Abstract
Background
Monocyte chemotactic protein-1 [MCP-1; chemokine C-C Ligand-2 (CCL-2)] is upregulated in ischemia-reperfusion injury and is a promising biomarker of inflammation in cardiac surgery.
Methods
We measured pre- and postoperative plasma MCP-1 levels in adult patients undergoing cardiac surgery to evaluate the association of perioperative MCP-1 levels with acute kidney injury (AKI) and mortality in TRIBE-AKI- a prospective, multicenter, observational cohort.
Results
Of the 972 participants in the study, 329 (34%) developed AKI and 45 (5%) developed severe AKI. During median follow-up of 2.9 (2.2–3.5) years, 119 (12%) participants died. MCP-1 levels were significantly higher in those who developed AKI and mortality as compared to those without AKI and mortality, respectively. Participants with preoperative MCP-1 level in the highest tertile (>196 pg/ml) had an increased AKI risk as compared to those in the lowest tertile [<147 pg/ml; OR, 1.43 (1.00–2.05)]; the association appeared similar but was not significant for the severe AKI outcome [OR, 1.48 (0.62–3.54)]. As compared with participants with preoperative MCP-1 level in the lowest tertile, those in the highest tertile had higher adjusted mortality risk [HR, 1.82 (1.40–2.38)]. Similarly, as compared to participants with postoperative MCP-1 level in the lowest tertile, those in the highest tertile had higher adjusted mortality risk [HR, 1.95 (1.09–3.49)].
Conclusions
Higher plasma MCP-1 is associated with increased AKI and mortality risk after cardiac surgery. MCP-1 could be used as a biomarker to identify high-risk patients for potential AKI prevention strategies in the setting of cardiac surgery.
Approximately two million cardiac surgeries are performed each year around the world. About 14% (280,000) of these patients experience the postoperative complication of acute kidney injury (AKI), which leads to increased length of hospital stay and healthcare expenditure(1–3). Identification of patients who are most susceptible to postoperative complications could improve patient outcomes and healthcare costs by targeted interventions.
Pre- and intraoperative inflammation is recognized as an important predictor of postoperative complications after cardiac surgery(4,5). Preoperative inflammation primes the kidney and other organs for intraoperative injury, and elevations of preoperative inflammatory markers are associated with adverse postoperative outcomes. Cardiac surgery is associated with periods of hypo-perfusion, tissue ischemia, contact of blood components with the bypass circuit, non-pulsatile blood flow, and operative trauma that all contribute to a pro-inflammatory state(6). Given the important role of inflammation in cardiac surgery, trials of anti-inflammatory interventions have been conducted in hopes of improving postsurgical outcomes(7–9). Studies are also attempting to discover biomarkers that can identify patients with subclinical inflammation who might be candidates for anti-inflammatory therapy in order to mitigate the risk of cardiac surgery.
Monocyte chemotactic protein-1 (MCP-1; chemokine C-C ligand-2) is a member of the chemokine family. Monocytes express CCR-2, the receptor for MCP-1, and MCP-1 regulates trafficking of monocytes from the bone marrow to inflamed tissue in response to inflammatory signals. In preclinical studies, MCP-1 expression was upregulated immediately after ischemia reperfusion injury(10) and MCP-1 inhibition was associated with lower cardiac fibrosis(11). In small human studies, MCP-1 expression increased immediately after cardiac surgery(12,13) and MCP-1 elevation was associated with AKI(14). Moreover, studies have used MCP-1 as a surrogate marker to quantify intra-operative inflammation after various interventions(13,15). However, the association of MCP-1 with post-cardiac surgery outcomes has not been examined in a large, prospective cohort.
We hypothesized that elevated perioperative plasma MCP-1 was a marker of unrecognized inflammation and would be associated with higher risk of adverse outcomes in patients undergoing cardiac surgery. In this study, we measured MCP-1 levels before and after cardiac surgery in participants of the Translational Research Investigating Biomarker Endpoints for Acute Kidney Injury (TRIBE-AKI) cohort, and tested its association with AKI and mortality.
PATIENTS AND METHODS
Participants
The TRIBE-AKI study is a prospective, multicenter, observational cohort of adults at high risk for developing AKI who underwent cardiac surgery [Coronary artery bypass grafting (CABG) and/or valve surgery]. Full study details have been previously published(16–18). In brief, we enrolled participants with at least one risk factor for AKI at five academic medical centers in North America (details in Supplemental Materials). Written informed consent was obtained from all patients or their proxy decision-makers. The TRIBE-AKI study was approved by the institutional review boards at each participating institution. We collected preoperative characteristics, operative details, and postoperative complications using definitions of the Society of Thoracic Surgeons(16).
Exposures
The exposures for this study were plasma MCP-1 levels at two time points: 1) preoperative concentration and 2) peak postoperative concentration in days 1–3 following surgery. Blood specimens were collected preoperatively and daily for up to five days after surgery. After a single freeze-thaw cycle of stored plasma samples, we measured MCP-1 on the Randox Evidence Investigator using a Randox-developed custom cytokine array (Randox Laboratories Ltd.) with a detection range of 12–1580 pg/ml and intra-assay coefficient of variation of 10–16% using manufacturer specifications. We blinded the personnel measuring the biomarkers to clinical outcomes.
Outcomes
The main outcomes for this study were occurrence of in-hospital AKI and all-cause mortality at complete cohort follow-up. AKI was defined as 0.3 mg/dl or >50% rise in serum creatinine from baseline or requiring dialysis during hospitalization. Severe AKI was defined as >100% rise in serum creatinine from baseline or requiring dialysis during hospitalization. Of the 329 AKI events, 327(99%) occurred within 7 days of surgery. The magnitude of changes in serum creatinine for AKI and severe AKI in our study correspond to KDIGO AKI stage 1 and AKI stage 2 or higher, respectively. All preoperative creatinine values were measured within two months prior to surgery. We estimated preoperative glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation.
We obtained mortality data after discharge through various mechanisms (and cross-referenced, when possible). For United States participants, we performed phone calls to patients’ homes, searched the National Death Index, and received hospital records. For participants from Canada, we used phone calls and data held at the Institute for Clinical Evaluative Sciences (ICES) to acquire vital status. These datasets were linked using unique, encoded identifiers and analyzed at the ICES. The death status and date of death were recorded through 12/31/2012. There was 100% vital status ascertainment on the cohort.
Statistical Analysis
We performed descriptive statistics and reported continuous variables as mean ± standard deviation or median (interquartile range), and categorical variables as frequency (percentage). Small cell counts are only presented for data collected by TRIBE-AKI (not from ICES data). Continuous variables were compared using Wilcoxon rank sum test or Fisher’s exact test, as appropriate. Categorical variables were compared using chi-square test. We performed pre- and postoperative MCP-1 correlation with each other, and with eGFR from corresponding time-points using Spearman correlations. We divided the population into MCP-1 concentration tertiles at preoperative and postoperative time points. We determined the association of MCP-1 tertiles with the AKI outcomes using logistic regression with the lowest tertile as the reference group. For the mortality outcome, we used Cox proportional hazards regression to estimate the hazard ratio of mortality with the lowest tertile of MCP-1 as the reference group. For the preoperative MCP-1 models, we adjusted the analyses for the following covariates: demographics [age, sex, race], comorbidities [diabetes, hypertension, heart failure, myocardial infarction], type of surgery (CABG or valve vs. all others), and evidence of kidney disease (preoperative albumin to creatinine ratio, preoperative eGFR). For the postoperative MCP-1 models, we additionally adjusted for surgical characteristics (CPB time>120 minutes, non-elective surgery), change in renal function (using change in serum creatinine), and baseline MCP-1 level. We performed two supplementary analyses: a) evaluation of association of each log increase in MCP-1 level with AKI and mortality, and b) evaluation of association of MCP-1 tertiles with mortality after excluding patients who experienced in-hospital mortality. We performed analyses in SAS version 9.3(SAS Institute, Cary, NC) and R 2.10.1(R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We enrolled 1219 patients to the TRIBE-AKI study from July 2007 through December 2009 (Supplemental Figure 1). Of these, 972 participants with adequate plasma sample for MCP-1 level measurement were included in the analysis. The mean age was 71 years and 68% were men. There were baseline participant differences across the preoperative plasma MCP-1 tertiles (Table 1). Patients in the highest tertile of MCP-1 were less likely to be white, had lower mean preoperative eGFR, and were more likely to have diabetes and hypertension. Baseline characteristics by postoperative MCP-1 tertiles had similar differences as the preoperative tertiles. Patients with longer cross-clamp time and who had on-pump surgery were more likely to be in the highest tertile of postoperative MCP-1 (Supplemental Table 1).
Table 1.
Patient Characteristics by Preoperative MCP-1 Tertiles
| Characteristic* | Overall (N=957) | MCP-1 Tertile‡ | P-value† | ||
|---|---|---|---|---|---|
| Lowest Tertile (N=319) | Middle Tertile (N=320) | Highest Tertile (N=318) | |||
| Demographics | |||||
| Age at surgery, mean(SD) | 71.7 (9.9) | 70.9 (10.4) | 72.6 (8.9) | 71.6 (10.2) | 0.08 |
| Men | 652 (68.1%) | 227 (71.2%) | 210 (65.6%) | 215 (67.6%) | 0.32 |
| White race | 894 (93.4%) | 307 (96.2%) | 298 (93.1%) | 289 (90.9%) | 0.02 |
| Medical History | |||||
| Diabetes | 379 (39.6%) | 104 (32.6%) | 123 (38.4%) | 152 (47.8%) | <0.001 |
| Hypertension | 758 (79.2%) | 240 (75.2%) | 249 (77.8%) | 269 (84.6%) | 0.01 |
| Heart Failure | 232 (24.2%) | 73 (22.9%) | 67 (20.9%) | 92 (28.9%) | 0.05 |
| LVEF<40% | 98 (10.2%) | 35 (11.0%) | 30 (9.4%) | 33 (10.4%) | 0.80 |
| Previous myocardial infarction | 247 (25.8%) | 77 (24.1%) | 83 (25.9%) | 87 (27.4%) | 0.02 |
| eGFR (mL/min/1.73m2), mean(SD) | 67.5 (19.5) | 72.8 (17.9) | 66.2 (18.1) | 63.3 (21.2) | <0.001 |
| eGFR<60 | 331 (35%) | 69 (22%) | 124 (39%) | 138 (43%) | <0.001 |
| Serum Creatinine (mg/dL), median[IQR] | 1.0 (0.9–1.2) | 1.0 (0.8–1.1) | 1.0 (0.9–1.2) | 1.1 (0.9–1.4) | <0.001 |
| Urine albumin to creatinine | 0.08 | ||||
| 30–300 | 265 (27.7%) | 85 (26.6%) | 83 (25.9%) | 97 (30.5%) | |
| >=300 | 59 (6.2%) | 13 (4.1%) | 27 (8.4%) | 19 (6.0%) | |
| Surgical Characteristics | |||||
| Elective Surgery | 766 (80.0%) | 262 (82.1%) | 261 (81.6%) | 243 (76.4%) | 0.14 |
| Surgery | |||||
| CABG or Valve | 748 (78.2%) | 259 (81.2%) | 247 (77.2%) | 242 (76.1%) | 0.33 |
| Off-pump | 86 (9.0%) | 38 (11.9%) | 24 (7.5%) | 24 (7.5%) | 0.20 |
| Perfusion time (minutes), mean(SD) | 112.3 (56.9) | 106.9 (58.6) | 109.4 (53.2) | 120.7 (58.0) | 0.005 |
| Cross-clamp time (minutes), mean(SD) | 76.7 (43.4) | 73.6 (44.7) | 75.4 (39.2) | 81.2 (45.8) | 0.08 |
Continuous variables are compared using Wilcoxon rank sum test or Fisher’s exact test, and categorical variables are compared using chi-square test.
Small cell counts are only presented for data collected by TRIBE-AKI and not from ICES data holdings.
Preoperative MCP-1 tertile cut-offs(pg/ml): T1: 12-147; T2: 147-196; T3: 196-1581.
LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate
Perioperative Plasma MCP-1 Levels
MCP-1 increased from preoperative median (IQR) level of 171 (135–212) pg/mL before surgery to a peak of 450 (307–735) pg/mL 0–6 hours after surgery (Figure 1). MCP-1 levels were higher in participants who developed AKI and mortality as compared to participants without AKI and mortality, respectively (Supplemental Table 2). Preoperative MCP-1 positively correlated with postoperative MCP-1 (R2=0.31) and negatively correlated with eGFR (R2=−0.22).
Figure 1. (A) MCP-1 Levels are Significantly Higher in Patients with AKI. (B) MCP-1 Levels are Significantly Higher in those with Mortality.
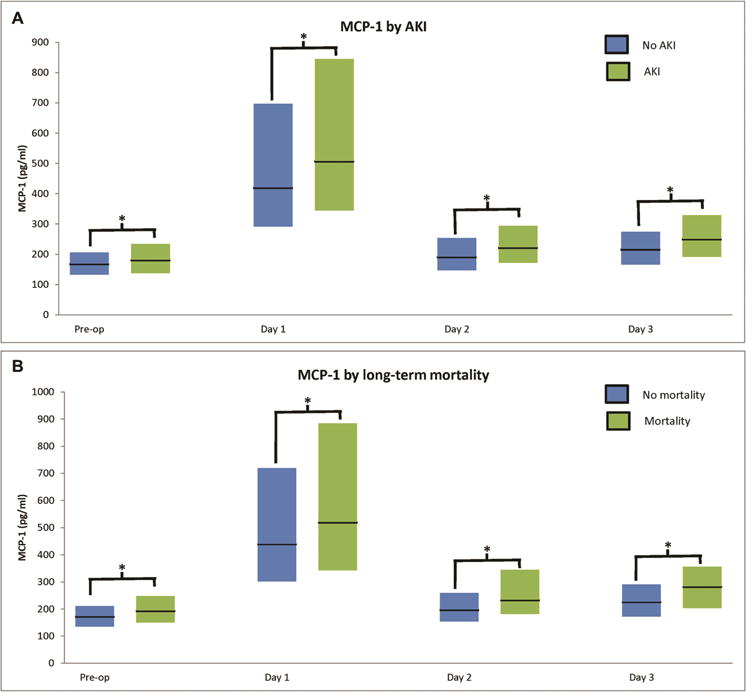
*For AKI: P<0.001 at all time-points. *For mortality: P=0.02 on first post-operative day, P=0.001 on pre-operative time-point, and P<0.001 at all other time-points.
Each bar represents interquartile range and horizontal line represents median. Day 1 refers to postoperative time 0–6 hours after surgery, day 2 corresponds to 48 hours after surgery, and day 3 corresponds to 72 hours after surgery.
Perioperative MCP-1 Levels and AKI Risk
Of the 972 participants, 329 (34%) developed AKI, 45 (5%) developed severe AKI, and 13 (1%) received acute dialysis. After adjusting for baseline characteristics and preoperative eGFR, the highest tertile of preoperative MCP-1 was associated with a higher odds ratio of AKI [Table 2; OR, 1.43 (1.00–2.05)]; the association appeared similar but was not significant for severe AKI [OR, 1.48 (0.62–3.54)]. Postoperative MCP-1 level showed similar trends of association as preoperative MCP-1 level, but was no longer statistically significant after covariate adjustment.
Table 2.
Association of MCP-1 with AKI
| Time point | Tertile (MCP-1 level in pg/ml) | AKI events (%) | Odds Ratio (95% CI)† | ||
|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | |||
| AKI (0.3 mg/dl or >50% rise in serum creatinine, or requiring dialysis) | |||||
| Pre-op | Log | 1.76 (1.29, 2.42) | 1.36 (0.97, 1.90) | 1.27 (0.90, 1.79) | |
| T1 (12–147) | 95 (30%) | Referent | Referent | Referent | |
| T2 (147–196) | 93 (29%) | 1.01 (0.72, 1.42) | 1.10 (0.76, 1.59) | 1.16 (0.80, 1.68) | |
| T3 (196–1581) | 133 (42%) | 1.78 (1.28, 2.47) | 1.48 (1.04, 2.11) | 1.43 (1.00, 2.05) | |
| Post-op | Log | 1.50 (1.21, 1.85) | 1.25 (0.98, 1.58) | 1.62 (1.03, 2.56) | |
| T1 (40–359) | 91 (28%) | Referent | Referent | Referent | |
| T2 (359–600) | 114 (35%) | 1.41 (1.01, 1.96) | 1.27 (0.88, 1.82) | 0.87 (0.45, 1.69) | |
| T3 (600–1581) | 124 (38%) | 1.65 (1.18, 2.30) | 1.29 (0.89, 1.87) | 1.63 (0.83, 3.19) | |
| Severe AKI (>100% rise in serum creatinine or requiring dialysis) | |||||
| Pre-op | Log | 1.68 (0.86, 3.27) | 1.27 (0.59, 2.72) | 1.19 (0.55, 2.60) | |
| T1 (12–147) | 9 (3%) | Referent | Referent | Referent | |
| T2 (147–196) | 12 (4%) | 1.34 (0.56, 3.23) | 1.21 (0.49, 2.99) | 1.15 (0.46, 2.89) | |
| T3 (196–1581) | 20 (6%) | 2.31 (1.04, 5.16) | 1.62 (0.69, 3.77) | 1.48 (0.62, 3.54) | |
| Post-op | Log | 1.88 (1.18, 2.99) | 1.57 (0.96, 2.56) | 2.07 (0.85, 5.04) | |
| T1 (40–359) | 8 (3%) | Referent | Referent | Referent | |
| T2 (359–600) | 19 (6%) | 2.46 (1.06, 5.70) | 2.40 (1.02, 5.69) | 1.05 (0.23, 4.84) | |
| T3 (600–1581) | 18 (6%) | 2.32 (1.00, 5.42) | 1.88 (0.78, 4.53) | 2.36 (0.55, 10.03) | |
Logistic regression
For preoperative biomarkers:
Model 1: Adjusted for age, sex, race, non-elective surgery, diabetes, hypertension, center, heart failure, myocardial infarction, preoperative urine albumin to creatinine ratio, and type of surgery (CABG or valve vs. all others).
Model 2: Model 1 + preoperative eGFR
For postoperative biomarkers:
Model 1: Adjusted for age, sex, race, CPB>120 minutes, non-elective surgery, preoperative eGFR, diabetes, hypertension, center, heart failure, myocardial infarction, preoperative urine albumin to creatinine ratio, and type of surgery (CABG or valve vs. all others)
Model 2: Model 1 + change in serum creatinine from preoperative to peak + baseline MCP-1 level
Perioperative MCP-1 Levels and AKI Duration
Of the 329 participants with AKI, 206(63%) had duration of 1–2 days, 98(29%) had duration of 3–6 days, and 25(8%) had duration of ≥7 days. The highest preoperative MCP-1 tertile was associated with longer AKI duration as compared to lowest tertile[OR: 1.45(1.03–2.05); Supplemental Table 3]. The highest postoperative MCP-1 tertile showed a trend towards longer AKI duration as compared to lowest tertile [OR: 1.36 (0.89–2.08)].
Perioperative MCP-1 Levels and Mortality Risk
During median follow-up of 2.9 (2.2–3.5) years, 119 (12%) participants died. Of the 119 deaths, 15 occurred during the index hospitalization. Median time from surgery to death for these in-hospital deaths was 10 (4–23) days. Participants in the highest pre- and postoperative MCP-1 tertile had higher death rates as compared to those in the lowest tertile (Table 3). After adjustment for baseline covariates, surgical characteristics and eGFR, participants in the highest pre- and postoperative MCP-1 tertiles had a higher mortality risk as compared to participants in the lowest pre- and postoperative MCP-1 tertile, respectively [Figure 2; preoperative: HR, 1.82 (1.40–2.38); postoperative: HR, 1.95 (1.09–3.49)]. We noted similar results when the association of mortality was analyzed using per-log increase in MCP-1 level (Table 3). We noted a similar association of MCP-1 level with long-term mortality after excluding participants with in-hospital deaths, except the association of postoperative MCP-1 level with mortality was no longer statistically significant (Supplemental Table 4 and Supplemental Figure 2).
Table 3.
Association of MCP-1 with Mortality
| Time point | Tertile (MCP-1 level in pg/ml) | Death rates* | Hazard Ratio (95% CI)† | ||
|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | |||
| Pre-op | Log | 1.54 (1.18, 2.01) | 1.43 (1.08, 1.90) | 1.40 (1.09, 1.81) | |
| T1 (12–147) | 33.4 | Referent | Referent | Referent | |
| T2 (147–196) | 44.3 | 1.33 (0.99, 1.78) | 1.34 (1.05, 1.70) | 1.32 (1.02, 1.71) | |
| T3 (196–1581) | 72.9 | 2.14 (1.62, 2.83) | 1.86 (1.40, 2.48) | 1.82 (1.40, 2.38) | |
| Post-op | Log | 1.56 (1.07, 2.27) | 1.54 (1.12, 2.10) | 1.59 (1.13, 2.25) | |
| T1 (40–359) | 37.3 | Referent | Referent | Referent | |
| T2 (359–600) | 45.9 | 1.21 (0.87, 1.70) | 1.28 (1.03, 1.59) | 1.20 (0.93, 1.55) | |
| T3 (600–1581) | 67.5 | 1.79 (0.96, 3.33) | 1.85 (1.11, 3.08) | 1.95 (1.09, 3.49) | |
Cox proportional hazards model
Per 1000 person-years
For preoperative biomarkers:
Model 1: Adjusted for age, sex, race, non-elective surgery, diabetes, hypertension, center, heart failure, myocardial infarction, preoperative urine albumin to creatinine ratio, and type of surgery (CABG or valve vs. all others).
Model 2: Model 1 + preoperative eGFR
For postoperative biomarkers:
Model 1: Adjusted for age, sex, race, CPB>120 minutes, non-elective surgery, preoperative eGFR, diabetes, hypertension, center, heart failure, myocardial infarction, preoperative urine albumin to creatinine ratio, and type of surgery (CABG or valve vs. all others)
Model 2: Model 1 + change in serum creatinine from preoperative to peak + baseline MCP-1 level
Figure 2. (A) Probability of Mortality after Cardiac Surgery by Preoperative MCP-1 Tertiles. (B) Probability of Mortality after Cardiac Surgery by Postoperative MCP-1 Tertiles.
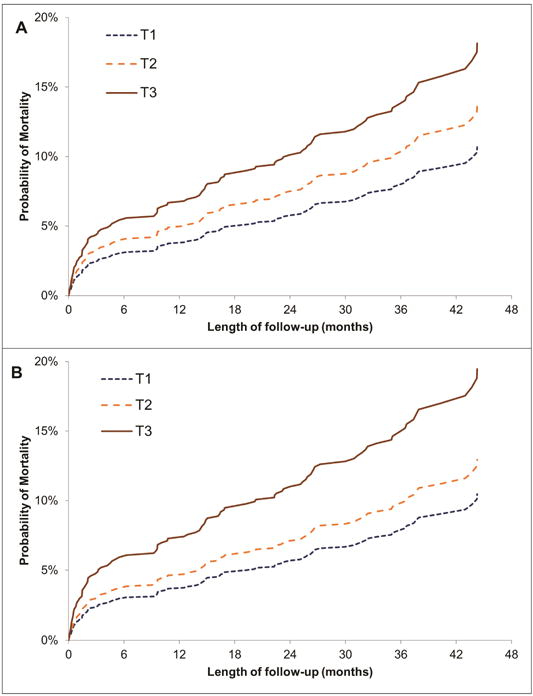
MCP-1 tertile cut-offs(pg/ml):
Preoperative: T1: 12-147; T2: 147-196; T3: 196-1581
Postoperative: T1: 40-359; T2: 359-600; T3: 600-1581
Median(IQR) length of follow-up: 2.9(2.2–3.5) years
COMMENT
Inflammation is associated with adverse post-cardiac surgery outcomes and biomarkers are being evaluated to detect patients with subclinical inflammation. Several trials of anti-inflammatory interventions have been conducted in hopes of improving outcomes after cardiac surgery(7,9). MCP-1 is a promising inflammatory biomarker that has been shown to increase in cardiac surgery and is associated with adverse postoperative outcomes in small clinical studies. In our large, multicenter, prospective cohort, we hypothesized that perioperative plasma MCP-1 would be associated with adverse outcomes. Our study shows that higher plasma MCP-1 level was associated with increased in-hospital AKI and mortality risk. Our findings suggest that, in a subset of patients undergoing cardiac surgery, unrecognized perioperative inflammation may contribute to higher postoperative mortality, and this inflammation can be detected by elevated plasma MCP-1 levels.
MCP-1 (CCL-2) is upregulated in the setting of inflammation. MCP-1 is the ligand for CCR-2 and CCR-2 is present almost exclusively on monocytes. Thus, in the setting of inflammation, MCP-1 attracts monocytes out of the bone marrow and recruits them to inflammatory sites(19). While MCP-1 is elevated in various renal diseases such as lupus nephritis, diabetic nephropathy, and transplant rejection, its role is not limited to the kidneys(19). MCP-1 is elevated in various other conditions including infection, allergic reactions, bone remodeling, atherosclerosis, and inflammatory bowel disease(20). Given the pathogenic role of MCP-1 in various disorders, MCP-1 receptor blocker has been developed and is now in early phase clinical trials(21).
There is preclinical and clinical evidence to suggest the role of MCP-1 as an inflammatory biomarker in cardiac surgery. Preclinical and clinical studies show that MCP-1 is increased immediately after ischemia-reperfusion injury and cardiac surgery(10,13). Studies have used a decrease in MCP-1 level as a surrogate marker for reduced postoperative inflammation from various interventions(13,15). MCP-1 level has also been associated with mortality in small studies. In a 32-patient study, higher serum MCP-1 level was associated with 10-fold higher mortality risk in acute respiratory distress syndrome(22). Similarly, in a 2270 patient study with unstable coronary syndromes with 10 months follow-up, higher MCP-1 level was associated with 54% higher risk of death(23). However, no study has examined the association of preoperative MCP-1 levels with mortality after cardiac surgery.
Similar to preclinical studies, we noted a 2.5-fold rise in MCP-1 after surgery and that patients with longer CPB time were more likely to have higher post-operative MCP-1 level. We found that patients in the highest tertile of MCP-1 before and after surgery were at increased risk of dying after cardiac surgery. Our results remained significant after adjustment for various confounders including diabetes, hypertension, surgical characteristics and eGFR. This association of higher MCP-1 level with mortality has been shown in the setting of coronary artery disease(24,25). We believe that this increase in mortality in patients with higher baseline and postoperative MCP-1 level may be due to unrecognized inflammation and resulting fibrosis in various tissues. Thus, while a major trial of untargeted anti-inflammatory therapy showed null results in cardiac surgery(9), MCP-1 may be able to select individuals who might benefit from targeted anti-inflammatory therapy.
Prior studies showed an association of elevated MCP-1 level with AKI in various settings(22,26). One small study of 100 subjects and 27% AKI events found a non-significant 4-fold AKI risk for patients in the highest MCP-1 tertile after surgery compared to the lowest tertile(14). Our study of over 900 patients sought to definitively establish this connection between MCP-1 and AKI. We found that preoperative plasma MCP-1 level was associated with AKI, and showed a similar, but statistically non-significant, association of preoperative plasma MCP-1 level with severe AKI. Postoperative MCP-1 showed similar associations as preoperative MCP-1, but was no longer statistically significant after we adjusted for demographics and surgical characteristics.
Our study has several important strengths. This is the largest study examining the association of MCP-1 with important post-cardiac surgery outcomes of AKI and mortality. Our sample size was adequate to detect clinically meaningful difference in outcomes. We were also able to reach 100% ascertainment of vital status. We adjusted our analysis for important covariates. Finally, personnel performing biomarker measurement were blinded to clinical data. Our study also has several limitations. We do not have data on cause of death. However, all-cause mortality is an important and clinically meaningful outcome. We did not have other traditional inflammatory markers such as erythrocyte sedimentation rate or C-reactive protein to compare with MCP-1. However, one study in patients with acute coronary syndrome demonstrated the ability of MCP-1 to predict long term mortality in addition to C-reactive protein, IL-6, troponin I, and N-terminal pro-brain natriuretic peptide(25).
In conclusion, our study shows that elevated perioperative plasma MCP-1 is associated with a higher AKI and mortality risk after cardiac surgery. MCP-1 may be a useful inflammatory biomarker that can be used to identify high-risk individuals for anti-inflammatory interventions in cardiac surgery.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hillis LD, Smith PK, Anderson JL, et al. 2011 accf/aha guideline for coronary artery bypass graft surgery: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;124(23):e652–735. doi: 10.1161/CIR.0b013e31823c074e. [DOI] [PubMed] [Google Scholar]
- 2.Brown PP, Kugelmass AD, Cohen DJ, et al. The frequency and cost of complications associated with coronary artery bypass grafting surgery: Results from the united states medicare program. Ann Thorac Surg. 2008;85(6):1980–1986. doi: 10.1016/j.athoracsur.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Kilic A, Shah AS, Conte JV, et al. Understanding variability in hospital-specific costs of coronary artery bypass grafting represents an opportunity for standardizing care and improving resource use. J Thorac Cardiovasc Surg. 2014;147(1):109–115. doi: 10.1016/j.jtcvs.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Balciunas M, Bagdonaite L, Samalavicius R, Griskevicius L, Vuylsteke A. Pre-operative high sensitive c-reactive protein predicts cardiovascular events after coronary artery bypass grafting surgery: A prospective observational study. Annals of cardiac anaesthesia. 2009;12(2):127–132. doi: 10.4103/0971-9784.53442. [DOI] [PubMed] [Google Scholar]
- 5.Franke A, Lante W, Fackeldey V, et al. Pro-inflammatory cytokines after different kinds of cardio-thoracic surgical procedures: Is what we see what we know? European journal of cardiothoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2005;28(4):569–575. doi: 10.1016/j.ejcts.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clinical journal of the American Society of Nephrology : CJASN. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 7.Garg AX, Vincent J, Cuerden M, et al. Steroids in cardiac surgery (sirs) trial: Acute kidney injury substudy protocol of an international randomised controlled trial. BMJ open. 2014;4(3):e004842. doi: 10.1136/bmjopen-2014-004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue T, Abe C, Sung SS, et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nachr+ splenocytes. J Clin Invest. 2016;126(5):1939–1952. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitlock RP, Devereaux PJ, Teoh KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (sirs): A randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10000):1243–1253. doi: 10.1016/S0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 10.Shimamoto A, Chong AJ, Yada M, et al. Inhibition of toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114(1 Suppl):I270–274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 11.Frangogiannis NG, Dewald O, Xia Y, et al. Critical role of monocyte chemoattractant protein-1/cc chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115(5):584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 12.de Mendonca-Filho HT, Pereira KC, Fontes M, et al. Circulating inflammatory mediators and organ dysfunction after cardiovascular surgery with cardiopulmonary bypass: A prospective observational study. Critical care. 2006;10(2):R46. doi: 10.1186/cc4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindholm EE, Aune E, Seljeflot I, Otterstad JE, Kirkeboen KA. Biomarkers of inflammation in major vascular surgery: A prospective randomised trial. Acta Anaesthesiol Scand. 2015;59(6):773–787. doi: 10.1111/aas.12466. [DOI] [PubMed] [Google Scholar]
- 14.Ejaz AA, Kambhampati G, Ejaz NI, et al. Post-operative serum uric acid and acute kidney injury. Journal of nephrology. 2012;25(4):497–505. doi: 10.5301/jn.5000173. [DOI] [PubMed] [Google Scholar]
- 15.Drapalova J, Kopecky P, Bartlova M, et al. The influence of deep hypothermia on inflammatory status, tissue hypoxia and endocrine function of adipose tissue during cardiac surgery. Cryobiology. 2014;68(2):269–275. doi: 10.1016/j.cryobiol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. Journal of the American Society of Nephrology. 2011;22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coca SG, Garg AX, Thiessen-Philbrook H, et al. Urinary biomarkers of aki and mortality 3 years after cardiac surgery. Journal of the American Society of Nephrology : JASN. 2014;25(5):1063–1071. doi: 10.1681/ASN.2013070742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moledina DG, Parikh CR, Garg AX, et al. Association of perioperative plasma neutrophil gelatinase-associated lipocalin levels with 3-year mortality after cardiac surgery: A prospective observational cohort study. PloS one. 2015;10(6):e0129619. doi: 10.1371/journal.pone.0129619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haller H, Bertram A, Nadrowitz F, Menne J. Monocyte chemoattractant protein-1 and the kidney. Current opinion in nephrology and hypertension. 2016;25(1):42–49. doi: 10.1097/MNH.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 20.Yadav A, Saini V, Arora S. Mcp-1: Chemoattractant with a role beyond immunity: A review. Clinica Chimica Acta. 2010;411(21–22):1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Kashyap S, Warner GM, Hartono SP, et al. Blockade of ccr2 reduces macrophage influx and development of chronic renal damage in murine renovascular hypertension. American journal of physiology Renal physiology. 2016;310(5):F372–384. doi: 10.1152/ajprenal.00131.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bautista E, Arcos M, Jimenez-Alvarez L, et al. Angiogenic and inflammatory markers in acute respiratory distress syndrome and renal injury associated to a/h1n1 virus infection. Experimental and molecular pathology. 2013;94(3):486–492. doi: 10.1016/j.yexmp.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 23.de Lemos JA, Morrow DA, Sabatine MS, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107(5):690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 24.Tunon J, Blanco-Colio L, Cristobal C, et al. Usefulness of a combination of monocyte chemoattractant protein-1, galectin-3, and n-terminal probrain natriuretic peptide to predict cardiovascular events in patients with coronary artery disease. Am J Cardiol. 2014;113(3):434–440. doi: 10.1016/j.amjcard.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Kavsak PA, Ko DT, Newman AM, et al. Risk stratification for heart failure and death in an acute coronary syndrome population using inflammatory cytokines and n-terminal pro-brain natriuretic peptide. Clinical chemistry. 2007;53(12):2112–2118. doi: 10.1373/clinchem.2007.090613. [DOI] [PubMed] [Google Scholar]
- 26.Bihorac A, Baslanti TO, Cuenca AG, et al. Acute kidney injury is associated with early cytokine changes after trauma. The journal of trauma and acute care surgery. 2013;74(4):1005–1013. doi: 10.1097/TA.0b013e31828586ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


