Aerosol Med. 1995;8:243–254. doi: 10.1089/jam.1995.8.243. [DOI] [PubMed] [Google Scholar]
- 34.Parthasarathy R, Gilbert B, Mehta K. Aerosol delivery of liposomal all-trans-retinoic acid to the lungs. Cancer Chemother Pharmacol. 1999;43:277–283. doi: 10.1007/s002800050895. [DOI] [PubMed] [Google Scholar]
- 35.Knight V, Gilbert B. Antiviral therapy with small particle aerosols. Eur J Clin Microbiol Infect Dis. 1988;7:721–731. doi: 10.1007/BF01975037. [DOI] [PubMed] [Google Scholar]
- 36.Corcoran TE, Venkataramanan R, Mihelc KM, et al. Aerosol deposition of lipid complex amphotericin-B (Abelcet) in lung transplant recipients. Am J Transplant. 2006;6:2765–2773. doi: 10.1111/j.1600-6143.2006.01529.x. [DOI] [PubMed] [Google Scholar]
- 37.Monforte V, Lopez-Sanchez A, Zurbano F, et al. Prophylaxis with nebulized liposomal amphotericin B for Aspergillus infection in lung transplant patients does not cause changes in the lipid content of pulmonary surfactant. J Heart Lung Transplant. 2013;32:313–319. doi: 10.1016/j.healun.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Farr SJ, Kellaway IW, Carman-Meakin B. Assessing the potential of aerosol-generated liposomes from pressurised pack formulations. J Control Release. 1987;5:119–127. [Google Scholar]
- 39.Vyas SP, Sakthivel T. Pressurized pack-based liposomes for pulmonary targeting of isoprenaline - development and characterization. J Microencapsul. 1994;11:373–380. doi: 10.3109/02652049409034255. [DOI] [PubMed] [Google Scholar]
- 40.Alouache AI, Kellaway IW, Taylor KMG, et al. Stability kinetics of HFA suspensions prepared with a fluoroalcohol and PEG-phospholipids. J Aerosol Med. 2006;20:373. [Google Scholar]
- 41.Radhakrishnan R, Mihalko PJ, Abra RM.Method and apparatus for administering dehydrated liposomes by inhalation. Google Patents US 4895719 A. 1990.
- 42.Lo Yl, Tsai JC, Kuo JH. Liposomes and disaccharides as carriers in spray-dried powder formulations of superoxide dismutase. J Control Release. 2004;94:259–272. doi: 10.1016/j.jconrel.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Ourique AF, Chaves Pdos S, Souto GD, et al. Redispersible liposomal-N-acetylcysteine powder for pulmonary administration: development, in vitro characterization and antioxidant activity. Eur J Pharm Sci. 2014;65:174–182. doi: 10.1016/j.ejps.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Seville PC, Kellaway IW, Birchall JC. Preparation of dry powder dispersions for non-viral gene delivery by freeze-drying and spray-drying. J Gene Med. 2002;4:428–437. doi: 10.1002/jgm.282. [DOI] [PubMed] [Google Scholar]
- 45.Payne NI, Browning I, Hynes CA. Characterization of proliposomes. J Pharm Sci. 1986;75:330–333. doi: 10.1002/jps.2600750403. [DOI] [PubMed] [Google Scholar]
- 46.Payne NI, Timmins P, Ambrose CV, et al. Proliposomes: a novel solution to an old problem. J Pharm Sci. 1986;75:325–329. doi: 10.1002/jps.2600750402. [DOI] [PubMed] [Google Scholar]
- 47.Alves GP, Santana MHA. Phospholipid dry powders produced by spray drying processing: structural, thermodynamic and physical properties. Powder Technol. 2004;145:139–148. [Google Scholar]
- 48.Rojanarat W, Changsan N, Tawithong E, et al. Isoniazid proliposome powders for inhalation-preparation, characterization and cell culture studies. Int J Mol Sci. 2011;12:4414–4434. doi: 10.3390/ijms12074414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hindle M. Soft Mist Inhalers: A Review of Current Technology: Drug Delivery Companies Report. Oxford: 2004. pp. 31–34. Autumn/Winter. [Google Scholar]
- 50.Elhissi A, Ahmed W. Advances in design and technology of devices manufactured for drug delivery applications. In: Jackson M, Davim JP, editors. Medical Device Manufacturing. New York: Nova; 2011. [Google Scholar]
- 51.Tamura G. Comparison of the aerosol velocity of Respimat® soft mist inhaler and seven pressurized metered dose inhalers. Allergol Int. 2015;64:390–392. doi: 10.1016/j.alit.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Deshpande D, Blanchard J, Srinivasan S, et al. Aerosolization of lipoplexes using AERx Pulmonary Delivery System. AAPS PharmSci. 2002;4:1–10. doi: 10.1208/ps040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mudumba S, Deshpande D, Sanders R, et al. Non-viral delivery of Cox-1 gene by the AERx® Pulmonary Delivery System. Respir Drug Delivery IX. 2004:353–355. [Google Scholar]
- 54.Ari A, Atalay OT, Harwood R, et al. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010;55:845–851. [PubMed] [Google Scholar]
- 55.Ehsan Z, Wetzel JD, Clancy JP. Nebulized liposomal amikacin for the treatment of pseudomonas aeruginosa infection in cystic fibrosis patients. Expert Opin Investig Drugs. 2014;23:743–749. doi: 10.1517/13543784.2014.895322. [DOI] [PubMed] [Google Scholar]
- 56.Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Bridges PA, Taylor KMG. Nebulisers for the generation of liposomal aerosols. Int J Pharm. 1998;173:117–125. [Google Scholar]
- 58.Elhissi AMA, Taylor KMG. Delivery of liposomes generated from proliposomes using air-jet, ultrasonic, and vibrating-mesh nebulisers. J Drug Deliv Sci Tech. 2005;15:261–265. [Google Scholar]
- 59.Elhissi AM, Ahmed W, Taylor KM. Laser diffraction and electron microscopy studies on inhalable liposomes generated from particulate-based proliposomes within a medical nebulizer. J Nanosci Nanotechnol. 2012;12:6693–6699. doi: 10.1166/jnn.2012.4566. [DOI] [PubMed] [Google Scholar]
- 60.Lehofer B, Bloder F, Jain PP, et al. Impact of atomization technique on the stability and transport efficiency of nebulized liposomes harboring different surface characteristics. Eur J Pharm Biopharm. 2014;88:1076–1085. doi: 10.1016/j.ejpb.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Leung KKM, Bridges PA, Taylor KMG. The stability of liposomes to ultrasonic nebulisation. Int J Pharm. 1996;145:95–102. [Google Scholar]
- 62.Elhissi AM, Faizi M, Naji WF, et al. Physical stability and aerosol properties of liposomes delivered using an air-jet nebulizer and a novel micropump device with large mesh apertures. Int J Pharm. 2007;334:62–70. doi: 10.1016/j.ijpharm.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 63.Cipolla D, Wu H, Gonda I, et al. Aerosol performance and long-term stability of surfactant-associated liposomal ciprofloxacin formulations with modified encapsulation and release properties. AAPS PharmSciTech. 2014;15:1218–1227. doi: 10.1208/s12249-014-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor KMG, McCallion ONM. Ultrasonic nebulisers for pulmonary drug delivery. Int J Pharm. 1997;153:93–104. [Google Scholar]
- 65.Dhand R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir Care. 2002;47:1406–1416. discussion 1416-1408. [PubMed] [Google Scholar]
- 66.Taylor KMG, Taylor G, Kellaway IW, et al. The stability of liposomes to nebulisation. Int J Pharm. 1990;58:57–61. [Google Scholar]
- 67.Niven RW, Schreier H. Nebulization of liposomes. I. Effects of lipid composition. Pharm Res. 1990;7:1127–1133. doi: 10.1023/a:1015924124180. [DOI] [PubMed] [Google Scholar]
- 68.Desai TR, Hancock RE, Finlay WH. A facile method of delivery of liposomes by nebulization. J Control Release. 2002;84:69–78. doi: 10.1016/s0168-3659(02)00264-x. [DOI] [PubMed] [Google Scholar]
- 69.Bruinenberg P, Blanchard JD, Cipolla DC, et al. Inhaled liposomal ciprofloxacin: once a day management of respiratory infections. Respir Drug Delivery IX. 2010;1:73–82. [Google Scholar]
- 70.Elhissi A, Gill H, Ahmed W, et al. Vibrating-mesh nebulization of liposomes generated using an ethanol-based proliposome technology. J Liposome Res. 2011;21:173–180. doi: 10.3109/08982104.2010.505574. [DOI] [PubMed] [Google Scholar]
- 71.Kamalaporn H, Leung K, Nagel M, et al. Aerosolized liposomal amphotericin B: a potential prophylaxis of invasive pulmonary aspergillosis in immunocompromised patients. Pediatr Pulmonol. 2014;49:574–580. doi: 10.1002/ppul.22856. [DOI] [PubMed] [Google Scholar]
- 72.Udhrain A, Skubitz KM, Northfelt DW. Pegylated liposomal doxorubicin in the treatment of AIDS-related Kaposi's sarcoma. Int J Nanomedicine. 2007;2:345–352. [PMC free article] [PubMed] [Google Scholar]
- 73.Green AE, Rose PG. Pegylated liposomal doxorubicin in ovarian cancer. Int J Nanomedicine. 2006;1:229–239. [PMC free article] [PubMed] [Google Scholar]
- 74.Konduri KS, Nandedkar S, Rickaby DA, et al. The use of sterically stabilized liposomes to treat asthma. Methods Enzymol. 2005;391:413–427. doi: 10.1016/S0076-6879(05)91023-9. [DOI] [PubMed] [Google Scholar]
- 75.Konduri KS, Nandedkar S, Duzgunes N, et al. Efficacy of liposomal budesonide in experimental asthma. J Allergy Clin Immunol. 2003;111:321–327. doi: 10.1067/mai.2003.104. [DOI] [PubMed] [Google Scholar]
- 76.Gautam A, Waldrep JC, Densmore CL, et al. Growth inhibition of established B16-F10 lung metastases by sequential aerosol delivery of P53 gene and 9-nitrocamptothecin. Gene Ther. 2002;9:353–357. doi: 10.1038/sj.gt.3301662. [DOI] [PubMed] [Google Scholar]
- 77.National Institute for Health and Clinical Excellence (NICE) Lung cancer: diagnosis and management. NICE guidelines CG121. 2011. http://www.nice.org.uk/nicemedia/live/13465/54202/54202.pdf (accessed May 31, 2015).
- 78.Zhou J, Zhao WY, Ma X, et al. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials. 2013;34:3626–3638. doi: 10.1016/j.biomaterials.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 79.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 80.Koshkina NV, Waldrep JC, Roberts LE, et al. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin Cancer Res. 2001;7:3258–3262. [PubMed] [Google Scholar]
- 81.Garbuzenko OB, Saad M, Betigeri S, et al. Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug. Pharm Res. 2009;26:382–394. doi: 10.1007/s11095-008-9755-4. [DOI] [PubMed] [Google Scholar]
- 82.Hitzman CJ, Wattenberg LW, Wiedmann TS. Pharmacokinetics of 5-fluorouracil in the hamster following inhalation delivery of lipid-coated nanoparticles. J Pharm Sci. 2006;95:1196–1211. doi: 10.1002/jps.20607. [DOI] [PubMed] [Google Scholar]
- 83.Antony GK, Dudek AZ. Interleukin 2 in cancer therapy. Curr Med Chem. 2010;17:3297–3302. doi: 10.2174/092986710793176410. [DOI] [PubMed] [Google Scholar]
- 84.Khanna C, Hasz DE, Klausner JS, et al. Aerosol delivery of interleukin 2 liposomes is nontoxic and biologically effective: canine studies. Clin Cancer Res. 1996;2:721–734. [PubMed] [Google Scholar]
- 85.Posch C, Weihsengruber F, Bartsch K, et al. Low-dose inhalation of interleukin-2 bio-chemotherapy for the treatment of pulmonary metastases in melanoma patients. Br J Cancer. 2014;110:1427–1432. doi: 10.1038/bjc.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wittgen BP, Kunst PW, van der Born K, et al. Phase I study of aerosolized slit cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 2007;13:2414–2421. doi: 10.1158/1078-0432.CCR-06-1480. [DOI] [PubMed] [Google Scholar]
- 87.Koshkina NV, Knight V, Gilbert BE, et al. Improved respiratory delivery of the anticancer drugs, camptothecin and paclitaxel, with 5% CO2-enriched air: pharmacokinetic studies. Cancer Chemother Pharmacol. 2001;47:451–456. doi: 10.1007/s002800000230. [DOI] [PubMed] [Google Scholar]
- 88.Chen J, Ping QN, Guo JX, et al. Effect of phospholipid composition on characterization of liposomes containing 9-nitrocamptothecin. Drug Dev Ind Pharm. 2006;32:719–726. doi: 10.1080/03639040500529077. [DOI] [PubMed] [Google Scholar]
- 89.Knight V, Kleinerman ES, Waldrep JC, et al. 9-nitrocamptothecin liposome aerosol treatment of human cancer subcutaneous xenografts and pulmonary cancer metastases in mice. Ann NY Acad Sci. 2000;922:151–163. doi: 10.1111/j.1749-6632.2000.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 90.Knight V, Koshkina NV, Waldrep JC, et al. Anticancer effect of 9-nitrocamptothecin liposome aerosol on human cancer xenografts in nude mice. Cancer Chemother Pharmacol. 1999;44:177–186. doi: 10.1007/s002800050965. [DOI] [PubMed] [Google Scholar]
- 91.Koshkina NV, Kleinerman ES, Waidrep C, et al. 9-nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice. Clin Cancer Res. 2000;6:2876–2880. [PubMed] [Google Scholar]
- 92.Tedesco KL, Berlin J, Rothenberg M, et al. A phase I study of concurrent 9-nitro-20(S)-camptothecin (9NC/Orathecin) and radiation therapy in the treatment of locally advanced adenocarcinoma of the pancreas. Radiother Oncol. 2005;76:54–58. doi: 10.1016/j.radonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Lawson KA, Anderson K, Snyder RM, et al. Novel vitamin E analogue and 9-nitro-camptothecin administered as liposome aerosols decrease syngeneic mouse mammary tumor burden and inhibit metastasis. Cancer Chemother Pharmacol. 2004;54:421–431. doi: 10.1007/s00280-004-0817-y. [DOI] [PubMed] [Google Scholar]
- 94.Rich TA, Winter K, Safran H, et al. Weekly paclitaxel, gemcitabine, and external irradiation followed by randomized farnesyl transferase inhibitor R115777 for locally advanced pancreatic cancer. Onco Targets Ther. 2012;5:161–170. doi: 10.2147/OTT.S33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghazanfari T, Elhissi AM, Ding Z, et al. The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int J Pharm. 2007;339:103–111. doi: 10.1016/j.ijpharm.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 96.Najlah M, Vali A, Taylor M, et al. A study of the effects of sodium halides on the performance of air-jet and vibrating-mesh nebulizers. Int J Pharm. 2013;456:520–527. doi: 10.1016/j.ijpharm.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 97.Saari SM, Vidgren MT, Herrala J, et al. Possibilities of formoterol to enhance the peripheral lung deposition of the inhaled liposome corticosteroids. Respir Med. 2002;96:999–1005. doi: 10.1053/rmed.2002.1393. [DOI] [PubMed] [Google Scholar]
- 98.Saari SM, Vidgren MT, Koskinen MO, et al. Regional lung deposition and clearance of 99mTc-labeled beclomethasone-DLPC liposomes in mild and severe asthma. Chest. 1998;113:1573–1579. doi: 10.1378/chest.113.6.1573. [DOI] [PubMed] [Google Scholar]
- 99.Batavia R, Taylor KM, Craig DQ, et al. The measurement of beclomethasone dipropionate entrapment in liposomes: a comparison of a microscope and an HPLC method. Int J Pharm. 2001;212:109–119. doi: 10.1016/s0378-5173(00)00584-6. [DOI] [PubMed] [Google Scholar]
- 100.Khan I, Yousaf S, Subramanian S, et al. Proliposome powders prepared using a slurry method for the generation of beclometasone dipropionate liposomes. Int J Pharm. 2015;496:342–350. doi: 10.1016/j.ijpharm.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 101.Guan P, Lu Y, Qi J, et al. Solidification of liposomes by freeze-drying: the importance of incorporating gelatin as interior support on enhanced physical stability. Int J Pharm. 2015;478:655–664. doi: 10.1016/j.ijpharm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 102.Koshkina NV, Golunski E, Roberts LE, et al. Cyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in mice. J Aerosol Med. 2004;17:7–14. doi: 10.1089/089426804322994415. [DOI] [PubMed] [Google Scholar]
- 103.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 104.Garbuzenko OB, Saad M, Pozharov VP, et al. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc Natl Acad Sci USA. 2010;107:10737–10742. doi: 10.1073/pnas.1004604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsia TC, Lin CC, Wang JJ, et al. Relationship between chemotherapy response of small cell lung cancer and P-glycoprotein or multidrug resistance-related protein expression. Lung. 2002;180:173–179. doi: 10.1007/s004080000091. [DOI] [PubMed] [Google Scholar]
- 106.Garbuzenko OB, Mainelis G, Taratula O, et al. Inhalation treatment of lung cancer: the influence of composition, size and shape of nanocarriers on their lung accumulation and retention. Cancer Biol Med. 2014;11:44–55. doi: 10.7497/j.issn.2095-3941.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaspar MM, Radomska A, Gobbo OL, et al. Targeted delivery of transferrin-conjugated liposomes to an orthotopic model of lung cancer in nude rats. J Aerosol Med Pulm Drug Deliv. 2012;25:310–318. doi: 10.1089/jamp.2011.0928. [DOI] [PubMed] [Google Scholar]
- 108.Verschraegen CF, Gilbert BE, Huaringa AJ, et al. Feasibility, phase I, and pharmacological study of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced malignancies in the lungs. Ann NY Acad Sci. 2000;922:352–354. doi: 10.1111/j.1749-6632.2000.tb07063.x. [DOI] [PubMed] [Google Scholar]
- 109.Koshkina NV, Gilbert BE, Waldrep JC, et al. Distribution of camptothecin after delivery as a liposome aerosol or following intramuscular injection in mice. Cancer Chemother Pharmacol. 1999;44:187–192. doi: 10.1007/s002800050966. [DOI] [PubMed] [Google Scholar]