Abstract
The Virtual Pediatric Airways Workbench (VPAW) is a patient-centered surgical planning software system targeted to pediatric patients with airway obstruction. VPAW provides an intuitive surgical planning interface for clinicians and supports quantitative analysis regarding prospective surgeries to aid clinicians deciding on potential surgical intervention. VPAW enables a full surgical planning pipeline, including importing DICOM images, segmenting the airway, interactive 3D editing of airway geometries to express potential surgical treatment planning options, and creating input files for offline geometric analysis and computational fluid dynamics simulations for evaluation of surgical outcomes. In this paper, we describe the VPAW system and its use in one case study with a clinician to successfully describe an intended surgery outcome.
Keywords: Surgery planning, virtual reality, 3D modeling
1. Introduction
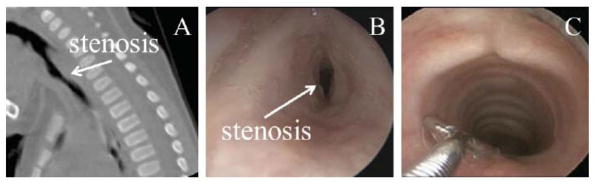
Airway problems in young children may cause life threatening respiratory difficulties, poor growth, aspiration, delay in speech development and long-term, debilitating illness. One common airway problem is subglottic stenosis, or narrowing of the airway below the vocal cords. An example of a subglottic stenosis is shown in Figure 1.
Figure 1.
Subglottic stenosis in one patient. A: CT sagittal slice showing stenosis. B: pre-operative laryngoscopy. C: 1-year post-operative laryngoscopy.
Treatment of children with subglottic stenosis may frequently be directed by factors other than just the severity of the stenosis, such as the clinician’s experience and preference and other institutional factors, rather than by published guidelines or quantitative airway measures. Improved, quantitative methods of evaluating treatment options hold the potential to improve patient care. Software tools that quantify airway pathology and potential virtually reconstructed airways may improve care of these children by indicating which intervention, if any, is warranted.
The Virtual Pediatric Airways Workbench (VPAW) is a comprehensive surgical planning software program that incorporates several airway computational modeling components. Starting from a CT image, VPAW can segment the airway with minimal user input. An editable geometric model can be derived from this segmentation. Free-form geometric editing is accomplished using 3D input devices and built-in sculpting tools. Several candidate geometries may be created for a single patient reflecting individual intended outcomes of different surgeries or different variations of a particular surgery. These sculpted candidate geometries can be evaluated via geometry-based comparisons to an atlas of normal airways and via computational fluid dynamics (CFD) simulations of a breathing cycle. Results of these comparisons may be used for quantitative insight into the different outcomes of potential interventions.
2. Methods
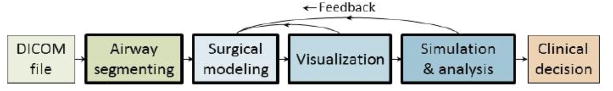
VPAW is a desktop program that uses the Visualization Toolkit (VTK) [1] interactive for visualization, the insight Toolkit [2] for image segmentation and editing, and the Virtual Reality Peripheral Network [3] to interface with 3D input devices. VPAW features four main components used in a surgical planning workflow as depicted in Figure 2: segmentation, interactive surgical modeling, visualization, and simulation.
Figure 2.
Surgical planning workflow using the segmentation, surgery modeling, visualization, and simulation components of VPAW (bold outline). Feedback from visualization, airflow simulation, and geometric analysis may be used to guide the surgical modeling.
2.1. Segmentation
The airway segmentation algorithm used in VPAW is based on the algorithm in Hong, et al. [4]. An initial Otsu thresholding on a CT scan identifies a Hounsfield value that separates air from tissue. The tissue portion is morphologically closed to create a mask of the patient’s head with the airway filled in, then an XOR operation is applied to the original threshold image and this head mask. The airway is identified as the largest connected component in the result. A second Otsu thresholding on the original CT with only voxels in the slightly dilated airway mask yields the airway.
2.2. Interactive Surgical Modeling
To mimic the operation of tissue removal from cold steel, debriders, or lasers used in endoscopic airway surgery, VPAW provides a spherical brush tool with adjustable radius that removes virtual tissue along the airway. This brush can be used to express the final intended geometric result obtained from open airway surgeries. 3D input devices, such as the Geomagic Touch X, can be used to control the 3D position of the brush. Simulated tissue may also be added to the geometry to express more complex surgeries. The spherical brush does not aim to simulate a real surgery, but rather serves as a sculpting tool to express an intended airway geometry resulting from a surgery.
VPAW represents airway geometry with a 3D grayscale image. Surfaces used for visualizing the airway are extracted from the grayscale image representation using a 3D contouring algorithm in VTK [1]. The grayscale image representation is initialized by masking the original CT image with the airway segmentation dilated morphologically by one voxel. This ensures that the grayscale image contains a layer of tissue so that the contouring algorithm can extract a smooth surface using trilinear interpolation.
Brush operations are implemented as modifications to the grayscale image. There are several advantages to this approach over direct modification of a polygonal surface mesh. First, topological changes to the airway geometry do not require any special treatment. Second, this approach avoids any need for self-intersection tests and special handling that are needed with direct modification of a polygonal mesh. Finally, no special remeshing steps are needed to handle modifications that cause large geometry distortions. Instead, local remeshing is accomplished with the contour algorithm used to extract the geometry from the grayscale image.
A 3D brush operation modifies voxels within the brush boundaries. Because Hounsfield values for air are less than those of tissue, increasing the volume of air inside the airway involves setting voxels in the image representation to values less than the air/tissue threshold value. Brush editing operations for opening the airway geometry involve setting each voxel value within the brush’s influence region to the minimum of a value from a brush-associated function and the current voxel value.
A brush-associated function could be as simple as setting voxels within the brush boundaries to a value less than the air/tissue boundary value and a larger value outside the brush. However, to maintain smoothness of the geometry presented to the editor, we do two things: 1) extend the range of influence of the brush to several voxels outside the brush, and 2) define a distance-based linear brush function such that the value at the center of the brush is similar to air, the value at the radius of the brush is the air/tissue threshold value, and values not at these points vary linearly with distance from the center. Defining the brush this way sets grayscale values in the geometry image so that the trilinear interpolation used by the contouring algorithm recovers the intended airway geometry bounds created by editing.
Extracting surface geometry from a large grayscale image via a contouring algorithm is computationally intensive, making it impractical to extract the entire surface after every edit. Instead, VPAW employs a grid-based subdivision approach to make interactive editing possible. The grayscale image representing the airway geometry is subdivided into 8 x 8 x 8 = 512 blocks. When an editing event occurs, only the portions of the surface geometry in the blocks that are within the influence region of the brush are updated. With a typical brush size, only a few blocks need updating which greatly reduces the time required to update the contour.
2.3. Visualization
VPAW incorporate best-known practices for conveying 3D shape information. This approach intentionally does not attempt to produce photorealistic imagery. Instead, it uses visualization techniques to increase viewer understanding of 3D shapes.
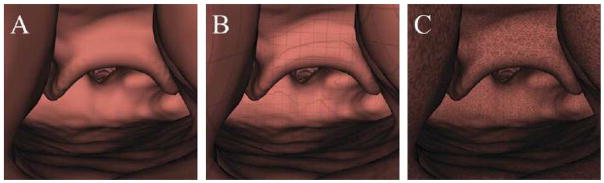
Stereo display, which effectively conveys depth information, is enabled in VPAW via consumer hardware. Two optional surface textures may be added to the otherwise solid-color surface display (Figure 3(a)). to improve shape perception in both mono and stereo display [5]. One texture is a grid pattern with lines spaced 1 millimeter apart to serve as an approximate ruler for estimating local feature sizes (Figure 3(b)). The other texture (Figure 3(c)) consists of high-frequency Perlin noise. Users initially disliked the noise texture because it did not look realistic, but over time felt that it provided the best shape perception. Surface texture is implemented with 3D textures set that repeat in all dimensions, and texture coordinates are set to the physical coordinates of the points.
Figure 3.
Available airway surface texturing modes that can be applied in VPAW. A: Semi-realistic solid surface. B: 1mm grid texture for approximate measuring of anatomical features. C: High-frequency Perlin noise texture for improved shape perception.
Aside from visual display, VPAW provides haptic touch of surface geometry in 3D devices with force feedback, such as the Geomagic Touch X. This provides tactile feedback that has proven useful for placing anatomical landmarks and for placing endpoints of a measuring tool. For example, touch can be used to identify the narrowest portion of a subglottic stenosis. While this particular structure can be readily visualized, the haptic feedback aids in the identification of this landmark.
To aid in communicating aspects of a patient’s airway to other clinicians, VPAW can create flythrough animations. A flythrough path is defined by creating a set of keyframes that store camera poses. The camera poses are interpolated between keyframes to produce smooth camera movement along the camera path.
2.4. Simulation and Analysis
Prior work shows that geometric and CFD-based analysis of airway geometry can be useful for determining which patients with subglottic stenosis require surgical intervention [6]. As a corollary, this analysis can be used to decide if an intended modeled surgery may be effective. VPAW provides mechanisms to export edited geometries for offline geometric and CFD-based analysis. Candidate geometries may be compared to an atlas of normal airways to determine if the outcome leads to a geometrically normal airway [4]. Candidate geometries can also be exported to files for a Lattice-Boltzmann fluid solver that has been accelerated with NVIDIA’s CUDA platform to run on graphics hardware accelerators for fast analysis turnaround time. VPAW extends the airway segmentations in these files to include a spherical region of air outside the nose, the surface of which provides a boundary condition so that airflow through the nostrils is taken into account.
3. Results
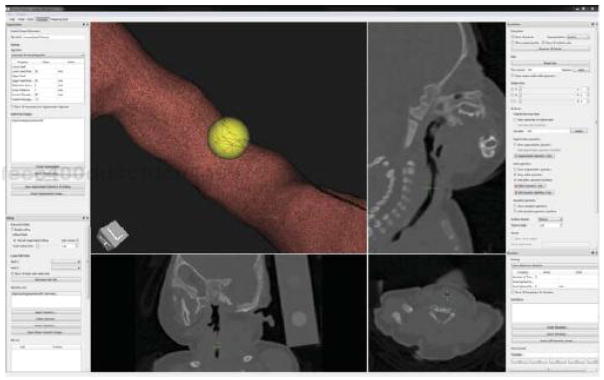
VPAW’s user interface (Figure 4) features a 3D view of the airway model loaded for editing. Within this view, tools such as the spherical brush, measuring stick, and annotation tool are also optionally displayed. Three additional views show axial, sagittal, and coronal planes from the CT image. These planes are updated to follow the center of the active tool.
Figure 4.
VPAW’s user interface. The airway surface (upper left) is displayed with a cutaway view where camera-facing portions are displayed as a wireframe and other portions are a solid textured surface. The center position of a 2mm spherical brush (yellow sphere) is displayed with green crosshairs in the three orthogonal views (top right and bottom).
As a case study, VPAW has been used retrospectively to describe the airway geometry intended in a surgery performed on a 9-month-old patient with subglottic stenosis. The clinician who performed the actual surgery used VPAW to model the results of the surgery as described in the operative note. The result of the surgical modeling was then compared to a follow-up CT scan taken approximately one year after surgery. Figure 5 shows the airway geometries in this retrospective study.
Figure 5.
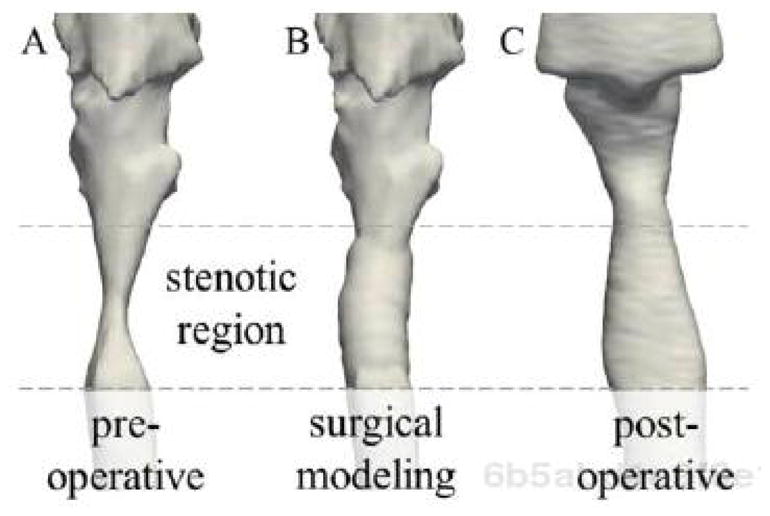
Comparison of airway geometry at stenotic region viewed externally from anterior. A: pre- operative airway when patient was 9 months old. B: intended surgical outcome described with VPAW. C: post-operative airway from follow-up CT scan obtained at 20 months.
We computed the cross-sectional areas at the narrowest part of the stenotic region in the pre-surgery airway geometry (5.4 mm2), the surgically modeled geometry (24.6 mm2), and the geometry from a one-year post operative follow-up CT scan (26.9 mm2). These results show that the modeled surgery compares favorably to the follow-up scan. The larger airway size in the one-year post-operative scan in the stenotic region is likely a result of patient growth, but may represent underestimation of the actual airway expansion achieved during surgery. In any case, the modeled results are similar to the actual outcome (minus the probable effects of growth), showing VPAW can be used to model a real-world surgical outcome.
4. Discussion
We developed VPAW to perform patient-specific surgical planning for pediatric patients with airway obstruction. While VPAW was developed for pediatric patients with airway obstruction, it could also be utilized for surgical modeling of adult patients’ airway obstruction, and may be useful for planning in other types of surgery such as voice, aspiration, colon polyp removal, and bone reshaping.
Acknowledgments
We gratefully acknowledge support from NIH/NHLBI grant 5R01HL105241 and NIH/NIDCR grant R43DE024334.
References
- 1.Schroeder WJ, Avila LS, Marlin KM, Hoffman W, Law C. The Visualization Toolkit User’s Guide. Kitware, Inc; Clifton Park, NY: 2001. [Google Scholar]
- 2.Johnson HJ, McCormick MM, Ibanez LI. The ITK Software Guide Book 1: Introduction and Development Guidelines. I. Kitware, Inc; Clifton Park, NY: 2015. [Google Scholar]
- 3.Taylor RM, II, Hudson TC, Seeger A, Weber H, Juliano J, Helser AT. VRPN: a device-independent, network-transparent VR peripheral system. Proceedings of the ACM symposium on virtual reality software and technology (VRST ‘01); New York, NY, USA: ACM; 2001. pp. 55–61. [Google Scholar]
- 4.Hong Y, Niethammer M, Johan A, Kimbell J, Pitkin E, Superfine R, Davis SD, Zdanski CJ, Davis B. A pediatric airway alias and its application to subglottic stenosis. Proceedings of the International Symposium on Biomedical Imaging (ISBI); pp. 1206–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ware C. Information Visualization: Perception for Design. 3. Morgan Kaufmann; New York: 2012. [Google Scholar]
- 6.Zdanski CJ, Davis SD, Hong Y, Miao D, Quammen C, Mitran S, Davis B, Niethammer M, Kimbell JS, Pitkin E, Fine J, Fordham L, Vaughn B, Superfine R. Quantitative assessment of the upper airway in infants and children with subglottic stenosis. The Laryngoscope. 2015 Jul 30; doi: 10.1002/lary.25482. [DOI] [PMC free article] [PubMed] [Google Scholar]