Abstract
A 4-year-old boy presented with loss of motor milestones following viral fever. On examination, the child had increased tone and exaggerated deep tendon reflexes. Magnetic resonance imaging of the brain showed white matter hyperintensities on T2-weighted images, which revealed partial inversion on fluid-attenuated inversion recovery images. Clinical exome sequencing revealed a novel homozygous mutation c.1270T>G: pCys424Gly in exon 11 of the EIF2B3 gene. This novel mutation is reported in this article along with a literature review.
KEYWORDS: Childhood ataxia with central nervous system hypomyelination, EIF2B3 gene mutation, Indian, leukodystrophy, vanishing white matter disease
INTRODUCTION
Vanishing white matter (VWM) disease or childhood ataxia with central nervous system hypomyelination (CACH) was first described by van der Knaap et al. in a series of nine children describing the clinical and radiological manifestation.[1] VWM disease is central nervous system white matter disease due to demyelination characterized by episodic deterioration following insults such as head trauma, infection with fever, and acute fright.[2] The phenotype of disease varies from mild adult-onset disease to most severe congenital form.[3] VWM disease is caused by mutation in the EIF2B15 gene encoding the subunits of the EIF2B.[3] We report the case of VWM disease with novel EIF2B3 mutation.
CASE REPORT
A 4-year-old boy born to a second-degree consanguineous marriage with normal birth history presented with regression of motor milestones. The regression occurred following an episode of febrile episode at 3 years 6 months when the child lost walking, sitting, and neck control over a period of 2 weeks. The child had two episodes of seizures with fever. On examination, the head circumference was 48 cm with weight of 14 kg. There were no neurocutaneous markers. There was increased tone of all four limbs with exaggerated deep tendon reflexes with extensor plantar response.
On investigations, complete blood count, liver function test, renal function test, serum ammonia, and serum lactate were all normal. Electroencephalogram done was normal. Axial T2-weighted image in magnetic resonance imaging (MRI) of the brain showed periventricular and lobar hyperintensities [Figure 1a] which revealed partial inversion on fluid-attenuated inversion recovery images [Figure 1b]. Axial T2-weighted image showed diffuse white matter hyperintensities [Figure 2a and b]. Axial T2-weighted images demonstrated bilateral central tegmental tract (white arrow) hyperintensities [Figure 3a] and cerebellar white matter (white triangle) hyperintensities [Figure 3b]. Based on clinical features and MRI of the brain, we considered possibilities of CACH. Clinical exome sequencing revealed a novel homozygous mutation c.1270T>G: pCys424Gly in exon 11 of the EIF2B3 gene.
Figure 1.
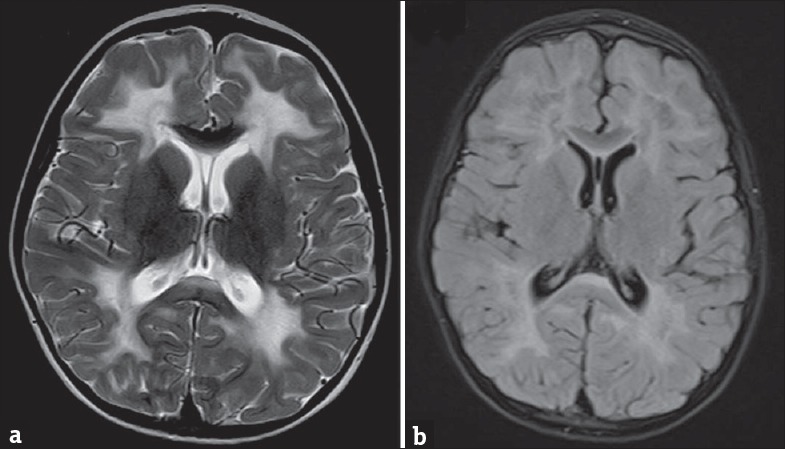
(a) Axial T2-weighted image of magnetic resonance imaging of brain showing periventricular and lobar hyperintensities which reveal partial inversion on fluid-attenuated inversion recovery images in (b)
Figure 2.
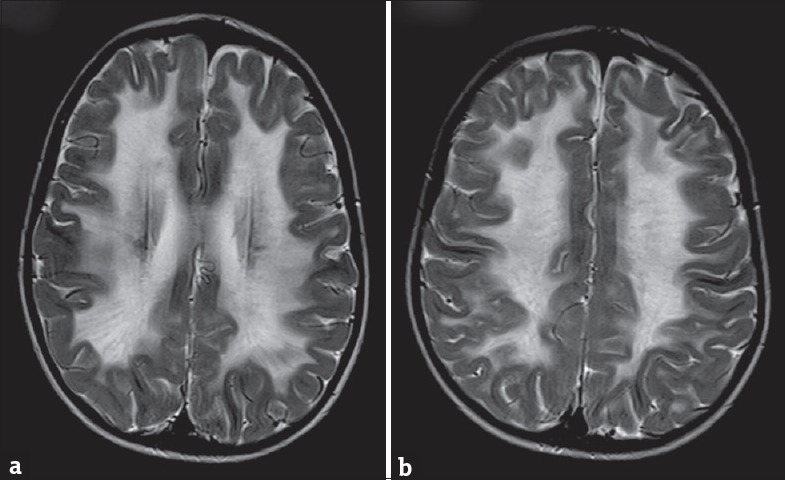
(a and b) Axial T2-weighted image of magnetic resonance imaging of brain showing diffuse white matter hyperintensities
Figure 3.
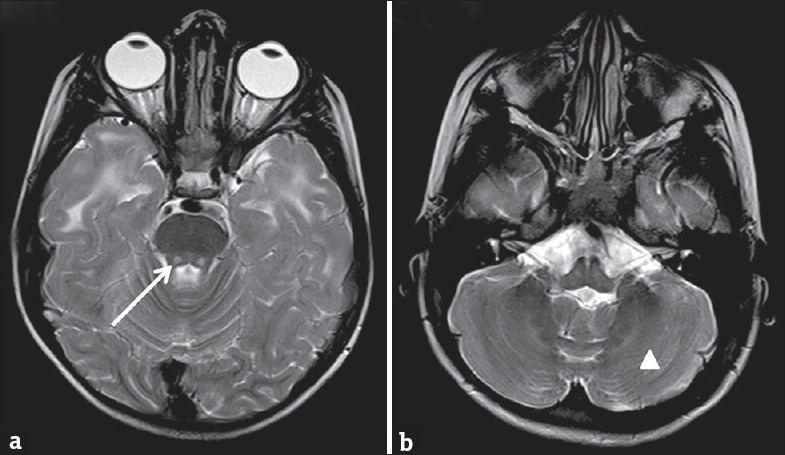
(a) Axial T2-weighted image of magnetic resonance imaging of brain demonstrating bilateral central tegmental tract (white arrow) hyperintensities and (b) showing cerebellar white matter (white triangle) hyperintensities
DISCUSSION
VWM disease is one of the common leukodystrophies.[4] Earlier cases have been reported from Europe, China, Japan, and India.[5] VWM disease is characterized by progressive ataxia and spasticity and periods of acute deterioration precipitated by febrile illness or head trauma. We suspected VWM disease based on clinical features and MRI findings. We confirmed on genetic testing. Mutations in the EIF2B1, EIF2B2, EIF2B3, EIF2B4, and EIF2B5 genes cause VWM disease. Mutation has been identified in all five of the genes of eIF2B protein. About 65% of mutation occurs in the EIF2B5 gene.[6] Approximately 4% to 7% of mutations in VWM disease are due to EIF2B3.[7] Wu et al. reported mutation in EIF2B3 account for 20% of the Chinese pediatric cases.[8] We report a novel mutation in this gene in a child with mild phenotype of VWM disease.
We are earlier reported case of VWM disease associated with ptosis and myoclonic seizures.[5] Fogli et al. found that 87% of 78 families with VWM disease had a mutation in four of the EIF2B genes. Sixty-two percent had mutation in the EIF2B5 gene. Three families (4%) had mutations in the EIF2B3 gene. Mean onset of disease was at 3.9 years. The disease severity ranged from no neurological signs to death. There was no correlation between type of gene mutation and age of onset or disease severity.[7] van der Lei et al. identified mutation in the EIF2B5 gene in 68% of 184 patients from a large database of VWM disease patients.[9] Matsukawa et al. reported adult-onset VWM disease with homozygous mutation in the EIF2B3 (L27Q; 606273.0005) gene.[10] La Piana et al. reported adult-onset VWM disease due to novel EIF2B3 mutation.[11] Mutations due to EIF2B3 gene are milder phenotype and indicate longer survival rate.
CONCLUSION
Homozygous mutation c.1270T>G: pCys424Gly in exon 11 of the EIF2B3 gene may be a causative factor for VWM disease. This can be used for novel diagnostic and prognostic marker in white matter diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to InterpretOmics India Pvt. Ltd., Bengaluru, Karnataka, India, for performing the genetic testing.
REFERENCES
- 1.van der Knaap MS, Barth PG, Gabreëls FJ, Franzoni E, Begeer JH, Stroink H, et al. Anew leukoencephalopathy with vanishing white matter. Neurology. 1997;48:845–55. doi: 10.1212/wnl.48.4.845. [DOI] [PubMed] [Google Scholar]
- 2.Bugiani M, Boor I, Powers JM, Scheper GC, van der Knaap MS. Leukoencephalopathy with vanishing white matter: A review. J Neuropathol Exp Neurol. 2010;69:987–96. doi: 10.1097/NEN.0b013e3181f2eafa. [DOI] [PubMed] [Google Scholar]
- 3.Pavitt GD, Proud CG. Protein synthesis and its control in neuronal cells with a focus on vanishing white matter disease. Biochem Soc Trans. 2009;37(Pt 6):1298–310. doi: 10.1042/BST0371298. [DOI] [PubMed] [Google Scholar]
- 4.van der Knaap MS, Breiter SN, Naidu S, Hart AA, Valk J. Defining and categorizing leukoencephalopathies of unknown origin: MR imaging approach. Radiology. 1999;213:121–33. doi: 10.1148/radiology.213.1.r99se01121. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Arya R, Raju KN, Kumar A, Scheper GC, van der Knaap MS, et al. Vanishing white matter disease associated with ptosis and myoclonic seizures. J Child Neurol. 2011;26:366–8. doi: 10.1177/0883073810381529. [DOI] [PubMed] [Google Scholar]
- 6.Pronk JC, van Kollenburg B, Scheper GC, van der Knaap MS. Vanishing white matter disease: A review with focus on its genetics. Ment Retard Dev Disabil Res Rev. 2006;12:123–8. doi: 10.1002/mrdd.20104. [DOI] [PubMed] [Google Scholar]
- 7.Fogli A, Schiffmann R, Bertini E, Ughetto S, Combes P, Eymard-Pierre E, et al. The effect of genotype on the natural history of eIF2B-related leukodystrophies. Neurology. 2004;62:1509–17. doi: 10.1212/01.wnl.0000123259.67815.db. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Pan Y, Du L, Wang J, Gu Q, Gao Z, et al. Identification of novel EIF2B mutations in Chinese patients with vanishing white matter disease. J Hum Genet. 2009;54:74–7. doi: 10.1038/jhg.2008.10. [DOI] [PubMed] [Google Scholar]
- 9.van der Lei HD, van Berkel CG, van Wieringen WN, Brenner C, Feigenbaum A, Mercimek-Mahmutoglu S, et al. Genotype-phenotype correlation in vanishing white matter disease. Neurology. 2010;75:1555–9. doi: 10.1212/WNL.0b013e3181f962ae. [DOI] [PubMed] [Google Scholar]
- 10.Matsukawa T, Wang X, Liu R, Wortham NC, Onuki Y, Kubota A, et al. Adult-onset leukoencephalopathies with vanishing white matter with novel missense mutations in EIF2B2, EIF2B3, and EIF2B5. Neurogenetics. 2011;12:259–61. doi: 10.1007/s10048-011-0284-7. [DOI] [PubMed] [Google Scholar]
- 11.La Piana R, Vanderver A, van der Knaap M, Roux L, Tampieri D, Brais B, et al. Adult-onset vanishing white matter disease due to a novel EIF2B3 mutation. Arch Neurol. 2012;69:765–8. doi: 10.1001/archneurol.2011.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]


