Abstract
Background
Firefighters have increased risk of cardiovascular disease and of sudden death from coronary heart disease on duty while suppressing fires. This study investigated the effect of firefighting activities, using appropriate personal protective equipment (PPE), on biomarkers of cardiovascular effects in young conscripts training to become firefighters.
Methods
Healthy conscripts (n = 43) who participated in a rescue educational course for firefighting were enrolled in the study. The exposure period consisted of a three-day training course where the conscripts participated in various firefighting exercises in a constructed firehouse and flashover container. The subjects were instructed to extinguish fires of either wood or wood with electrical cords and mattresses. The exposure to particulate matter (PM) was assessed at various locations and personal exposure was assessed by portable PM samplers and urinary excretion of 1-hydroxypyrene. Cardiovascular measurements included microvascular function and heart rate variability (HRV).
Results
The subjects were primarily exposed to PM in bystander positions, whereas self-contained breathing apparatus effectively abolished pulmonary exposure. Firefighting training was associated with elevated urinary excretion of 1-hydroxypyrene (105%, 95% CI: 52; 157%), increased body temperature, decreased microvascular function (−18%, 95% CI: -26; −9%) and altered HRV. There was no difference in cardiovascular measurements for the two types of fires.
Conclusion
Observations from this fire extinction training show that PM exposure mainly occurs in situations where firefighters removed the self-contained breathing apparatus. Altered cardiovascular disease endpoints after the firefighting exercise period were most likely due to complex effects from PM exposure, physical exhaustion and increased core body temperature.
Electronic supplementary material
The online version of this article (10.1186/s12940-017-0303-8) contains supplementary material, which is available to authorized users.
Keywords: Cardiovascular disease, Firefighter, Ultrafine particles
Background
Firefighters have high risk of on-duty death due to cardiovascular diseases, whereas the life time risk is similar to the general population [1]. It has been shown that deaths from coronary heart disease were most frequent among firefighters who were actively engaged in suppressing fires, whereas those with non-emergency duties had the lowest mortality among on-duty firefighters [2]. The excess mortality has been attributed to various factors such as smoke, physical exhaustion, hyperthermia, dehydration and mental stress. Controlled studies of 3 h during fire extinction showed that firefighters had decreased left ventricular contractility and stroke volume, tachycardia and increased microvascular vasodilation within the first 30 min after cessation of the activities [3, 4]. Several studies have demonstrated that exposure to heat, associated with increased body temperature, increases the peripheral arterial compliance, shear stress and blood flow [5, 6]. Exercise also increases the body temperature and evokes a number of hemodynamic changes, including vasodilation [7]. Above all, these results demonstrate an immediate and possibly transient effect of exercise and increased body temperature on the cardiovascular physiology.
Exposure to particulate matter (PM) from combustion of carbon-based materials such as fossil fuels is associated with increased risk of morbidity and mortality of cardiovascular diseases [8]. Firefighters may be exposed to PM when they remove their self-contained breathing apparatus while not actively engaged in fire suppression activities. Bystander exposure to smoke can therefore occur and diesel exhaust from fire trucks or pumps operated by firefighters may constitute additional sources of PM exposure. A meta-analysis of epidemiological studies has shown an inverse relationship between exposure to particulate air pollution and heart rate variability (HRV) [9]. Likewise a number of studies have documented associations between exposure to PM and cardiovascular disease endpoints such as vasomotor dysfunction and progression of atherosclerosis in animal models and humans [10, 11].
The chemical composition of the smoke varies substantially from one fire to another. Fires in urban settings typically give rise to very complex mixtures because of the combustion of household equipment, whereas combustion of wood can be considered as a more “clean” type of smoke. Studies on controlled exposure to wood smoke have indicated little effect on microvascular vasomotor function [12, 13], whereas HRV was decreased [14]. To the best of our knowledge, no studies have assessed biomarkers for cardiovascular disease after controlled exposure to more complex fuels than wood, such as plastic or household materials.
The aim of the present study was to assess whether firefighting activities, using appropriate personal protective equipment (PPE), were associated with cardiovascular effects in young subjects training to become firefighters. The subjects participated in smoke diving exercises to supress wood fires with or without additional items that occur in “real” fires (i.e. electrical cords and mattresses). Markers of cardiovascular function and risk factors included vasomotor function measurements by reactive hyperemia index (RHI) and cardiac autonomic nervous system regulation by HRV. Personal exposure to polycyclic aromatic hydrocarbons (PAH) was assessed by urinary excretion of 1-hydroxypyrene (1-OHP), which is a widely used biomarker of exposure to combustion products in environmental and occupational settings [15]. Biomarkers of cardiovascular risk obtained after the firefighting exercise were compared to control measurements performed 2 weeks before and 2 weeks after the firefighting course, respectively.
Methods
Subjects
The subjects were healthy conscripts who participated in a rescue specialist educational course, a nine-month education under the Danish Emergency Management Agency in 2015 and 2016. Self-reported pregnancy, smoking, and drug or alcohol misuse were exclusion criteria. Fifty-four subjects were enrolled in the study in four different campaigns. One female subject dropped out of the education and cardiovascular endpoints were not measured from additional 10 subjects for logistic reasons (5 subjects in each of the campaigns 3 and 4). Consequently, the final study population consisted of 32 males and 11 females. The subjects were recruited from four consecutive training classes (campaigns): campaign 1) covered 8 conscripts in the summer; 2) 11 conscripts, autumn; 3) 17 conscripts, winter; and 4) 17 conscripts, spring. Table 1 shows the characteristics of the subjects. The distribution of female subjects between campaigns varied from 17 to 36%. The age of the participants varied from 18 to 26 years. Seventy-two percent of the subjects had a body mass index (BMI) between 18.5 and 24.9 kg/m2 and 28% of the subjects had BMIs between 25 and 30 kg/m2.
Table 1.
Characteristics of the subjects
| Characteristic | Male (n = 32) | Female (n = 11) | Total (n = 43) |
|---|---|---|---|
| Age (years) | 21.0 ± 1.3 | 21.5 ± 2.1 | 21.1 ± 1.6 |
| Height (cm)a | 181.4 ± 6.7 | 172.1 ± 3.2 | 179.0 ± 7.2 |
| Weight (kg)a | 78.3 ± 11.4 | 67.6 ± 10.0 | 75.6 ± 11.9 |
| BMI (kg/m2) | 23.7 ± 2.6 | 22.8 ± 3.0 | 23.5 ± 2.7 |
| Subjects with allergies (n)a | 10 | 3 | 13 |
| cBL.HR (bpm) | 65.8 ± 6.6 | 66.9 ± 7.4 | 66.0 ± 6.7 |
BMI body mass index, cBL.HR average baseline heart rate from the two control measurements. Values are number or mean ± SD
aSelf-reported information
Study protocol
The design was a human exposure study, where the participants were studied in three exposure scenarios, serving as their own controls. In each campaign, the blood sampling and physiological measurements after each exposure scenario were conducted at the same time of the day, separated by around 14 days with the exception of campaign 3 where only 7 days separated the second and third exposure scenario due to the Christmas holiday. During the first exposure scenario, subjects were in a classroom receiving theoretical information. During the second exposure scenario, the subjects participated in a 3-day smoke diving training program with various types of activities in a constructed firehouse and in a flashover container. The exercises increased in complexity as the participants acquired skills and they were wearing full PPE, including a self-contained breathing apparatus. In the third exposure scenario, the subjects were having another module component of their education unrelated to firefighting. The first and third scenarios were control measurements, whereas the second period was the exposure situation. We designed two different types of fires. The subjects supressed fires of standard wooden EUR pallets in absence (campaign 1 and 2) or presence (campaign 3 and 4) of foam mattresses and electrical cords. New material (one-third of a mattress and 2 m electrical cord) was added to the fires as each team of smoke divers entered the building. In total, during each day of the 3-day smoke-diving course, 6 mattresses and 20 m of electrical cords were burned. The foam mattresses were purchased in IKEA; they consisted of polyurethane (28 kg/m3) with a cover fabric (64% polyester and 36% cotton) and the weight of each mattress was 6 kg. A recycling station delivered the electrical cords.
Exposure assessment
The smoke exposure was assessed with various stationary and person-borne equipment for PM measurements that measured either the particle number or mass concentrations. The supplement contains further description of the exposure setting, including type and location of PM monitors. Personal exposure to PM was assessed immediately before, during and immediately after the fire extinction exercise for 3 subjects in the first campaign. It was not possible to obtain personal PM exposure for all subjects due to a limited number of personal monitors. We therefore focussed on determining whether PM exposure occurred when the subjects were wearing PPE, including self-contained breathing apparatus. We used the urinary excretion of 1-OHP as a biomarker of PAH exposure, whereas PAH is used as an exposure marker of PM and smoke. The subjects delivered morning urine samples on the measurement day for the control measurements and on the day after the exposure situation. The half-life of 1-OHP is 6–35 h [16], thus the 1-OHP measurement captures the exposure period, although exposures closest to the sampling contributes the most. Reverse-phase HPLC was used for the quantitative measurement of 1-OHP in urine using a previously published method [17]. We standardized for diuresis with the concentration of creatinine as used in other studies [15].
We assessed the impact of fire-related activities on the body temperature in an auxiliary experiment conducted during a smoke diving module course in 2016. The subjects performed smoke-diving exercises or acquired skills in a flashover container. Body temperature was recorded before, immediately after, and more than 20 min after fire-related activities using an ear thermometer (ThermoScan® 7, Braun GmbH, Kronberg, Germany). Two different activities were monitored: fire-suppression in the firehouse (7 to 10 min inside the firehouse with suppression or rescuing tasks to perform) and flashover container (30 min sitting inside a container with fire). It was not possible to organize a stringent exposure scenario due to logistic implications of the exercise, as some participants had to do fire extinction exercises several times or they hurried on to other exercises.
Cardiovascular measurements
RHI and HRV measurements were primary outcomes, which were measured non-invasively using the portable EndoPAT2000 (Itamar Medical Ltd., Israel) as previously described [18]. Briefly, finger-mountable pneumatic sensors were placed on the index fingers measuring pulse volume changes through three test stages: a baseline recording (6–7 min), a brachial arterial occlusion of one of the arms, induced by inflation of a blood pressure cuff to a supra-systolic pressure (5 min), and a post-occlusion recording of the induced reactive hyperemia response (5 min). Blood pressure measurements were done with a single measurement using one aneroid sphygmomanometer, before the peripheral arterial tonometry (PAT) measurement. From the baseline recording, the EndoPAT device determines the HRV based on measurement over 5 min. The HRV results include time domain measures (SDNN, pNN50 and RMSSD), high (HF) and low frequency (LF) components as well as the LF/HF ratio. Additionally the device determines the baseline heart rate (BL.HR) and the augmentation index (AI). All the measures were done in a quiet room with the subjects resting in a seated position. The measurements in the second exposure scenario were carried out between 20 min to 3 h after cessation of the fire extinction exercise.
Statistical analysis
We used R statistical language and the package lme4 [19] to perform a linear mixed effects analysis of the relationship between the cardiovascular endpoints and exposure. As fixed effects, we used factorial variables of exposure (before/exposure/after) and sex (male/female) and continuous variable of BMI (without interaction terms) into the model. The exposure term in the statistical analysis was either exposure period (i.e. one exposure and two non-exposure periods within each campaign) or type of fire (i.e. wood or wood with mattresses and electrical cords). Inclusion of campaign or the type of fire in the statistical analysis using the exposure period as predictor did not alter the size of the exposure-outcome relationship; thus we have reported results that have not been adjusted for effects related to campaigns. As random effects, we used by-subject intercepts. P-values were obtained with the function glht from multcomp [20]. The percent changes were obtained by dividing the estimate change with the intercept value from the mixed model graph line and multiplying with 100. As the RHI was expressed on a logarithmic scale, the percent change was obtained directly from the effect estimate using the expression: (expestimate − 1)*100. The biomarker of exposure was also analysed with the same mixed model function, using the creatinine-adjusted urinary 1-OHP concentration, sex and BMI as fixed effects. The analysis of the association between the fire extinction exercise and urinary excretion of 1-OHP demonstrated a skewed distribution of residuals. A cubic root transformation of the data and removal of one outlier did not change the statistical significance of the association; thus, we have reported the statistics of the non-transformed data. Welch t-test was used to compare the difference in means of effect change between exposed and unexposed scenarios between the different types of fire. Paired t-test was used to compare the mean body temperature difference between different exposure conditions. P-values <0.05 were considered statistically significant. Since many of the assessed biomarkers are inter-dependent, correction for multiple testing was not performed.
Results
Exposure to particulate matter
The PM exposure assessment showed that the PPE with the self-contained breathing apparatus very efficiently protected the conscripts from PM exposure by inhalation during fire-suppression activities. The mean particle number concentrations in the inhalation zone inside the self-contained breathing apparatus during fire suppression activities were less than 1000 particles/cm3 (Additional file 1: Table S2). We were unable to assess PM levels in the fire room, but at the floor landing above the fire extinction exercises, the total PM mass concentration was 32 mg/m3. The subjects were exposed to higher particle number concentrations in situations when they were not wearing the self-contained breathing apparatus. This occurred when they received instructions or feedback at locations that were considered as “safe zones”. The mean aerosol particle number concentrations in the inhalation zone varied substantially among the subjects when they were not wearing the self-contained breathing apparatus (50,000–250,000 particles/cm3). Further information on the exposure assessment is available in the supplemental material.
Urinary excretion of 1-hydroxypyrene
Figure 1 shows the creatinine-adjusted urinary 1-OHP concentrations in the three exposure scenarios (control measurement before, exposure and control measurement after). Results from 6 males were excluded due to missing data for the exposure measurement (n = 5) or for both control measurements (n = 1). The exposure during the fire extinction exercise increased the urinary excretion of 1-OHP by 105% (95% CI: 52,157%) based on the mixed effects model. The association was especially driven by campaign 2 (Additional file 1, Figure S10).
Fig. 1.
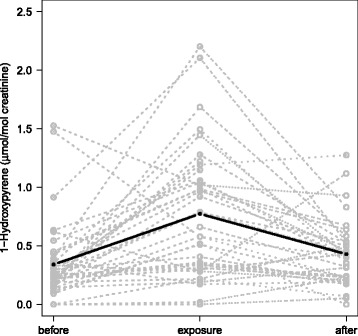
Creatinine-adjusted urinary concentration of 1-hydroxypyrene in three exposure scenarios (before and after as control measurements, and exposure measurement). Grey symbols and dashed lines are individual results in each subject. Black line is a graphical output of the mixed effect model
Effect of fire-suppression activity on the body temperature
The fire extinction exercise in the firehouse increased the body temperature (average increase = 1.1°C, 95% CI: 0.7, 1.4, n = 16, p < 0.001, paired t-test) immediately after the exercise. This was followed by an average decrease of 1.6°C (95% CI: -2.0, −1.1, n = 13, p < 0.001, paired t-test), compared to the temperature immediately after the exercise, measured at 60 min or more after the exercise. Following the flashover container exercise, we observed an average increase of 0.8 °C (95% CI: 0.6, 1.0, n = 8, p < 0.001, paired t-test) followed by an average decrease of 1.3 °C (95% CI: -1.8, −0.8, n = 7, p < 0.001, paired t-test), measured after 20 min and compared to the temperature immediately after the exercise. It should be noted that carryover effects cannot be ruled out as the subjects did both exercises on the same day in relatively close succession.
Cardiovascular measurements
Figure 2 presents the effect of exposure to firefighting on the cardiovascular endpoints. One female subject was eliminated from RHI analysis and one male subject was eliminated from HRV analysis, due to missing data for both control measurements. Exposure to firefighting was associated with decreased levels of RHI and time domain HRV. Table 2 presents the estimated changes for each of the cardiovascular measurements between different exposure scenarios showing a significant effect of exposure to firefighting as categorical variable on RHI, HRV both in time and frequency domains and in baseline heart rate. The mean baseline PAT signal amplitude was only modestly altered after the fire extinction exercise (change of −0.03%, p < 0.001). However adjustment for the baseline PAT signal in the statistical model did not substantially change the exposure-effect relationship of cardiovascular measurements (e.g. the percent change in RHI was decreased from −21.9% (95% CI: -32.0,-10.3) to −16.5% (95% CI: -26.1, −5.6). There was no significant difference between campaigns in the exposure-effect relationship for any of the cardiovascular measurements. There were no statistically significant relationship between LnRHI and HRV measurements and urinary 1-OHP excretion (Additional file 1: Table S6). Addition of information on self-reported allergies in the statistical model did not affect the exposure-effect relationship. Outcome average results for each exposure scenario are presented in Additional file 1: Table S5.
Fig. 2.
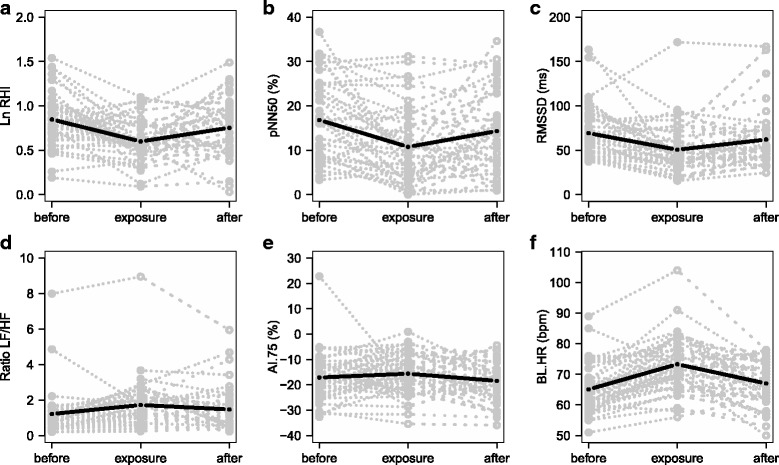
Cardiovascular endpoints in the three exposure scenarios (before and after as control measurements, and exposure measurement). Natural base log of reactive hyperemia index (one subject was eliminated due to missing data in both control measurements) (a), time domain heart rate variability in pNN50 (b) and RMSSD (c), frequency domain heart rate variability (d), augmentation index corrected for 75 bpm (e) and baseline heart rate (f). Grey symbols and dashed lines are individual results in each subject. Black line is a graphical output of the mixed effect model. pNN50, proportion of successive NN intervals differing by more than 50 milliseconds divided by total number of NN intervals; RMSSD, square root of the mean squared differences of successive NN intervals; bpm, beat per minute; ms, millisecond
Table 2.
Percent change (95% confidence interval) in outcome levels estimated by mixed effects model adjusted for sex and body mass index
| Outcome | Exposure vs Before | Exposure vs After | After vs Before | Exposure vs Unexposeda |
|---|---|---|---|---|
| RHI b | −21.9 (−32.0,-10.3)*** | −14.3 (−25.3, −1.6)** | −8.9 (−20.7, 4.6) | −18.0 (−26.0, −9.2)*** |
| SDNNb | −17.3 (−28.3, −6.4)** | −10.3 (−21.7, 1.2) | −7.9 (−18.9, 3.1) | −13.2 (−22.9, −3.6)** |
| pNN50b | −36.1 (−52.3, −19.9)*** | −25.1 (−43.4, −6.8)** | −14.7 (−31.0, 1.6) | −28.6 (−45.3, −11.8)*** |
| RMSSDb | −26.9 (−41.0, −12.7)*** | −18.6 (−33.9, −3.4)* | −10.2 (−24.4, 4.1) | −21.5 (−33.4, −9.7)*** |
| LFb | 27.0 (11.3, 42.8)*** | 17.8 (3.7, 31.9)* | 7.8 (−8.1, 23.7) | 21.1 (7.9, 34.2)** |
| HFb | −15.4 (−26.2, −4.7)** | −4.4 (−16.1, 7.4) | −11.6 (−22.4, −0.7)* | −9.1 (−19.2, 1.0) |
| LF/HFb | 41.4 (15.0, 67.9)** | 17.1 (−3.9, 38.2) | 20.7 (−6.0, 47.4) | 26.4 (6.7, 46.2)** |
| SP | −4.4 (−8.0, −0.7)* | −0.3 (−4.2, 3.5) | −4.0 (−7.7, −0.3)* | −2.4 (−5.7, 1.0) |
| DP | −3.2 (−8.6, 2.3) | 5.8 (−0.2, 11.8) | −8.5 (−14.0, −3.0)** | 1.1 (−3.5, 5.7) |
| AI.75 | −8.8 (−24.8, 7.3) | −15.3 (−30.2, −0.4)* | 7.7 (−8.3, 23.8) | −12.2 (−26.4, 2.1) |
| BL.HR | 12.7 (9.0, 16.3)*** | 9.4 (5.8, 12.9)*** | 3.0 (−0.7, 6.7) | 11.0 (7.7, 14.3)*** |
RHI reactive hyperemia index, SDNN standard deviation of all NN intervals, pNN50 proportion of successive NN intervals differing by more than 50 milliseconds divided by total number of NN intervals, RMSSD square root of the mean squared differences of successive NN intervals, LF power in low frequency range (0.04–0.15 Hz) in ms2, HF power in high frequency range (0.15–0.4 Hz) in ms2, LF/HF ratio LF(ms2)/HF(ms2), SP systolic blood pressure (mmHg), DP diastolic blood pressure (mmHg), AI.75 augmentation index corrected for 75 bpm, BL.HR baseline heart rate (bpm)
Results are percent change from the mixed effect model in Fig. 1 except for RHI where percent change was obtained directly from the effect estimate due to the logarithmic transformation. The data are based on 43 individuals with measurements in both fire extinction exercise and control exposure condition (measurements of control exposure condition were missing for one subject in RHI and heart rate variability outcomes)
*,**,*** Significantly different (p < 0.05, p < 0.01 and p < 0.001 respectively)
a Unexposed corresponds to the mean between “Before” and “After” for each subject
b One subject was eliminated due to missing data in both control measurements
Table 3 presents the average effect change for each of the cardiovascular endpoints between exposure and unexposed situations for the two different types of fire: wood and wood with mattresses and electrical cords. The results show no difference between the two different types of fire for our primary outcomes, except for blood pressure, where a statistically significant difference was observed.
Table 3.
Within-subject effect change between exposure and unexposed situations for two different types of fires: wood pallets and wood pallets with mattresses and electrical cords
| Outcome | Differenceawith pallet fuel | Differenceawith mixed fuel | Welch t-test p-value |
|---|---|---|---|
| LnRHI | −0.1 ± 0.3 | −0.3 ± 0.3 | 0.166 |
| SDNN | −9.3 ± 24.9 | −9.8 ± 22.2 | 0.942 |
| pNN50 | −0.05 ± 0.1 | −0.04 ± 0.1 | 0.879 |
| RMSSD | −14.2 ± 29.8 | −14.1 ± 22.9 | 0.991 |
| LF | 12.8 ± 71.0 | 56.6 ± 74.2 | 0.075 |
| HF | −18.7 ± 71.0 | −15.1 ± 54.7 | 0.857 |
| LF/HF | 0.2 ± 0.7 | 0.5 ± 1.0 | 0.174 |
| SP | −8.2 ± 12.4 | 1.6 ± 11.8 | 0.013 |
| DP | −3.3 ± 7.2 | 3.9 ± 10.5 | 0.012 |
| AI.75 | 2.9 ± 7.8 | 1.6 ± 9,1 | 0.614 |
| BL.HR | 6.2 ± 6.7 | 8.1 ± 7.8 | 0.400 |
LnRHI natural logarithm of the reactive hyperemia index, SDNN standard deviation of all NN intervals, pNN50 proportion of successive NN intervals differing by more than 50 milliseconds divided by total number of NN intervals, RMSSD square root of the mean squared differences of successive NN intervals, LF power in low frequency range (0.04–0.15 Hz) in ms2, HF power in high frequency range (0.15–0.4 Hz) in ms2, LF/HF ratio LF(ms2)/HF(ms2), SP systolic blood pressure (mmHg), DP diastolic blood pressure (mmHg), AI.75 augmentation index corrected for 75 bpm, HR baseline heart rate (bpm). Values are mean ± SD
aAverage difference between the exposed and unexposed situations within each subject
Discussion
The present study showed that participation in fire extinction exercise did not cause PM exposure during firefighting using the PPE with self-contained breathing apparatus, whereas PM exposure occurred when the self-contained breathing apparatus was taken off in areas considered safe. Participation in firefight training resulted in exposure to PAHs in terms of increased urinary excretion of 1-OHP, increased body temperature and with cardiovascular risk markers in terms of both decreased microvascular function and changed HRV.
In the present study, there was no association between urinary excretion of 1-OHP and cardiovascular risk markers. Urinary excretion of 1-OHP has been established as a reliable biomarker of internal dose of PAHs in populations exposed to urban air pollution [21]. Our results demonstrate that the subjects were exposed to PAHs, although we did not appoint sources of PAHs in the present study. PAH exposure occurs both by inhalation of PM and by dermal exposure to soot [22]. Our results indicate that the exposure to PAH is a weak predictor of cardiovascular risk markers as compared to other risk factors such as physical exhaustion and heat. Both of these alter blood flow. Nevertheless, it should be noted that the firefighting exercises encompassed simultaneous exposure to smoke, heat and physical activity. It is not possible to separate the effect of smoke exposure on cardiovascular endpoints from that of heat and physical activity in the present study. It is possible that the observed short-term vascular effects predominantly reflects effects related to increased blood flow in order to ameliorate peripheral built-up of waste products from the physical exercise and reduce the core body temperature related to the last of the smoke diving exercises in the 3-day course. We did not observe any difference in the microvascular function and HRV between fires with or without mattresses and electrical cords. In parallel to the biomarkers of cardiovascular risk described in the present study, PAH exposure on skin and biomarkers of inflammation and genotoxicity in blood were assessed for the 53 study subjects [23]. Firefighting did not affect blood levels of C-reactive protein, serum amyloid A, IL6 and IL8 concentrations, whereas there was increased level of oxidatively damaged DNA (i.e. formamidopyrimidine DNA glycosylase sensitive sites measured by the comet assay) in peripheral blood mononuclear cells compared to the mean of two control measurements performed 2 weeks before and 2 weeks after the fire fighting course [23]. The results suggest systemic oxidative stress, which is linked to cardiovascular disease.
The assessment of ambient air levels of PM indicated high concentrations inside and outside the firehouse. PM inside the firehouse came from the fire, whereas outdoor exposure represents dispersion of smoke from the firehouse and exhaust from a diesel-driven fire truck near the entrance of the firehouse. Other studies have demonstrated elevated levels of 1-OHP in subjects participating in firefighting exercises using diesel as fuel [24] and real-life fires [25]. Firefighting activities on wood fire have yielded rather low urinary levels of 1-OHP, whereas other types of urinary hydroxylated PAHs have been elevated post-exposure [26–28]. We did not obtain information on the total personal exposure to PM because of limited number of samplers and because we chose to assess PM exposure during firefighting while wearing PPE for more subjects instead of assessing whole day exposure for one or two subjects. During firefighting there was little PM inhalation exposure because the self-contained breathing apparatus was a highly efficient barrier toward particles. Pulmonary exposure was only observed when the subjects were not wearing the full PPE. The exposure assessment indicated substantial PM exposure in the areas considered safe.
We found a decreased microvascular function, measured by RHI, after the fire extinction exercise compared to the no-exposure scenario. A decreased microvascular function, using EndoPAT, has previously been described in exposure studies on air pollution particles in susceptible groups such as elderly [18, 29], whereas mixed results were reported for young and healthy subjects [30, 31]. Likewise, short-term controlled exposure studies on diesel exhaust have shown associations with reduced vasodilatory response [32]. However, short-term controlled exposure to high concentrations of wood smoke, i.e. several hundred micrograms per cubic meter, have demonstrated unaltered or even increased vasodilation response [12, 13]. Low level of flow-mediated vasodilation corrected for shear stress is a risk factor to cardiovascular disease in firefighters; other additive risk factors are Framingham risk score and carotid intima-media thickness [33].
Altered HRV was manifested in both time and frequency domains. The fire extinction exercise was associated with decreased time domain HRV measures (SDNN, pNN50 and RMSSD), reduced high frequency components (HF), increased low frequency components (LF) and increased LF/HF ratio. Overall, it indicates an imbalance in the autonomic activation of the heart with reduced vagal activity and increased sympathetic activity. A meta-analysis has recently shown reduced measures of HRV in humans after exposure to particulate air pollution [9], whereas a review of panel studies concluded that the studies did not convincingly show inverse associations between ambient air PM2.5 concentrations and HRV [34]. Two controlled studies reported no association between short-term diesel exhaust (100–300 μg/m3 for 2 h) and HRV [35, 36]. However, a short-term controlled exposure to wood smoke study (314 μg/m3 for 3 h) showed reduced HRV and increased heart rate during a 1-h post-exposure period [14]. Reduced HRV has been shown to be associated with increased risk of a first cardiovascular event in people without cardiovascular diseases [37].
Despite the demand for physical fitness, firefighters as a group seem to harbour several risk factors for cardiovascular diseases. In a recent study on young career firefighters (<45 years), increased risk of sudden cardiac death was found to be largely attributed to obesity, hypertension and smoking [38]. Therefore, to avoid effect modification due to lifestyle factors, we used young and non-smoking conscripts who were generally healthy in our study. It is generally acknowledged that exposure to air pollution has an immediate effect, e.g. precipitation of myocardial infarction, and a chronic effect related to progression of atherosclerosis. Consequences of this difference in effect are apparent in the risk estimates from short-term and long-term exposure in epidemiological studies, whereas a time-integrated exposure metric suggests a monotonic exposure-effect relationship [39]. Our study is by design revealing short-term effects on both the vasculature and myocardium. The observation suggests that a reduced microvascular vasodilation response would be associated with increased peripheral resistance and progression toward hypertension and left ventricular cardiac overload due to backward failure. Indeed, HRV is reduced in patients with hypertension [40]. Increased physical workload, heat and dehydration also can be independent risk factors for increased risk of mortality from coronary heart disease among on-duty firefighters, whereas conditional risk factors for cardiovascular disease such as obesity, dyslipidemia, hypertension and diabetes may put certain subjects in high-risk category for sudden cardiac death.
Conclusions
In the present study, exposure of human volunteers following a 3-day firefighting training program with various types of exercises in a firehouse was associated with altered cardiovascular effects in terms of decreased microvascular function and altered HRV. The subjects were very efficiently protected against pulmonary PM exposure when using the full personal protective equipment including the self-contained breathing apparatus. Significant PM exposure was observed when the subjects took off their self-contained breathing apparatus in areas considered safe. Fire extinction exercises were associated with increased urinary 1-OHP levels indicating exposure to PAH. However, the association between urinary excretion of 1-OHP and cardiovascular effects was not statistically significant in models that included smoke exposure as categorical variable. Physical activity and heat are also conditions that occur during the fire extinction exercise, which alter blood flow. Thus, the altered cardiovascular responses after fire extinction exercises are most likely due to complex effects from PM exposure, physical exhaustion and increased core body temperature.
Additional file
Supplementary material. (DOC 4338 kb)
Acknowledgments
The technical assistance from Anne Abildtrup, Ulla Tegner and Inge Christiansen is gratefully acknowledged. A special thanks goes to the Danish Emergency Management Agency where the measurements took place. We are also grateful to the study participants for the considerable time and willingness put into this study. We established a reference group which includes stakeholders from e.g. fire brigades, trade unions and The Danish Emergency Management Agency. We thank the reference group for involvement in the overall study design.
Funding
The research leading to these results has received funding from The Danish Working Environment Research Fund (BIOBRAND, grant 34–2014-09 / 20,140,072,567), Danish Centre for Nanosafety, grant 20,110,092,173/3 and Danish Centre for Nanosafety II).
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 1-OHP
1-hydroxypyrene
- AI
Augmentation index
- BL.HR
Base line heart rate
- BMI
Body mass index
- CI
Confidence interval
- DP
Diastolic blood pressure
- HF
High frequency
- HPLC
High performance liquid chromatography
- HRV
Heart rate variability
- LF
Low frequency
- PAH
Polycyclic aromatic hydrocarbons
- PAT
Peripheral arterial tonometry
- PM
Particulate matter
- pNN50
proportion of normal-to-normal intervals differing by more than 50 miliseconds
- PPE
Personal protective equipment
- RHI
Reactive hyperemia index
- RMSSD
Root mean square of the successive differences
- SDNN
Standard deviation of normal-to-normal intervals
- SP
Systolic blood pressure
Authors’ contributions
MHGA collected the data on vasculature effects and body temperature, assisted in the exposure measurements, analysed the results, and wrote the first draft of the manuscript. ATS designed and coordinated the study, supervised the data analysis and the writing of the manuscript. PBP measured and reported the exposure. SL designed the study and was a major contributor in the analysis and interpretation of results. AMH supervised the analysis and report of 1-OHP and was a contributor in writing the manuscript. IKK assisted in the exposure assessment. JEP collected and reported the data from the questionnaires. NE designed the study and contributed to the analysis and interpretation of results. ECN assisted in the collection of data of body temperature. PAC assisted in the exposure assessment and contributed to the writing manuscript. AHG contributed to the analysis of 1-OHP and the writing the manuscript. UV designed and supervised the study and was a major contributor in writing the manuscript. PM designed and supervised the study and was a major contributor to the analysis, interpretation and writing of the manuscript. All authors read and approved the final manuscript.
Authors’ information
Correspondence regarding this study should be addressed to UV (ubv@nrcwe.dk) or PM (pemo@sund.ku.dk).
Ethics approval and consent to participate
The Danish Committee on Health Research Ethics of the Capital Region (H-15003862) approved the study and study subjects participated in information meeting and provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12940-017-0303-8) contains supplementary material, which is available to authorized users.
Contributor Information
Maria Helena Guerra Andersen, Email: mhar@sund.ku.dk.
Anne Thoustrup Saber, Email: ats@nrcwe.dk.
Peter Bøgh Pedersen, Email: pbbp@teknologisk.dk.
Steffen Loft, Email: stl@sund.ku.dk.
Åse Marie Hansen, Email: asemarie.hansen@sund.ku.dk.
Ismo Kalevi Koponen, Email: ikk@nrcwe.dk.
Julie Elbæk Pedersen, Email: juliep@cancer.dk.
Niels Ebbehøj, Email: Niels.Erik.Ebbehoej@regionh.dk.
Eva-Carina Nørskov, Email: ecn@maersk-nielsen.dk.
Per Axel Clausen, Email: pac@nrcwe.dk.
Anne Helene Garde, Email: ahg@nrcwe.dk.
Ulla Vogel, Email: ubv@nrcwe.dk.
Peter Møller, Phone: +45 3532 7654, Email: pemo@sund.ku.dk.
References
- 1.Soteriades ES, Smith DL, Tsismenakis AJ, Baur DM, Kales SN. Cardiovascular disease in US firefighters: a systematic review. Cardiol Rev. 2011;19(4):202–215. doi: 10.1097/CRD.0b013e318215c105. [DOI] [PubMed] [Google Scholar]
- 2.Kales SN, Soteriades ES, Christophi CA, Christiani DC. Emergency duties and deaths from heart disease among firefighters in the United States. N Engl J Med. 2007;356(12):1207–1215. doi: 10.1056/NEJMoa060357. [DOI] [PubMed] [Google Scholar]
- 3.Fahs CA, Yan H, Ranadive S, Rossow LM, Agiovlasitis S, Echols G, Smith D, Horn GP, Rowland T, Lane A, et al. Acute effects of firefighting on arterial stiffness and blood flow. Vasc Med. 2011;16(2):113–118. doi: 10.1177/1358863X11404940. [DOI] [PubMed] [Google Scholar]
- 4.Fernhall B, Fahs CA, Horn G, Rowland T, Smith D. Acute effects of firefighting on cardiac performance. Eur J Appl Physiol. 2012;112(2):735–741. doi: 10.1007/s00421-011-2033-x. [DOI] [PubMed] [Google Scholar]
- 5.Carter HH, Spence AL, Atkinson CL, Pugh CJ, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol. 2014;114(4):859–865. doi: 10.1007/s00421-013-2817-2. [DOI] [PubMed] [Google Scholar]
- 6.Ganio MS, Brothers RM, Shibata S, Hastings JL, Crandall CG. Effect of passive heat stress on arterial stiffness. Exp Physiol. 2011;96(9):919–926. doi: 10.1113/expphysiol.2011.057091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefferts WK, Heffernan KS, Hultquist EM, Fehling PC, Smith DL. Vascular and central hemodynamic changes following exercise-induced heat stress. Vasc Med. 2015;20(3):222–229. doi: 10.1177/1358863X14566430. [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 9.Pieters N, Plusquin M, Cox B, Kicinski M, Vangronsveld J, Nawrot TS. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: a meta-analysis. Heart. 2012;98(15):1127–1135. doi: 10.1136/heartjnl-2011-301505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moller P, Mikkelsen L, Vesterdal LK, Folkmann JK, Forchhammer L, Roursgaard M, Danielsen PH, Loft S. Hazard identification of particulate matter on vasomotor dysfunction and progression of atherosclerosis. Crit Rev Toxicol. 2011;41(4):339–368. doi: 10.3109/10408444.2010.533152. [DOI] [PubMed] [Google Scholar]
- 11.Moller P, Christophersen DV, Jacobsen NR, Skovmand A, Gouveia AC, Andersen MH, Kermanizadeh A, Jensen DM, Danielsen PH, Roursgaard M, et al. Atherosclerosis and vasomotor dysfunction in arteries of animals after exposure to combustion-derived particulate matter or nanomaterials. Crit Rev Toxicol. 2016;46(5):437–476. doi: 10.3109/10408444.2016.1149451. [DOI] [PubMed] [Google Scholar]
- 12.Forchhammer L, Moller P, Riddervold IS, Bonlokke J, Massling A, Sigsgaard T, Loft S. Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Part Fibre Toxicol. 2012;9:7. doi: 10.1186/1743-8977-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter AL, Unosson J, Bosson JA, Langrish JP, Pourazar J, Raftis JB, Miller MR, Lucking AJ, Boman C, Nystrom R, et al. Effect of wood smoke exposure on vascular function and thrombus formation in healthy fire fighters. Part Fibre Toxicol. 2014;11:62. doi: 10.1186/s12989-014-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unosson J, Blomberg A, Sandstrom T, Muala A, Boman C, Nystrom R, Westerholm R, Mills NL, Newby DE, Langrish JP, et al. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Part Fibre Toxicol. 2013;10:20. doi: 10.1186/1743-8977-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen AM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies--a review. Int J Hyg Environ Health. 2008;211(5–6):471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Jongeneelen FJ, van Leeuwen FE, Oosterink S, Anzion RB, van der Loop F, Bos RP, van Veen HG. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990;47(7):454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen AM, Poulsen OM, Christensen JM, Hansen SH. Determination of 1-hydroxypyrene in human urine by high-performance liquid chromatography. J Anal Toxicol. 1993;17(1):38–41. doi: 10.1093/jat/17.1.38. [DOI] [PubMed] [Google Scholar]
- 18.Brauner EV, Forchhammer L, Moller P, Barregard L, Gunnarsen L, Afshari A, Wahlin P, Glasius M, Dragsted LO, Basu S, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177(4):419–425. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- 19.Bates D, Machler M, Bolker BM, Walker SC. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 20.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 21.Demetriou CA, Raaschou-Nielsen O, Loft S, Moller P, Vermeulen R, Palli D, Chadeau-Hyam M, Xun WW, Vineis P. Biomarkers of ambient air pollution and lung cancer: a systematic review. Occup Environ Med. 2012;69(9):619–627. doi: 10.1136/oemed-2011-100566. [DOI] [PubMed] [Google Scholar]
- 22.IARC. Painting, firefighting, and shiftwork. In: Monographs on the Evaluation of the Carcinogenic Risks to Humans vol 98. Edited by International Agency for Research on Cancer, vol. 98; 2010: 9–764. [PMC free article] [PubMed]
- 23.Andersen MH, Saber AT, Clausen PA, Pedersen JE, Lohr M, Kermanizadeh A, Loft S, Ebbehoj N, Hansen AM, Pedersen PB, et al. Association between polycyclic aromatic hydrocarbons exposure and peripheral blood mononuclear cell DNA damage in human volunteers during fire extinction exercises. Mutagenesis. 2017; in press [DOI] [PubMed]
- 24.Moen BE, Ovrebo S. Assessment of exposure to polycyclic aromatic hydrocarbons during firefighting by measurement of urinary 1-hydroxypyrene. J Occup Environ Med. 1997;39(6):515–519. doi: 10.1097/00043764-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Caux C, O'Brien C, Viau C. Determination of firefighter exposure to polycyclic aromatic hydrocarbons and benzene during fire fighting using measurement of biological indicators. Appl Occup Environ Hyg. 2002;17(5):379–386. doi: 10.1080/10473220252864987. [DOI] [PubMed] [Google Scholar]
- 26.Fernando S, Shaw L, Shaw D, Gallea M, VandenEnden L, House R. Verma DK, Britz-McKibbin P, McCarry BE. Evaluation of Firefighter Exposure to Wood Smoke during Training Exercises at Burn Houses. Environ Sci Technol. 2016;50(3):1536–1543. doi: 10.1021/acs.est.5b04752. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira M, Slezakova K, Alves MJ, Fernandes A, Teixeira JP, Delerue-Matos C, Pereira MD, Morais S. Firefighters' exposure biomonitoring: Impact of firefighting activities on levels of urinary monohydroxyl metabolites. Int J Hyg Environ Health. 2016;219(8):857–866. doi: 10.1016/j.ijheh.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Robinson MS, Anthony TR, Littau SR, Herckes P, Nelson X, Poplin GS, Burgess JL. Occupational PAH exposures during prescribed pile burns. Ann Occup Hyg. 2008;52(6):497–508. doi: 10.1093/annhyg/men027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmingsen JG, Rissler J, Lykkesfeldt J, Sallsten G, Kristiansen J, Moller PP, Loft S. Controlled exposure to particulate matter from urban street air is associated with decreased vasodilation and heart rate variability in overweight and older adults. Part Fibre Toxicol. 2015;12:6. doi: 10.1186/s12989-015-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brauner EV, Moller P, Barregard L, Dragsted LO, Glasius M, Wahlin P, Vinzents P, Raaschou-Nielsen O, Loft S. Exposure to ambient concentrations of particulate air pollution does not influence vascular function or inflammatory pathways in young healthy individuals. Part Fibre Toxicol. 2008;5:13. doi: 10.1186/1743-8977-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weichenthal S, Hatzopoulou M, Goldberg MS. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: a cross-over study. Part Fibre Toxicol. 2014;11:70. doi: 10.1186/s12989-014-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barath S, Mills NL, Lundback M, Tornqvist H, Lucking AJ, Langrish JP, Soderberg S, Boman C, Westerholm R, Londahl J, et al. Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions. Part Fibre Toxicol. 2010;7:19. doi: 10.1186/1743-8977-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123(2):163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 34.Buteau S, Goldberg MS. A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ Res. 2016;148:207–247. doi: 10.1016/j.envres.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Peretz A, Kaufman JD, Trenga CA, Allen J, Carlsten C, Aulet MR, Adar SD, Sullivan JH. Effects of diesel exhaust inhalation on heart rate variability in human volunteers. Environ Res. 2008;107(2):178–184. doi: 10.1016/j.envres.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong H, Rappold AG, Caughey M, Hinderliter AL, Graff DW, Berntsen JH, Cascio WE, Devlin RB, Samet JM. Cardiovascular effects caused by increasing concentrations of diesel exhaust in middle-aged healthy GSTM1 null human volunteers. Inhal Toxicol. 2014;26(6):319–326. doi: 10.3109/08958378.2014.889257. [DOI] [PubMed] [Google Scholar]
- 37.Hillebrand S, Gast KB, de Mutsert R, Swenne CA, Jukema JW, Middeldorp S, Rosendaal FR, Dekkers OM. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–749. doi: 10.1093/europace/eus341. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Teehan D, Farioli A, Baur DM, Smith D, Kales SN. Sudden cardiac death among firefighters </=45 years of age in the United States. Am J Cardiol. 2013;112(12):1962–1967. doi: 10.1016/j.amjcard.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pope CA., 3rd Mortality effects of longer term exposures to fine particulate air pollution: review of recent epidemiological evidence. Inhal Toxicol. 2007;19(Suppl 1):33–38. doi: 10.1080/08958370701492961. [DOI] [PubMed] [Google Scholar]
- 40.Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32(2):293–297. doi: 10.1161/01.HYP.32.2.293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. (DOC 4338 kb)
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.


