Abstract
The Haller's organ (HO), unique to ticks and mites, is found only on the first tarsus of the front pair of legs. The organ has an unusual morphology consisting of an anterior pit (AP) with protruding sensilla and a posterior capsule (Cp). The current thinking is that the HO's main function is chemosensation analogous to the insect antennae, but the functionality of its atypical structure (exclusive to the Acari) is unexplained. We provide the first evidence that the HO allows the American dog tick, Dermacentor variabilis, to respond to infrared (IR) light. Unfed D. variabilis adults with their HOs present were positively phototactic to IR. However, when the HOs were removed, no IR response was detected. Ticks in these experiments were also attracted to white light with and without the HOs, but were only positively phototactic to white light when the ocelli (primitive eyes) were unobstructed. Covering the eyes did not prevent IR attraction. A putative TRPA1 receptor was characterized from a D. variabilis-specific HO transcriptome we constructed. This receptor was homologous to transient receptor potential cation channel, subfamily A, member 1 (TRPA1) from the pit organ of the pit viper, python, and boa families of snakes, the only receptor identified so far for IR detection. HO scanning electron microscopy (SEM) studies in the American dog tick showed the AP and Cp but also novel structures not previously described; the potential role of these structures in IR detection is discussed. The ability of ticks to use IR for host finding is consistent with their obligatory hematophagy and has practical applications in tick trapping and the development of new repellents.
Keywords: American dog tick, Dermacentor variabilis, Haller's organ, Infrared, TRPA1, Light
Graphical abstract
1. Introduction
Ticks are responsible for transmitting the majority of arthropod vector-borne disease agents in the U.S., and the incidence of tick-borne disease is on the rise because of globalization, population growth, people moving to rural areas, and climate change (Sonenshine et al., 2014; Spach et al., 1993). The American dog tick, Dermacentor variabilis, the focus of this study, is a hard tick (family Ixodidae) that lives a non-nidicolous lifestyle in North and South America. D. variabilis vectors the causative agent (the bacterium Rickettsia rickettsia) for Rocky Mountain spotted fever (RMSF) as well as other serious human pathogens (Calhoun, 1954, Steiert et al., 2002). Transmission occurs from the tick to humans during blood feeding; once established in the host, RMSF can cause severe headaches, nausea, vomiting, and death if not treated within approximately the first week of symptom onset (Biggs, 2016). The number of reported cases of RMSF in the U.S. has been on the rise, with less than 500 cases reported in 1993 and 2500 cases in 2008 (CDC, 2010).
Understanding how ticks find their host is of utmost importance to disease prevention and personal protection from tick bites. Early morphological observations, generally made with light microscopy in the late 19th and early 20th century, revealed an abundance of sensory sensilla scattered across the surface of ticks as well as patches localized to specific areas of the palps and tarsus I of each foreleg. General differences in morphology and orientation were noticed, but the examination of fine detail was limited by the microscopic capabilities of the day (Haller, 1881; Nuttall et al., 1908). Work in the 1970s, fueled by the advent of electron microscopy and improved electrophysiology, further advanced our characterization of the different sensillar types associated with these regions. The foretarsal sensory organ, commonly referred to as the Haller's organ (HO) in ticks, is a sensilla-rich structure thought to be mostly chemosensory. The HO is unique to the Acari and not found in any other animals (Ivanov et al., 1983; Balashov, 1979; Foelix et al., 1972; Roshdy et al., 1972). Some of its structure like the capsule is not typical of a chemosensory organ. However, the current consensus is that the HO is functionally analogous to the insect antenna (Sonenshine et al., 2014).
In insects, there are two types of IR-sensing organs: (Type 1) photomechanic sensilla found in Melanophila beetles (Evans, 1964; Schmitz et al., 1997; Schmitz et al., 1998) and (Type 2) photothermal microbolometers found in the Merimna Australian fire-beetles (Schmitz et al., 2000; Schmitz et al., 2001; Schmitz et al., 2002) that are structurally and functionally similar to the pit organ IR detectors in snakes (Gracheva et al., 2010). Gracheva et al. (2010) used an unbiased transcriptional profiling method to identify the IR receptors in snakes as transient receptor potential cation channel, subfamily A, member 1 (TRPA1) channels. They have not been studied for their role in IR perception elsewhere. The only report of IR detection in the Acari was in the spiny rat mite, Laelaps echidnina, by Bruce (1971) where IR detection was localized to the forelegs; however, no specific region of tarsus I (including the HO), specific sensilla, or receptors were shown to be involved in IR detection (Bruce, 1971). Since ticks are obligatory blood feeders, there is similar morphology in the HO to IR-receptor organs in insects and snakes, and we had preliminary HO-specific transcriptomic data of non-chemosensory receptor channels in the tick foreleg, the current study was conducted to determine if ticks would be responsive to IR and to determine the role of the HO in this response.
2. Materials and methods
2.1. Ticks
Unfed, virgin adult (male and female) American dog ticks, Dermacentor variabilis (Ixodida: Ixodidae), were provided by both Dr. Daniel E. Sonenshine in the Department of Biological Sciences at Old Dominion University (Norfolk, VA) and Lisa Coburn from the Department of Entomology and Plant Pathology, and manager of the Tick Rearing Facility at Oklahoma State University (Stillwater, OK). Ticks were obtained from two sources to ensure our results were not specific to one strain. Upon arrival, ticks were maintained at 26±1°C and 80%±5% relative humidity on a 16:8 light/dark cycle until needed for assays or imaging.
2.2. Scanning Electron Microscopy
For scanning electron microscopy (SEM; Fig. 1), methods were adapted from Sonenshine et al. (1984). Ticks were sacrificed by freezing at -80°C for at least 2 h, removed, washed immediately 3 times in 70% ethanol:distilled water, and stored in fresh 70% ethanol:distilled water in a 1.5 uL microcentrifuge tube until imaging was performed. SEM was performed at the Analytical Instrumentation Facility at North Carolina State University (Raleigh, NC) where tick specimens were removed from the 70% ethanol, air-dried for approximately 10 mins, mounted on a metal plug with double-sided mounting tape before being coated with 100-200 Å of a gold-palladium mixture (60 Au/40 Pd), and scanned with a Hitachi S-3200N variable pressure scanning microscope.
Fig. 1.
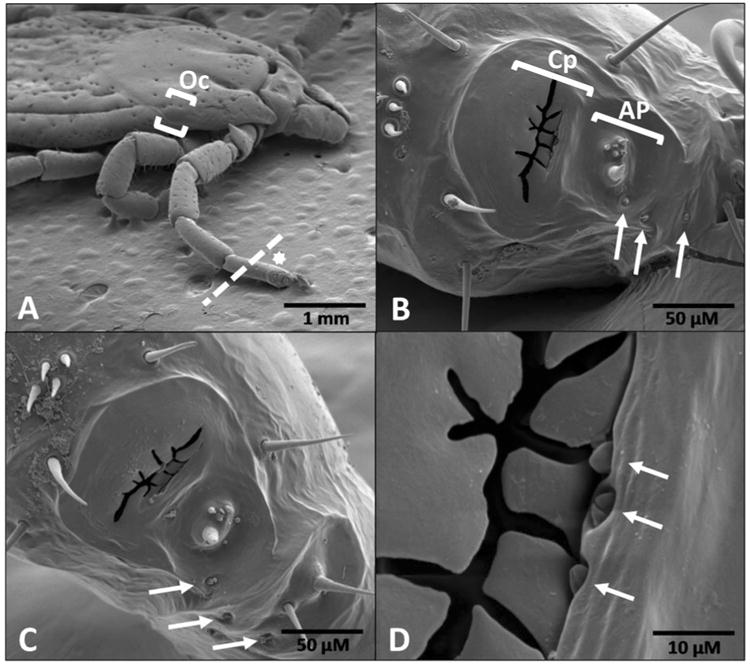
Scanning electron micrographs of Dermacentor variabilis Haller's organ (HO) and associated structures. (A) female, dorsal view at 25×, dotted line where tarsus I including HO was removed, (B) female, dorsal view of HO anterior pit and capsule at 500×, (C) male, dorsal view of HO anterior pit and capsule at 500×, and (D) female, dorsal view, aperture opening of capsule at 2500×. Arrows in panels B-D indicate undescribed structures resembling auricular or companiform sensilla that may serve as IR detectors or assist in this function in both male and female D. variabilis. The white star in panel A denotes the location of the HO (star just above structure). The ocellus (primitive eye) is located between the brackets in panel A. The dotted line denotes the location where the HO was ablated for the corresponding trials. Oc = ocellus, Cp = capsule, AP = anterior pit.
2.3. Behavioral Bioassays
Choice bioassays were conducted to assess IR versus visible light taxis for the ocelli versus HOs of the American dog tick. We designed the assay to limit interference from extraneous sources. The test arena (Fig. 2A) was 25 cm from the start to finish in “Direction I” and (at a right angle) 25 cm from the start to finish in “Direction II”. The test arena was a flat white plastic tray. Assay conditions were 24±1°C and 45±5% relative humidity in total darkness (except for the light source being tested) in a walk-in incubator. Unfed, virgin male and female adult D. variabilis with both HOs intact or removed and/or both ocelli intact or disrupted (i.e., ocelli obscured with black paint) were incubated for 5 h under assay conditions in complete darkness prior to bioassay. Each HO was removed by amputation with a sharp razor blade by cutting through tarsus I just distal to the tibia-tarsus I joint (Fig. 1A, dotted line). Very little if any hemolymph was lost from the wound, and therefore the cut leg was not sealed. The coating used to cover the ocelli was black nail polish (Wet N'Wild, Los Angeles, CA) that was applied, allowed to dry and then applied and dried a second time. There was no visible indication of cracks in the paint before or after bioassays were conducted. We did not measure light penetration through these two layers of paint since the results from the bioassays suggested the treatment was effective in preventing the detection of white light. The IR (880 nm) was produced from an Evolva T20 instrument (Dexcel International Co., Guangdong, China) and the visible light (450-650 nm in the visible light spectrum) by a BYB Super Bright 9 LED (BYB limit Co., United Kingdom). These light sources were commercially available at a reasonable cost for exploratory studies like those being conducted here, spanned the visible to IR range as was needed, produced similar shaped beams of light that spanned the distance of our test area, and were most compatible with our bioassay approach. Fig. 2B shows a typical light projection on the arena surface. Ticks did not respond to the light sources when they were turned off. Using a Ryobi infrared thermometer (Ryobi Limited, Hiroshima, Japan), we observed no measurable difference in temperature (±0.1°C) at the surface of the light sources where light was projected (or any other surface of the flashlight tested) or anywhere on the surface of the test arena (5 mins after the light was turned on) that was different from the ambient incubator temperature. This indicates that there was no measurable convection heat emanating from the light sources, and the tick responses were only to the detection of radiant energy.
Fig. 2.
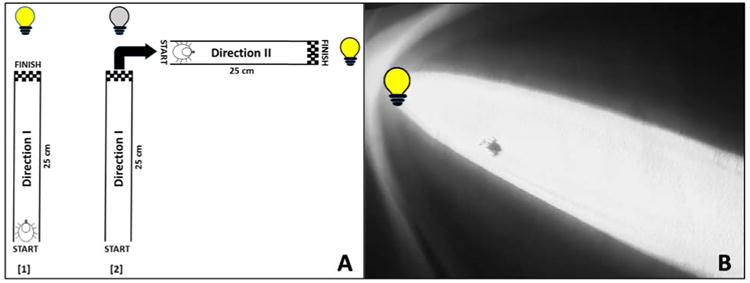
Arena calibration points and video screenshot. (A) Choice arena where two of the ports at right angles to each other were fitted with identical light sources (either visible light or infrared), and (B) high definition, IR-capable video camera capture of bioassay trial where HO and ocelli were present and unobstructed (tick moving toward IR light). At the beginning of each assay a single tick was placed at the start and a light source was illuminated (yellow bulb, lane 1). After crossing the finish line of “Direction I” the first light source was turned off (grey bulb, lane 2) and the second light source (at a right angle) was immediately illuminated (yellow bulb, lane 2). Once the tick traveled from the start to the finish of “Direction II” the assay was over. Any deviation out of the field of the light beam (and not correcting toward the light source) was considered non-responsive. Movement toward each light source was considered non-responsive if the tick took longer than 1 minute to move within 2.5 cm of the illuminated source. Yellow bulbs denote lights that were turned on while the grey bulb represents a light that was turned off.
At the beginning of each assay, a single tick (randomly selected with no consideration of sex) was placed at a pre-determined start location, labeled “Start” within the arena (Fig. 2A), and its movement visualized (and recorded) in response to IR or visible light exposure using a high resolution, video IR-capable camera (Canon XA25, Tokyo, Japan). The zoom capability of this camera allowed for visualization approximately 1 m from the test arena, and the observer was never closer to the test arena than the camera during any single trial. In total darkness, there was no positive or negative tick taxis relative to the camera. There were 4 possible responses from a tick once the assay began: (1) movement toward the light source, (2) movement away from the light source, (3) random movement, or (4) no movement at all. Ticks that did not move at all after a minimum of 30 sec were removed and excluded from the assay (i.e., no movement) and not included in the results, which resulted in 5 of our 115 tick trials being excluded (approximately 4%). No ticks were observed to be repelled by the light source. Ticks that constantly changed direction without regard for the light source were considered non-light responsive (not positively or negatively phototactic). Therefore, two end points were recorded, attraction to the light source or a non-response to the light source. In each assay, a single tick was challenged twice; a successful response was not only movement towards a light source (from “Start” to “Finish”) in Direction I (Fig. 2A, lane 1), but the ability to correct its movement when a new light source was introduced at a right angle to the initial challenge, Direction II (Fig. 2A, lane 2). If a tick successfully navigated from start to finish in “Direction I,” the first light source was turned off and a second light source of identical type was immediately projected into the arena at a right angle. If the tick did not move toward the second light source, then the overall movement was considered a non-response. Successful movement of a tick from start to finish in both Direction I and Direction II in the same trial was considered positive phototaxis. The light sources were arranged at a right angle to help demonstrate that the ticks were only responding to the light sources and no other potential cues like light, noise, CO2, air movement or heat sources within the incubator. The results were statistically evaluated using chi-square analysis under the null hypothesis that the expected proportion for either choice (response or no response) was 0.50 (Microsoft Excel and Powerpoint. Redmond, Washington: Microsoft, 2013).
2.4. Transcriptomic Analysis
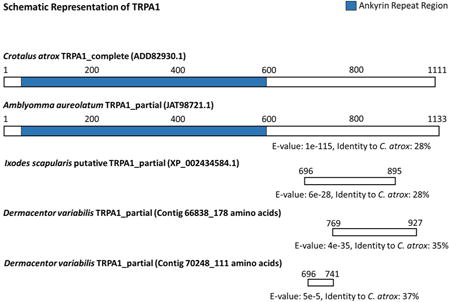
A transcriptome was constructed from the front pair of legs just proximal to the HO extending to the end of the leg from unfed virgin adult D. variabilis males using the Illumina Hi-Seq 2000 platform (Illumina, San Diego, CA) (Carr, 2015). Briefly, data sets generated by Hi-Seq were cleaned, trimmed, and assembled de novo using the CLC pipeline assembler and scaffolder (Qiagen, Valencia, CA). Blast2Go (BioBam, Valencia, Spain) and the GenBank non-redundant (nr) database were then used for functional annotation. A BLASTX and BLASTN search of the Haller's organ transcriptome using the western diamondback rattlesnake IR-detecting TRPA1 revealed the presence of 5 putative partial TRPA1 transmembrane proteins. Those partial TRPA1 sequences covered a range of e-values; therefore, we chose to focus on the two contigs with the lowest e-values and longest sequence lengths for further analysis (contig 66838 and contig 70248). Both contigs were used to search against the non-redundant (nr) protein sequence database using BLASTX with a maximum expect threshold of 10. The contigs aligned closely to a nearly full-length TRPA1 receptor in Amblyomma aureolatum and a partial TRPA1 receptor found in Ixodes scapularis, both tick species. An alignment of a TRPA1 known to be involved in IR detection in the western diamondback rattlesnake, Crotalus atrox, was constructed with the putative TRPA1s from A. aureolatum, I. scapularis and our 2 contigs from the D. variabilis HO transcriptome using the MUSCLE ver. 3.9.31 algorithm (Edgar, 2004). A summary of the alignment result is included at the bottom of Table 1. C. atrox was chosen for the alignments because it was one of three pit-bearing snake species whose TRPA1 was shown bioinformatically, anatomically and functionally to serve as an IR detector (Gracheva et al., 2010). Furthermore, of the three species whose TRPA1s were described, C. atrox aligned most closely at the amino acid level with our contigs from the HO transcriptome. We define our HO-specific transcriptome as the sum of all the messenger RNAs that were expressed in dissected forelegs (including the HO) that were removed from multiple adult male D. variabilis specimens and pooled together for sequencing.
Table 1.
Top 5 BLASTP hits for two HO-specific RNA-Seq contigs putatively assigned as TRPA1s exclusive to the HO of adult Dermacentor variabilis from our transcriptome (top) and schematic representation of putative HO-specific TRPA1 partial transcripts in D. variabilis from our transcriptomes aligned with a full-length TRPA1 from C. atrox and a putative TRPA1 from I. scapularis and A. aureolatum using DELTA-BLAST (bottom).1
| Sequence | Desc. | Top 5 Hits | Hit Acc. | E-value | Sim. | Bit-Score | Len. | Pos. |
|---|---|---|---|---|---|---|---|---|
| Contig 66838 | TRPA1* | A. aureolatum | JAT98721.1 | 1e-124 | 98 | 385 | 178 | 174 |
| TRPA1* | I. scapularis | XP_002434584.1 | 8e-82 | 85 | 252 | 143 | 122 | |
| TRPA1* | C. borealis | APG53778.1 | 1e-55 | 51 | 198 | 167 | 86 | |
| TRPA1* | H. americanus | APG53784.1 | 3e-53 | 50 | 186 | 175 | 87 | |
| TRPA1* | L. anatina | XP_013406001.1 | 5e-51 | 48 | 185 | 165 | 79 | |
| Contig 70248 | TRPA1* | A. aureolatum | JAT98721.1 | 8e-59 | 91 | 204 | 111 | 101 |
| TRPA1* | I. scapularis | XP_002434584.1 | 2e-43 | 83 | 151 | 81 | 67 | |
| TRPA1* | C. borealis | APG53778.1 | 2e-21 | 44 | 97.8 | 112 | 49 | |
| TRPA1* | S. mimosarum | KFM57166.1 | 3e-21 | 43 | 96.7 | 116 | 50 | |
| TRPA1* | P. tepidariorum | XP_015910181.1 | 7e-21 | 44 | 95.9 | 117 | 51 | |
| ||||||||
(*) indicates putatively assigned TRPA1 (i.e. partial transcript, TRPA1 homolog, or TRPA1-like protein); “Desc.” is contig identification assignment; “Hit Acc.” is accession number for match to contig; “Sim.” is similarity score between contig and hit, i.e. the extent to which the two sequences are related; “Len.” is amino acid alignment length between contig and hit; “Pos.” is number of exact amino acid matches between query and subject sequence. The numbers on the schematic (bottom) above the protein sequence illustrations represent amino acid positions. BLASTP = protein-protein BLAST; DELTA-BLAST = Domain Enhanced Lookup Time Accelerated BLAST. Matching organisms: Amblyomma aureolatum, Ixodes scapularis (blacklegged tick), Cancer borealis (Jonah crab), Homarus americanus (American lobster), Lingula anatina (duck mussel), Stegodyphus mimosarum (velvet spider), Parasteatoda tepidariorum (common house spider).
3. Results and discussion
3.1. Tick Forelegs Detect IR Light
Ticks are obligatory blood feeders often on warm-blooded animals. This lifestyle requires ticks to locate a host multiple times each generation to progress from larvae to nymphs, from nymphs to adults, in some tick species to progress through multiple nymphal stages, and for female ticks to develop eggs. Although there has been research on chemical cues that might be attractive to ticks and assist in host finding and there is a general view that heat is a component of host finding, no one has previously considered the possibility that ticks might be able to detect IR light (Lahille, 1905; Lees, 1948; Sonenshine et al., 2014). There are organs in the nasal cavity of snakes for IR detection that are used for prey location. There are also IR-sensing organs in some beetles that are used for locating forest fires to take advantage of new food sources and for mating after fire events (Gracheva et al., 2010; Schmitz et al., 2000). Furthermore, there is one study in mites suggesting that the front pair of legs was important in IR light detection (Bruce, 1971). Finally in our studies of the HO transcriptome in the American dog tick where we were examining mechanisms for chemoreception, we found receptor proteins that could be involved in light reception (discussed in more detail later). In toto, these findings led to examining whether ticks could detect IR.
Currently, all visual perception in ticks is attributed to the ocelli found on the dorso-lateral surface just above the second pair of legs (Fig. 1A, single ocellus in brackets). In contrast, the HO (Fig. 1) has traditionally been regarded as a chemosensory organ analogous to insect antennae (Ivanov et al., 1983; Balashov, 1979; Roshdy et al., 1972; Sonenshine et al., 2014). We designed a bioassay to assess IR versus visible light taxis for the ocelli versus HOs in the American dog tick. Fig. 2A shows a schematic diagram of the test area dimensions and relative positioning of the light source being tested during any single trial. Fig. 2B shows the appearance of the light on the arena surface and is a screenshot of an assay in which the tick is moving toward the light source. Fig. 2B displays IR detected by an IR video camera; the arena appears completely dark to the human eye. Selected video footage (Appendix A) demonstrates the response of D. variabilis to both IR and visible light under the experimental conditions described below.
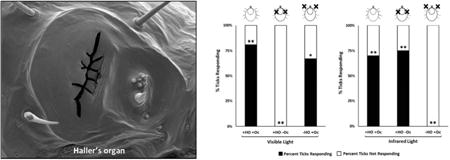
We tested the response of male and female virgin adult American dog ticks to white (visible) light and IR under the following conditions: (1) HOs and ocelli intact, (2) HOs removed and ocelli intact, and (3) HOs intact and ocelli blocked. A summary of the results for the different conditions tested is provided in Fig. 3. References to the removal of the HO was defined as the amputation of the end of the leg from just distal to the tibial-tarsus I joint to the end of the leg (Fig. 1A, dotted line). This included the HO and any other possible sensory structures found on the amputated part of the leg. Unfed D. variabilis with their HOs and ocelli (Oc) left intact (+HO +Oc) were positively phototactic to white (visible) light at a distance of 25 cm (Fig. 3A). The proportion of ticks responding to white light with their HOs and Oc intact was 0.81 (Fig. 3A; first bar on left). Using a chi-squared test of homogeneity of proportions with the null hypothesis being the ticks have a 50:50 chance of responding or not responding to the light source (a proportionality of 0.5), the difference in proportion observed in the test, 0.8, was significant from 0.5 (degrees of freedom, d.f. = 1, n = 42, χ² = 28.6, p ≤ 0.001). With the HOs left intact but the Oc occluded with black paint (+HO –Oc; Fig. 3A, second bar from the left), no ticks were attracted to the white light (d.f. = 1, n = 12, χ² = 12.0, p ≤ 0.001). It was clear the HO was not involved in attraction to the visible light. With the HOs removed by amputation and the Oc intact (-HO +Oc; Fig. 3A, third bar from the left), American dog ticks moved toward the white light. The proportion of ticks responding to white light with their HOs ablated was 0.67 (d.f. = 1, n = 12, χ² = 6.0, p ≤ 0.01). This treatment is also important in showing that removal of the tarsi on the front pair of legs does not affect the ability of the ticks to walk towards light under the conditions of our bioassay.
Fig. 3.
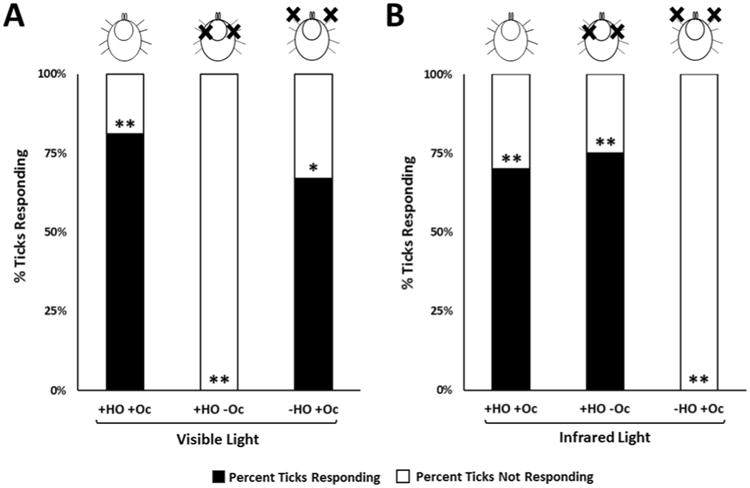
Dermacentor variabilis choice assay conditions and results. (A) Response of adult ticks to visible light with Haller's organs (HOs) removed or ocelli (Oc) blocked. (B) Response of adult ticks to infrared light with Haller's organs (HOs) removed or ocelli (Oc) blocked. “+HO +Oc” means that both the HOs and Oc were intact for those trials. “+HO -Oc” means that the HOs were intact and the Oc were covered with black paint for those trials. “-HO +Oc” means that the HOs were removed and the Oc were intact for those trials. A black “X” on the illustrations above each bar graph represents where the ticks' HOs were ablated or Oc were blocked. The frequency response was analyzed using a chi-squared test of homogeneity of proportions under the null hypothesis that the expected proportion for either choice (response or no response) was 0.50. Response to either visible or infrared light was significant at P ≤ 0.001 (**), P ≤ 0.01 (*).
Fig. 3B shows that ticks were attracted to IR presented at 880 nm and that the tarsi on the front pair of legs only and not the ocelli or tarsi on any of the other legs are responsible for this attraction. Unfed adult D. variabilis with their HOs and ocelli (Oc) left intact (+HO +Oc; Fig. 3B, left bar) were positively phototactic to IR at 880 nm. The proportion of ticks responding to IR light with their HOs and Oc intact was 0.70 which was significantly different from the null hypothesis, 0.5 (d.f. = 1, n = 20, χ² = 10.8, p ≤ 0.001). Seventy percent of the ticks assayed moved to the IR light, 30 percent moved about randomly. The proportion of ticks responding to IR light with their HOs intact and Oc covered (+HO -OC; Fig. 3B, second bar from the left) was 0.75 (d.f. = 1, n = 16, χ² = 9.6, p ≤ 0.001). These studies show that the ocelli are not involved in IR attraction or the black paint which covered the ocelli did not block the IR light. When the HO was removed and the ocelli left intact (-HO +Oc; Fig. 3B, third bar from the left), no ticks were attracted to the IR light (d.f. -1, n = 10), χ² = 10.0, p ≤ 0.001). The finding that covering the Oc with black paint with the HO intact had no impact on attraction to IR but removal of the HO and leaving the Oc eliminated IR attraction clearly rules out the Oc as being involved in IR detection. Also, the removal of the HO (the tarsi on the front pair of legs) had no impact on the ability of the ticks to crawl to visible light (Fig. 3A). Therefore, it is likely that removing the tarsi on the front pair of legs is the reason the ticks did not crawl to the IR light (Fig. 3B).
In summary, these results suggest in D. variabilis that visible light is detected by the ocelli and IR by the tarsi on the front pair of legs only. The assumption is made that the IR detection involves the HO or sensory structures closely associated with the HO, since the HO is exclusive to the tarsi on the front legs and is the most prominent sensory organ which is not found on the other walking legs.
3.2. Mechanism of IR detection in ticks
Understanding the mechanism of IR detection in ticks could lead to novel methods for disruption of this system and prevention of blood feeding on hosts. In insects, there are two different types of IR sensing organs: (Type 1) photomechanic IR receptors and (Type 2) photothermal receptors. Type 1 receptors, found in insects, are thought to be adapted from hair mechanoreceptors (Schmitz et al., 1997; Vondran et al., 1995). They contain a single sensory dendrite suspended at the bottom of a pressurized fluid-filled chamber. IR absorption and conversion to heat by the organ increases its internal pressure which is detected by stretch-gated ion channels (possibly TRPs) via physical deflection of the dendrite. In Type 2 IR organs found in both insects and snakes (Gracheva et al., 2010; Schmitz et al., 2002), a thin layer of cuticle (which appears circular in shape from the outside) covers an air filled pit. This cover internally contains a large multipolar neuron (Schmitz et al., 2000; Schmitz et al., 2001), and the air-filled pit below is open to the outside by a narrow slit between the cuticular cap and the rest of the body. The cap is attached by a single pedicel and contains the main branches of the cap sensor neurons. When IR light is absorbed by the cuticular cap, the increase in temperature of the cap is detected by thermal sensors in the cap sensory dendrites. The IR receptor protein detecting the temperature change is a transient receptor potential cation channel, subfamily A, member 1, or TRPA1 only studied so far in snakes (Gracheva et al., 2010). The air-filled cavity below the cuticular cap is thought to increase the sensitivity of the organ by enhancing thermal insulation and reducing thermal mass of the cap (Schmitz et al., 2002).
The HO in ticks is composed of two main areas, an anterior pit (AP) and a proximal capsule (Cp) (Fig. 1B). In adult male and female D. variabilis, there are 6 sensilla in the AP, which is the same number found in the AP of adult blacklegged ticks, Ixodes scapularis, and the meadow tick, Dermacentor reticulatus (Buczek et al., 2002; Homsher et al., 1975). In contrast, adult lone star ticks, Amblyomma americanum, and several soft tick species have 7 sensilla in their AP. The sensilla in the AP of both male and female American dog ticks appeared to be a mixture of different types of chemosensory sensilla similar to that described in A. americanum (Foelix et al., 1972). The capsule (Fig. 1B-D) is approximately 125 microns (μ) front to back, 75 μ along the long axis of the leg (Fig. 1B-C) and with a pit below 30-40 μ in depth in D. variabilis. The pit cover has a thin, jagged, aperture approximately 60 μ long and 1-3 μ wide (Fig. 1B-C). The capsule pit contains sensilla (not visible in Fig. 1) surrounded by dozens of cuticular projections of differing morphology called pleomorphs, which are much more easily seen in species whose capsule is partly or completely open (Klompen et al., 1993; Roshdy et al., 1972). In I. scapularis, the capsule has a rounded larger opening which shows a single central projection from the pit bottom (Homsher et al., 1975). In the soft tick, Ornithodoros rostratus, there is no visible cover and the pit is filled with hundreds of long, thin pleomorphs that morphologically resemble branching marine corals or filiform papillae found on the surface of the human tongue under high magnification (Klompen et al., 1993).
The morphology of the American dog tick capsule appears morphologically to be a hybrid between Type 1 and Type 2 receptors described in insects and snakes. The D. variabilis capsule is similar externally to the Type 2 IR receptor organ found on the fore coxae of the Australian ‘little ash beetle’, Acanthocnemus nigricans. In this beetle, like in snakes, an innervated disk overlays an air-filled cavity, absorbs IR radiation, and the increase in cuticular temperature is measured by temperature receptors within the disk. The circular cap in insects and snakes appears as a slit in the American dog tick. Whether the slit cover is innervated in D. variabilis has never been investigated. The capsule pit in D. variabilis may be fluid-filled like Type 1 receptors. Histological studies show there are secretory cells associated with the American dog tick capsule pit which would release their products directly into the pit space (Balashov, 1979). They differ from Type 1 IR organs in that the pore leading from the fluid-filled pit to the outside appears as a slit in D. variabilis. We found a number of other sensory structures associated with the HO (shown with arrows, Fig. 1C-D). The Type 1 IR organs in insects are 170-320 μ by 80-150 μ in size on the outside and 17-100 μ in depth. Type 2 IR organs in insects are 150-180 μ across. So the structures indicated by the arrows (approximately 2 μ) are likely not involved in IR detection based on their size. However, we cannot completely rule out a possible role in IR detection based on their size alone, and further research is needed. In some cases like in Fig. 1B-C, the structures are similar in external appearance to auricular or companiform sensilla. Companiform sensilla have traditionally been linked with mechanosensation including functioning as stretch receptors (Beadle, 1973; Obenchain et al., 1982; Schmitz et al., 2000). These structures (Fig. 1B-D) may also be involved in the detection of humidity (Obenchain et al., 1982; Vondran et al., 1995; Woolley, 1972). The best candidate for IR detection in ticks is the capsule area of the HO based on its sharing similarities with both Type 1 and Type 2 IR detection organs in insects and snakes.
3.3. Molecular clues for IR detection in ticks
Snakes are the only organisms so far where specific IR receptor proteins (and not just the proposed mechanism of IR detection) were characterized at the molecular level. The IR receptor protein detecting temperature change in snakes is a transient receptor potential cation channel, subfamily A, member 1, or TRPA1 (Gracheva et al., 2010). This TRP superfamily of ion channels plays important roles in various sensory functions in a wide variety of animal species, from vision and hearing to taste and mechanosensation. TRPs are 6-transmembrane cation-permeable channels that mediate the entry of positively charged ions like sodium, calcium, and magnesium. TRPs contain ankyrin repeats composed of 33 amino acids that are organized into α-helices connected by β-hairpin motifs. These ankyrin repeats, which vary in number between TRP types and different animal species are likely involved in protein-protein interactions and appear to play a critical role in their sensitivity to a wide variety of stimuli. TRPs are categorized into 8 distinct sub-families in metazoans, and we identified representatives of several of these sub-families in our HO-specific transcriptome. TRPA1 can function as a stress receptor but has also been associated with temperature sensitivity and more recently IR detection (Gracheva et al., 2010; Nilius et al., 2012; Paulsen et al., 2015; Ramsey et al., 2006).
We found contigs in our HO-specific transcriptome from Illumina sequencing that aligned to several different types of TRP, including two contigs (contig 66838 and contig 70248) that aligned to putative TRPA1s from both I. scapularis, and A. aureolatum. In Table 1, the top 5 hits based on e-value are listed for both of our HO-specific contigs along with data points describing where the strongest alignments occurred. Contig 66838 was 98% similar to a putative TRPA1 in A. aureolatum (top hit with e-value 1e-124) where 174 amino acids of our query sequence matched a 178 amino acid stretch of the subject sequence. The next four hits were I. scapularis (blacklegged tick), C. borealis (Jonah crab), H. americanus (American lobster), and L. anatina (duck mussel) with e-values of 8e-82, 1e-55, 3e-53 and 5e-51, respectively. Contig 70248 was 91% similar to a putative TRPA1 in A. aureolatum (top hit with e-value 8e-59) where 101 amino acids of our query sequence matched a 111 amino acid stretch of the subject sequence. The next four hits were I. scapularis, C. borealis, S. mimosarum (velvet spider), and P. tepidariorum (common house spider) with e-values of 2e-43, 2e-21, 3e-21 and 7e-21, respectively.
Our HO-specific contigs also aligned with Crotalus atrox, the western diamondback rattlesnake, and other pit-bearing snake species studied by Gracheva et al. (2010) in a region immediately following the conserved ankyrin repeats associated with all TRPs. At the bottom of Table 1 we show an illustration of the alignment performed with the MUSCLE algorithm (Edgar, 2004) showing where our contigs aligned with the snake and other tick species at the amino acid level whose TRPA1s (full or partial) have been identified. This alignment with the snake TRPA1 is significant because a functional relationship has been established between the receptor and IR detection. This relationship has not been established in insects. Contig 66838 has a 35% identity and an e-value of 4e-35 when compared to the C. atrox complete TRPA1. Contig 70248 has a 37% identity and an e-value of 5e-5 when compared to the C. atrox complete TRPA1. The A. aureolatum putative TRPA1 (accession number JAT98721.1) that aligns with both of our putative TRPA1 contigs has a 28% identity and an e-value of 1e-115 when compared to the C. atrox complete TRPA1. The I. scapularis putative TRPA1 (accession number XP_002434584.1) that aligns with both of our putative TRPA1 contigs has a 28% identity and an e-value of 6e-28 when compared to the C. atrox complete TRPA1. Identity is defined as, “the extent to which two (nucleotide or amino acid) sequences have the same residues at the same positions in an alignment, often expressed as a percentage (Fassler et al., 2011).” Our analysis suggests that the HO contains TRPA1 that may be responsible for IR detection in ticks similar to the function of TRPA1 in several pit-bearing snake species. However, longer contiguous sequence data are needed for these putative TRPA1s from the HO for a more definitive functional assignment.
4. Conclusion
In summary, herein we presented evidence that demonstrates that adult D. variabilis is positively phototactic to IR light and that the organ for IR detection is found exclusively on the front tarsi. Morphological comparisons of the HO on the tarsi of the front legs of American dog ticks to IR receptors in insects and snakes suggest the capsule area of the HO might be responsible for the tick IR detection. A putative TRPA1 transcript was found in an American dog tick HO-specific transcriptome which was similar to the TRPA1 receptor in the snake, C. atrox, which was shown to be involved in IR detection. Tick attraction to IR by the American dog tick adult suggests this could be a mechanism for host location and/or selection of feeding sites on the host; more studies are needed to better understand the importance of IR detection in tick biology. Since D. variabilis adults were positively phototactic to light, an obvious practical application is the use of light attraction for tick trapping. The advantage of the use of IR versus white light attraction, the former would limit trapping only to animals that can detect and are attracted to IR.
Supplementary Material
Highlights.
The Haller's organ is not only a chemosensory organ; D. variabilis uses it to see.
American dog ticks use their Haller's organs (HOs) to perceive infrared (IR) light.
American dog ticks use their ocelli to perceive visible light.
TRPA1, responsible for IR detection in insects and snakes, is found in the D. variabilis HO.
Acknowledgments
This work was funded by grants to RMR and DES from NIH (1R21AI096268) and NSF (IOS-0949194). RM and AC were also supported in part by a Graduate Student Teaching Assistantship from the Department of Entomology at North Carolina State University.
Abbreviations
- HO
Haller's organ
- Oc
ocelli
- AP
anterior pit
- Cp
capsule
- IR
infrared
- TRPA1
transient receptor potential cation channel, subfamily A, member 1
- SEM
scanning electron microscopy
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at (insert video destination here).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balashov Yu S., editor. Atlas of the electron microscopic anatomy of ixodid ticks. Nauka Publishers; Leningrad: 1979. [Google Scholar]
- Beadle D. Muscle attachment in the tick, Boophilus decoloratus Koch (Acarina: Ixodidae) International Journal of Insect Morphology and Embryology. 1973;2:247–255. [Google Scholar]
- Biggs HM. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recommendations and Reports. 2016;65 doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- Bruce WA. Perception of infrared radiation by the spiny rat mite Laelaps echidnina (Acari: Laelapidae) Annals of the Entomological Society of America. 1971;64:925–931. [Google Scholar]
- Buczek A, Buczek L, Kusmierz A, Olszewski K, Jasik K. Acarid Phylogeny and Evolution: Adaptation in Mites and Ticks. Springer; Netherlands: 2002. Ultrastructural investigations of Haller's organ in Dermacentor reticulatus (Fabr.)(Acari: Ixodida: Ixodidae) pp. 227–231. [Google Scholar]
- Calhoun EL. Natural occurrence of tularemia in the lone star tick, Amblyomma americanum (Linn.), and in dogs in Arkansas. The American journal of tropical medicine and hygiene. 1954;3(2):360–366. doi: 10.4269/ajtmh.1954.3.360. [DOI] [PubMed] [Google Scholar]
- Carr AL. Doctor of Philosophy. North Carolina State University; 2015. Profiling of acarine attractants and chemosensation. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Rocky mountain spotted fever (RMSF): symptoms, diagnosis, and treatment. [accessed 16.10.16];2010 https://www.cdc.gov/rmsf/index.html.
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WG. Infra-red receptors in Melanophila acuminata DeGeer. Nature. 1964;202:211–211. doi: 10.1038/202211a0. [DOI] [PubMed] [Google Scholar]
- Fassler J, Cooper P. BLAST glossary. [accessed 16.12.30];2011 https://www.ncbi.nlm.nih.gov/books/NBK62051/
- Foelix RF, Axtell RC. Ultrastructure of Haller's organ in the tick Amblyomma americanum (L.) Z Zellforsch Mikrosk Anat. 1972;124:275–292. doi: 10.1007/BF00355031. [DOI] [PubMed] [Google Scholar]
- Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller G. Vorlaufige bemerkungen uber das gehororgan der Ixodiden. Zool Anz. 1881;4:165–166. [Google Scholar]
- Homsher PJ, Sonenshine DE. Scanning electron microscopy of ticks for systematic studies: Fine structure of Haller's organ in ten species of Ixodes. Transactions of the American Microscopical Society. 1975:368–374. [PubMed] [Google Scholar]
- Ivanov VP, Leonovich SA. Chapter VIII. Sensory organs. In: Balashov Yu S., editor. An Atlas of Ixodid Tick Ultrastructure. Nauka Publishers; Leningrad: 1983. English translation, Entomological Society of America, Special Publication. [Google Scholar]
- Klocke D, Schmitz A, Soltner H, Bousack H, Schmitz H. Infrared receptors in pyrophilous (“fire loving”) insects as model for new un-cooled infrared sensors. Beilstein journal of nanotechnology. 2011;2:186–197. doi: 10.3762/bjnano.2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompen JS, Oliver JH., Jr Haller's organ in the tick family Argasidae (Acari: Parasitiformes: Ixodida) J Parasitol. 1993;79:591–603. [PubMed] [Google Scholar]
- Lahille F. Contribution à l'étude des Ixodidés de la République Argentine. Printing Bureau of the Meteorological Office; Argentina: 1905. [Google Scholar]
- Lees A. The sensory physiology of the sheep tick, Ixodes ricinus L. Journal of Experimental Biology. 1948;25:145–207. [Google Scholar]
- Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflügers Archiv-European Journal of Physiology. 2012;464:425–458. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- Nuttall GHF, Cooper WF, Robinson LE. On the structure of “Haller's Organ” in the Ixodoidea. Parasitology. 1908;1:238–242. [Google Scholar]
- Obenchain FD, Galun R, editors. Physiology of Ticks: Current Themes in Tropical Science. Vol. 1. Pergamon Press; Oxford, United Kingdom: 1982. [Google Scholar]
- Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Roshdy MA, Foelix RF, Axtell RC. The subgenus Persicargas (Ixodoidea: Argasidae: Argas). 16. Fine structure of Haller's organ and associated tarsal setae of adult A. (P.) arboreus Kaiser, Hoogstraal, and Kohls. J Parasitol. 1972;58:805–816. [PubMed] [Google Scholar]
- Schmitz H, Bleckmann H. The photomechanic infrared receptor for the detection of forest fires in the beetle Melanophila acuminata (Coleoptera: Buprestidae) Journal of Comparative Physiology A. 1998;182:647–657. [Google Scholar]
- Schmitz H, Bleckmann H, Mürtz M. Infrared detection in a beetle. Nature. 1997;386:773. [Google Scholar]
- Schmitz H, Schmitz, Bleckmann H. A new type of infrared organ in the Australian “fire-beetle” Merimna atrata (Coleoptera: Buprestidae) Naturwissenschaften. 2000;87:542–545. doi: 10.1007/s001140050775. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Schmitz A, Bleckmann H. Morphology of a thermosensitive multipolar neuron in the infrared organ of Merimna atrata (Coleoptera, Buprestidae) Arthropod Structure & Development. 2001;30:99–111. doi: 10.1016/s1467-8039(01)00028-7. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Schmitz A, Trenner S, Bleckmann H. A new type of insect infrared organ of low thermal mass. Naturwissenschaften. 2002;89:226–229. doi: 10.1007/s00114-002-0312-4. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, Homsher PJ, Carson KA, Wang VD. Evidence of the role of the cheliceral digits in the perception of genital sex pheromones during mating in the American dog tick, Dermacentor variabilis (Acari: Ixodidae) Journal of medical entomology. 1984;21:296–306. doi: 10.1093/jmedent/21.3.296. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, Roe RM, editors. Biology of Ticks. I. Oxford University Press; New York: 2014. [Google Scholar]
- Sonenshine DE, Roe RM, editors. Biology of Ticks. II. Oxford University Press; New York: 2014. [Google Scholar]
- Spach DH, Liles WC, Campbell GL, Quick RE, Anderson DE, Jr, Fritsche TR. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936–947. doi: 10.1056/NEJM199309233291308. [DOI] [PubMed] [Google Scholar]
- Steiert JG, Gilfoy F. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector Borne and Zoonotic Diseases. 2002;2(2):53–60. doi: 10.1089/153036602321131841. [DOI] [PubMed] [Google Scholar]
- Vondran T, Apel KH, Schmitz H. The infrared receptor of Melanophila acuminata De Geer (Coleoptera: Buprestidae): ultrastructural study of a unique insect thermoreceptor and its possible descent from a hair mechanoreceptor. Tissue Cell. 1995;27:645–658. doi: 10.1016/s0040-8166(05)80020-5. [DOI] [PubMed] [Google Scholar]
- Woolley TA. Some sense organs of ticks as seen by scanning electron microscopy. Transactions of the American Microscopical Society. 1972;91(1):35–47. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


