Abstract
Introduction
The increased availability of immunotherapeutic agents for the treatment of a wide array of cancer in the general oncology practice setting will reveal rare and unique toxicities.
Materials and methods
The mechanism of cardiac allograft rejection in the context of PD-1 antibody therapy was explored in a patient with cutaneous squamous cell cancer complicating long-standing cardiac allograft. Immune cell infiltrate in the myocardium and peripheral blood lymphocyte repertoire were assessed using myocardial biopsy and temporal analysis of peripheral blood samples. The efficacy of high-intensity immunosuppression to reverse graft rejection was explored.
Results
Endomyocardial biopsy showed acute moderate diffuse cellular rejection with a predominant population of CD3+, CD8+ and CD4+ infiltrating lymphocytes; peripheral blood circulating lymphocytes showed a high frequency of proliferating and activated CD8+ T cells expressing PD-1 compared to a normal control. There was no difference in the activation and proliferation of CD4+ T cells compared to a normal control. Cardiac function improved following high-intensity immunosuppression and patient survived for up to 7 months after discontinuation of nivolumab.
Conclusions
Immune checkpoint inhibitors should be avoided in allograft recipients but high-intensity immunosuppression is effective to salvage allograft rejection induced by these agents.
Keywords: Cancer, Immunotherapy, Rejection, Allotransplant, PD-1, Antibody
Introduction/case report
Patient is a 49-year-old Caucasian male, orthotopic heart transplant recipient in 1996 secondary to familial dilated cardiomyopathy. His post-transplant course over the ensuing 18 years was complicated by grade IIIA (acute moderate, diffuse) cellular rejection diagnosed on routine surveillance endomyocardial biopsy during his sixth annual post-transplant. This was successfully treated with high-dose oral prednisone tapered off over 3 weeks with resolution of cellular rejection on repeat endomyocardial biopsy. Patient continued on chronic low-dose prednisone maintenance along with his original immunosuppressive regimen of tacrolimus and mycophenolate mofetil without recurrence of cellular rejection and preserved cardiac allograft function. The patient was diagnosed with early cardiac allograft vasculopathy (CAV) during his eleventh annual post-transplant cardiac catheterization with distal tapering of coronary arteries. Mycophenolate mofetil was switched to sirolimus and repeat cardiac catheterization following adjustment of his immunosuppressive regimen showed no significant cellular rejection. He also developed grade 2 cardiac allograft vasculopathy that was successfully treated with percutaneous coronary intervention with drug eluting stents to proximal and mid left anterior descending (LAD) artery during the thirteenth annual cardiac catheterization. Cardiac allograft vasculopathy was stable overall with normal systolic function and hemodynamics through the eighteenth annual surveillance catheterization.
In 2012, 16 years post-transplant, the patient was diagnosed with cutaneous squamous cell carcinoma involving the left upper chest and shoulder for which he underwent Mohs procedure and radiation therapy. Two years later, he had metastatic recurrence to the axillary lymph nodes and lungs, which was treated with local radiation to the axilla and systemic chemotherapy (carboplatin and docetaxel). In preparation for systemic chemotherapy for recurrent squamous cell carcinoma, sirolimus was held while prednisone and tacrolimus were continued. A routine cardiac catheterization 4 months after completion of chemotherapy was notable for a 70% proximal left anterior descending artery stenosis, which was bypassed with a drug eluting stent. Patient was asymptomatic and had normal cardiac systolic function. Sirolimus was subsequently restarted after completion of cytotoxic chemotherapy. Due to lack of response to frontline cytotoxic chemotherapy, he was treated off-label with an anti-PD-1 antibody, nivolumab in May, 2015 by his local oncologist. Five days after the administration of the first dose of nivolumab, the patient experienced progressively increasing dyspnea on exertion, decreased urine output, and generalized swelling and required therapeutic thoracentesis to drain approximately 1200 ml of pleural fluid (Fig. 1a). He was subsequently admitted to his local hospital in cardiogenic shock, 2 weeks after receiving nivolumab, and required immediate initiation of inotrope therapy with dobutamine. A transthoracic echocardiogram showed left ventricular ejection fraction (EF) of 25% compared to a recent EF of 55% measured 3 weeks prior to his hospitalization. Coronary angiography showed no new stenosis in comparison with a recent surveillance angiography 3 weeks previously (Fig. 1b). On the recommendation of his transplant team, patient was initiated on high-dose methylprednisolone along with sirolimus and tacrolimus and was subsequently transferred for further management by his primary transplant team at our institution with a working diagnosis of acute transplant rejection.
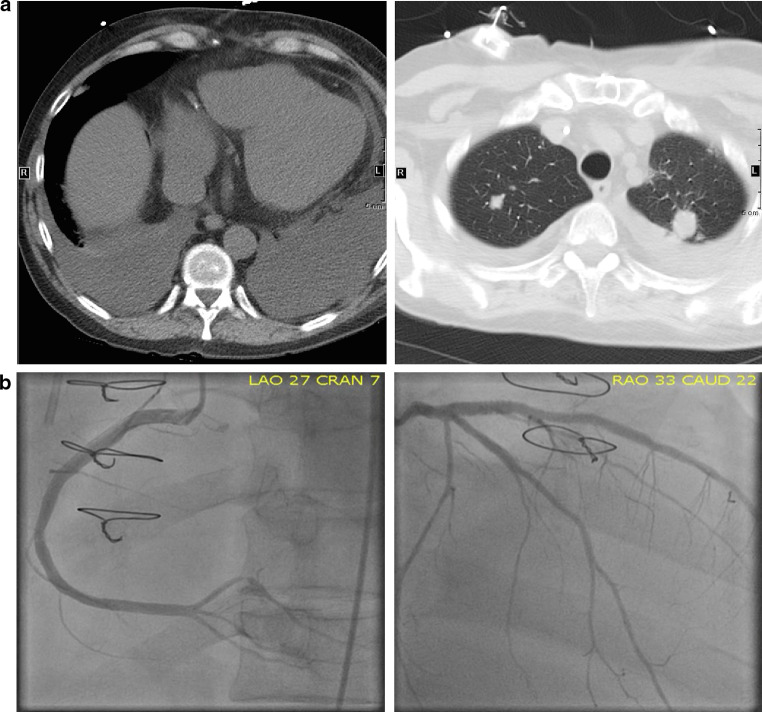
Fig. 1.
Cross sectional chest imaging. a CT scan of the chest showing bilateral pulmonary metastatic nodules, new onset of moderate bilateral pleural effusion but no evidence of significant pericardial effusion 5 days after administration of nivolumab. b Coronary angiography via left heart catheterization showing unchanged left main coronary artery, eccentric stenosis in the proximal LAD and patent stents in mid LAD and ramus intermedius. There was patency of the stents in the proximal-mid segments of LAD and ramus intermedius arteries but 70% stenosis in the distal right posterior descending artery consistent with findings on the most recent routine coronary angiography performed approximately 3 weeks prior to presentation
Hospital course
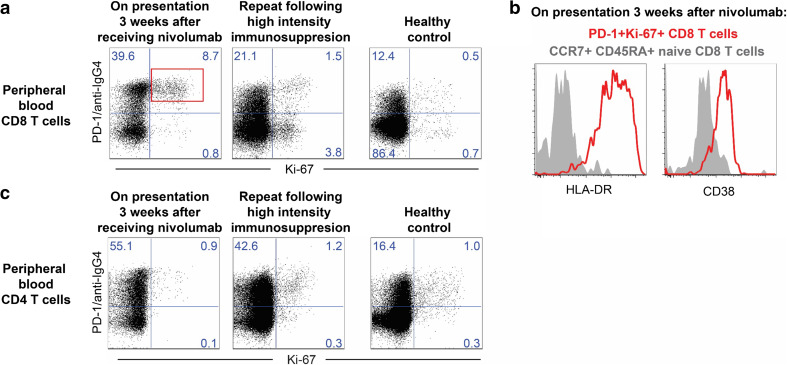
On admission to the Emory University Hospital coronary care unit, the patient was noted to have a blood pressure of 127/82 while on inotropes (dobutamine), heart rate 105 and respiratory rate 18. Other pertinent physical findings include moderate respiratory distress, generalized anasarca, pulmonary crackles, bilateral pitting edema and elevated jugular venous distension to 12 cm at 45°. Laboratory evaluation revealed an elevated brain natriuretic peptide (3213 pg/ml) and troponin I (4.63 ng/mg; peaked at 7.1 ng/ml) along with poor renal function (serum creatinine of 5.38 mg/dl; recent historical baseline of 1.86 mg/dl). Due to the rapid development of acute decompensated systolic heart failure in the context of receiving an immune-modulating therapy, there was a high suspicion for acute allograft rejection precipitated by nivolumab therapy. The patient was continued on pulsed high-dose intravenous methylprednisolone (500 mg IV Q12 h) for 4 days along with sirolimus and increased dose of tacrolimus adjusted for kidney function. No donor-specific antibodies were identified. An endomyocardial biopsy was performed and showed a grade IIIA (acute moderate diffuse) cellular rejection with a predominant population of CD3+, CD8+ and CD4+ infiltrating lymphocytes (Fig. 2). As part of an IRB-approved immune checkpoint therapy biomarker profiling program, peripheral blood circulating lymphocytes were characterized by flow cytometry at presentation to the Emory coronary care unit and after 1 week of high-intensity immunosuppression. At the time of admission, there was a high frequency of proliferating CD8+ T cells expressing PD-1 (Fig. 3a), a significant proportion of which were highly activated as evidenced by expression of CD38 and HLA-DR (Fig. 3b). PD-1-expressing cells were identified by an anti-human IgG4 staining as previously described [1]. Repeat analysis 1 week later showed a greater than fivefold reduction in the proportion of proliferating CD8+ T cells expressing PD-1 to a level comparable to that of a healthy control (Fig. 3c). In contrast, there was no difference in the activation and proliferation of CD4+ T cells on presentation or following 1 week of high-intensity immunosuppression in comparison with a health control (Fig. 3c).
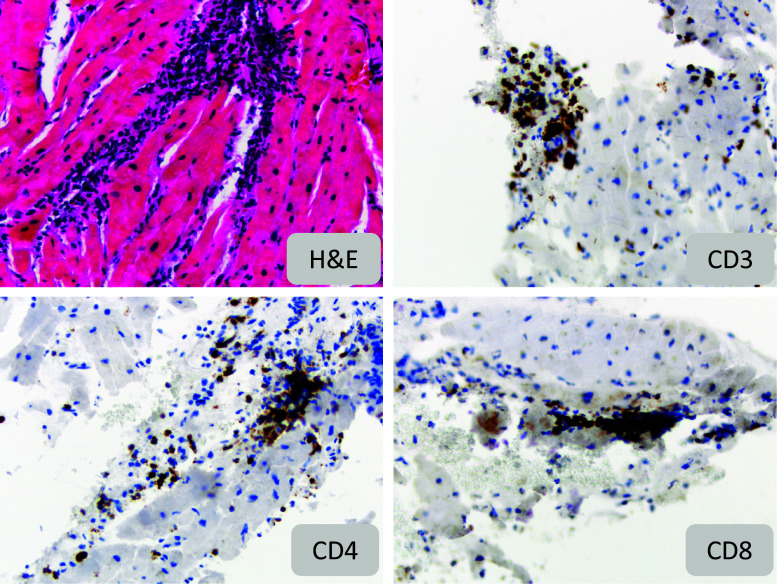
Fig. 2.
Histopathology and immunohistochemistry for immune cells in myocardial biopsy: Hematoxylin/Eosin and immunohistochemistry stains (×200 magnification) for CD3, CD4, and CD8 on endomyocardial biopsy showing acute moderate diffuse (grade IIIA) cellular rejection with a predominant population of CD3+, CD8+ and CD4+ infiltrating lymphocytes
Fig. 3.
Peripheral blood circulating lymphocyte characterization by flow cytometry. a Dot plots show proliferation (Ki-67) and PD-1 expression (PD-1 and anti-hIgG4) on peripheral blood CD8+ CD3+ T cells, from index patient and a control healthy volunteer. PD-1-expressing cells were identified by an anti-human IgG4 staining as previously described [1]. b Dot plot shows activation (HLA-DR and CD38 expression) of PD-1 + Ki67 + CD8 T cells (red dots, gate as shown in a) compared to total CD8 T cells (black dots) from index patient 3 weeks after receiving nivolumab. c Dot plots show proliferation (Ki-67) and PD-1 expression (PD-1 and anti-hIgG4) on peripheral blood CD4+ CD3+ T cells, from index patient and a control healthy volunteer
Hemodynamics improved by Day 5 of high-intensity immunosuppression confirmed on repeat transthoracic echocardiogram, which showed improved cardiac function with an EF of 40% (from 25% 2 weeks previously). The patient was subsequently weaned off hemodialysis and ionotropic support with stable renal function. He elected to pursue symptom-focused care support and was discharged home in stable clinical condition approximately 10 days after his initial transfer to our facility. The patient subsequently reestablished cardiac care closer to home and died 8 months after discharge from our institution.
Discussion
Programmed cell death protein 1 (PD-1 and CD279) is a regulatory protein expressed by activated T cells and an established physiologic regulator of immune function. Aberrant expression of the PD-1 ligand (PD-L1) by tumor cells leading to evasion of antitumor immunity has been implicated in cancer development and progression [2–4]. Pharmacological blockade of the PD-1 pathway has been validated as a treatment strategy in multiple tumor types with regulatory approval of anti-PD-1 therapy as standard of care treatment for cancer patients [5–12]. Nivolumab and pembrolizumab (PD-1 receptor blocking antibodies) as well as atezolizumab (PD-1 ligand-blocking antibody) selectively block engagement between PD-1 receptor and its cognate ligands, PD-L1/PD-L2, leading to the restoration of T cell-mediated anti-tumor immunity [1]. Following efficacy demonstrated in clinical trials, these agents are now available for use in the general oncology clinic setting outside the restrictive monitoring required on clinical trials. The increased use of these agents in various categories of patients who may not fit the profile of patients enrolled on clinical trials is bound to result in previously unreported complication and toxicity profile with this class of agents. The case presented in this report illustrates an example of unique toxicities to be anticipated, as this class of cancer therapies becomes standard treatment option in the general oncology community.
Immune checkpoint inhibitors confer meaningful clinical and survival benefit to a significant proportion of patients with advanced incurable cancer [5–12]. However, by enhancing the host’s anti-tumor response, this approach can also engender unwanted side effects. Immune-related adverse events (irAE) resulting from host immune response hyperactivation by these immune checkpoint inhibitors are generally mild to moderate in severity but can be severe and even fatal in occasional cases. Well-described irAE range from skin rash and arthralgia to immune-mediated colitis, endocrinopathies, pneumonitis, hepatitis and nephritis. In preclinical studies, ablation of the PD-1 encoding gene in BALB/c mice resulted in high titer of IgG autoantibodies against cardiac troponin I, which was associated with a phenotype akin to dilated cardiomyopathy [13, 14]. In addition, PD-1 ablation in MRL mice resulted in fatal myocarditis with increased infiltration of the myocardium by CD4, CD8 T cells and myeloid cells along with a high titer of autoantibodies against cardiac myosin [15]. These observations suggest an important role of PD-1 in limiting T cell-mediated inflammatory responses in the heart [16]. Other cases of myocarditis and acute heart failure have been reported in cancer patients treated with PD-1 inhibitors [17–19]. However, cardiac toxicity is not a common adverse event associated with this class of agents. To our knowledge, there has not been a prior report of a cardiac transplant patient suffering allograft organ rejection as a complication of PD-1—targeted therapy. However, PD-1 blockade led to early rejection of renal allograft in a patient with cutaneous squamous cell carcinoma treated with nivolumab [20]. Contrarily, the use of CTLA-4 inhibitors in renal allograft recipients seemed to be better tolerated and did not result in renal graft rejection [21]. The differences in outcome in the limited number of graft recipients treated with CTLA-4 and PD-1 inhibitors may be related to the important role of PD-1 in the induction and maintenance of transplantation tolerance [22, 23].
The rarity to date of allograft rejection as a complication of PD-1 inhibitor therapy is due to the fact that allograft patients were ineligible to participate in the pivotal trials that established the efficacy of these agents. However, these agents have now become available for routine treatment of cancer patients outside the controlled clinical trial setting. Off-label use of these agents is likely to increase especially in patient populations where the safety of these agents have not been established but for which where there are no specific contraindications based on the official prescribing information from the US Food and Drug Administration [24, 25].
Our case highlighted the challenges of immunotherapy for managing cancers in allograft recipients with a predictable toxicity of graft rejection resulting from increased immune activation. It also demonstrated the efficacy of high-intensity immunosuppression regimen for graft salvage in situations where graft rejection complicates the use of this class of agents and the need to manage such complex cases at tertiary centers with needed expertise.
Acknowledgements
This work was supported in part by grant support from the US National Institute of Health Grant 1K23CA164015 (TK Owonikoko).
Abbreviations
- CAV
Cardiac allograft vasculopathy
- LAD
Left anterior descending
- EF
Ejection fraction
- irAE
Immune-related adverse event
- IRB
Institutional review board
Compliance with ethical standards
Conflict of interest
T. K Owonikoko and S. S Ramalingam received research funding and served on advisory board for Astra Zeneca, Bristol-Myers Squib, Merck and Genentech. All other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27(4):195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K- Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K- Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 10.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, Hiai H, Honjo T, Okazaki T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–452. doi: 10.1093/intimm/dxq026. [DOI] [PubMed] [Google Scholar]
- 16.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188(10):4876–4884. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. doi: 10.1186/s40425-015-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, Voegeli M, Cathomas G, Zippelius A, Mertz KD. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer. 2016;4:13. doi: 10.1186/s40425-016-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipson EJ, Bagnasco SM, Moore J, Jr, Jang S, Patel MJ, Zachary AA, Pardoll DM, Taube JM, Drake CG. Tumor regression and allograft rejection after administration of anti-PD-1. N Engl J Med. 2016;374(9):896–898. doi: 10.1056/NEJMc1509268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipson EJ, Bodell MA, Kraus ES, Sharfman WH. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. 2014;32(19):e69–e71. doi: 10.1200/JCO.2013.49.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, Shin T, Blazar BR, Rothstein DM, Sayegh MH, Najafian N. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179(8):5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, Sharpe AH, Auchincloss H, Jr, Sayegh MH, Najafian N. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174(11):6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 24.MERCK HIGHLIGHTS OF PRESCRIBING INFORMATION: KEYTRUDA® (pembrolizumab) for injection. Initial U.S. Approval (2014)
- 25.Bristol-Myers-Squibb HIGHLIGHTS OF PRESCRIBING INFORMATION: OPDIVO (nivolumab) injection, for intravenous use. Initial U.S. Approval (2014)