Abstract
Objective
To evaluate the expression of PD-L1 (programmed death 1 ligand 1, PD-L1) and its clinical significance in breast invasive ductal carcinoma.
Methods
Tumor samples were collected from 64 cases of breast invasive ductal carcinoma patients, and tumor adjacent normal breast tissue were obtained as normal control. The expression of PD-L1 were examined by immunohistochemical staining and real time PCR assay, its correlations with patients’ clinical pathological characteristics were analyzed.
Results
PD-L1 was found to be over-expressed in 24 of 64 (37.5%) breast invasive ductal carcinoma samples, while in 1 of 22 (4.5%) tumor adjacent normal breast tissue which indicated PD-L1 was higher expressed in breast invasive ductal carcinoma samples than the tumor adjacent normal breast tissue (P < 0.05). PD-L1 positive expression was associated with clinical pathological characteristics of TNM stage and pathology grading (P < 0.05). However, PD-L1 positive expression was not correlated with age (P > 0.05), menstruation status (P >0.05), family history of breast cancer (P > 0.05), tumor diameter (P > 0.05), lymph node metastasis (P > 0.05) and tumor location (P > 0.05).
Conclusion
PD-L1 may play an important role in invasive ductal carcinoma, which could be a potential indicator for advanced clinical stage and poor prognosis.
Keywords: Programmed death 1 ligand 1, Breast invasive ductal carcinoma, Clinical characteristics
1. Introduction
Breast cancer is one of leading cause of cancer related death worldwide [1]. In western countries, most breast cancer cases can be diagnosed at an early stage given the availability of technology for early detection [2]. However, more than 40% of breast cancer cases admitted to hospitals in developing countries are at advanced stages [3]. Although effective chemotherapy, endocrine therapy, and trastuzumab-targeted therapy have been used in the clinical treatment of breast cancer, metastasis disease is still not cured. One-third of patients with advanced breast cancer eventually experience metastases and death [4]. Therefore, the early diagnosis and treatment of breast cancer is essential to its prognosis.
Programmed death ligand 1 (PD-L1), an immunoglobulin superfamily haplotype I transmembrane glycoprotein associated with apoptosis, binds to the programmed cell death-1 (PD-1) receptor [5]. It has been reported that PD-L1 expression is up regulated in human tumor cell lines of ovarian cancer, lymphoma, and etc, suggesting that it has a close relationship with tumorigenesis and development [6]. Numerous studies focused on PD-L1 expression in various malignant carcinomas, such as laryngeal cancer, colon cancer, and lung cancer. However, few studies on PD-L1 expression in breast cancer tissue have been conducted. This study aims to investigate PD-L1 expression in breast cancer by immunohistochemical staining and quantitative real-time PCR assay, which are commonly used in clinical pathology laboratory. And further discussion about the association between PD-L1 expression and clinical pathological characteristics in breast invasive ductal carcinoma patients was necessary.
2. Materials and methods
2.1. Patients
This study collected 64 cases of paraffin-embedded tissue specimens of breast invasive ductal carcinoma. All specimens were from patients with complete medical records in the pathology department of Lishui central hospital and who were admitted from February 2013 to March 2015. The patients inclusion criteria were: The patients were diagnosed with invasive ductal carcinoma with pathology confirmation; The patients had relative complete medical records; The patients did not received any preoperative chemoradiation or biotherapy; Written informed consents were obtained from all of the patients for their samples. The histological type of all cases was invasive ductal carcinoma. Moreover, 22 cases of normal peritumorial breast tissues (normal breast tissue taken from over 5 cm away from the edge of the breast tumor) were also collected. All the cases were female and did not receive radiotherapy or chemotherapy prior to surgery. The clinical data was complete. There were 28 cases with tumor diameters < 2 cm and 36 cases with tumor diameters ≥ 2 cm. Histopathological grading of tissue samples showed 18 cases of grade I tumors and 46 cases of grades II or III tumors. Tumor staging in accordance with the criteria of TNM staging of breast cancer by AJCC (2002) revealed that there were 42 cases in stages I or II and 22 cases in stage III.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
2.2. Immunohistochemistry assay
Paraffin-embedded specimens were cut into 4-μm sections. After dewaxing, dehydration in benzene, and hydration, the sections were stained by the streptavidin-peroxidase immunohistochemical method (SP method). The specific steps were described in the kit’s instruction manual. PD-L1 protein expression was detected for all specimens. A positive control was established for each staining. PBS was used as a negative control instead of a primary antibody.
2.3. Real-time quantitative PCR
RNA was extracted from the specimen with the following steps: dewaxing, tissue crushing, stratification, sedimentation, drying, and dissolution. The specific steps were performed according to the manufacturer’s instructions. Total RNA purity and concentration were determined by ultraviolet spectrophotometry photometer. Reverse transcription process was performed in accordance with the manufacturer’s instructions. The Ct value of the measured gene was corrected as follows: the Ct value of the internal reference gene GAPDH was subtracted from the Ct value of the target gene PD-L1 to represent the relative copy number of the gene to be measured: ΔCt = CtPLD-L1 - CtGAPDH. The average of each group ΔCt was then calculated.
2.4. PD-L1 positive and negative expression criteria
PD-L1 presents a positive color of yellow to brown particles in the cytoplasm. PD-L1 expression was assessed with semi-quantitative integration by combining the percentage of staining intensity and positive cells (immunohistochemical scores, IHS), IHS = a × b [7], where a represents the staining intensity and b represents the percentage of positive cells. The whole field of view of the section was first observed at low magnification. Then, five high power fields (×400) were randomly selected to view cancer cells and cancer stroma. The grading for “a” was as follows: 0 = no coloring, 1 point = light yellow, 2 points = brown yellow and 3 points = dark brown. The grading for “b” was as follows: 1 point = ≤10%, 2 points = 10%–50%, 3 points = >50%. When the two scores were multiplied, a result of ≥ 3 points indicated positive expression.
2.5. Statistical analysis
Analysis was conducted by SPSS 18.0 statistical software. PD-L1 expression and its relationship with clinicopathological features of breast cancer patients were analyzed by χ2 test. qRT-PCR results were analyzed by t test, where P<0.05 signified a statistically significant difference.
3. Results
3.1. Expression of PD-L1 in breast invasive ductal carcinoma and normal tissues
Immunohistochemical staining results showed that PD-L1 protein was highly expressed in invasive ductal carcinoma tissues with the positive rate of 37.5% (24/64). PD-L1 proteins were mainly located in the cytoplasm of cancer cells and cancer stromal lymphocytes (Figure 1). The 22 cases of peritumorial breast tissues lowly expressed PD-L1 protein at a positive rate of 4.5% (1/22). The difference between invasive ductal carcinoma and normal tissue was statistically different (P< 0.05).
Figure 1.
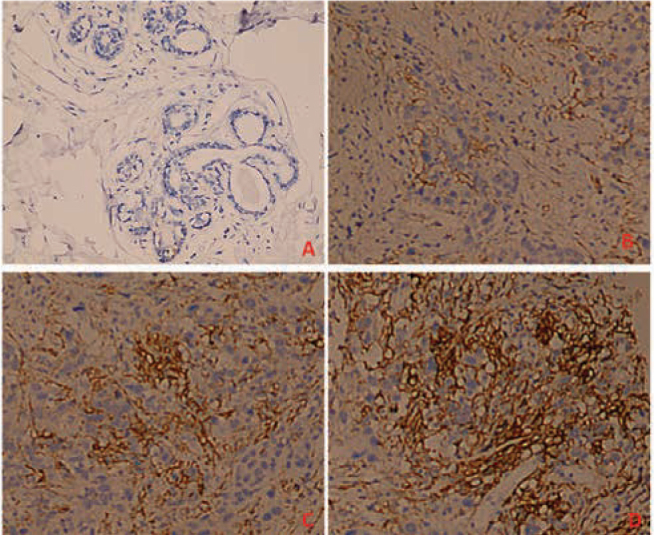
PD-L1 expression in breast cancer tissues and normal peritumorial tissues. (A: PD-L1 negatively expressed in normal breast tissues; B: PD-L1 lowly expressed in infiltrating ductal carcinoma tissues; C: PD-L1 highly expressed in infiltrating ductal carcinoma tissues) ×20.
3.2. Expression of PD-L1 and clinicopathological features of patients
PD-L1 expression was not significantly correlated with age, menopause history, family history, tumor size, lymph node metastasis, and tumor location (Pall>0.05). However, PD-L1 expression was correlated with clinical stage (P<0.05) and histopathological tumor grade (P<0.05), as shown in Table 1.
Table 1.
PD-L1 expression and clinical characteristics of patients with breast invasive ductal carcinoma [n(%)]
| Clinical characteristics | n=64 | Positive(n=24) | Negative (n=40) | Chi-square | P |
|---|---|---|---|---|---|
| Age | 0.40 | 0.52 | |||
| <55 | 19 | 6(31.6) | 13(68.4) | ||
| ≥55 | 45 | 18(40.0) | 27(60.0) | ||
| Tumor location | 0.05 | 0.82 | |||
| Upper outer quadrant | 58 | 22(37.9%) | 36(62.1) | ||
| Others | 6 | 2(33.3) | 4(66.7) | ||
| Menopause | 0.01 | 0.93 | |||
| No | 11 | 4 (36.4) | 7 (63.6) | ||
| Yes | 53 | 20 (37.7) | 33 (62.3) | ||
| Tumor diameter | 0.07 | 0.79 | |||
| <2 | 28 | 10(35.7) | 18(64.3) | ||
| ≥2 | 36 | 14(38.9) | 22(61.1) | ||
| Lymph node metastasis | 0.02 | 0.90 | |||
| Yes | 30 | 11(36.7) | 19(63.3) | 0.29 | |
| No | 34 | 13(38.2) | 21(61.8) | ||
| Clinical stage | 5.33 | 0.02 | |||
| I – II | 42 | 20 (47.6) | 22 (52.4) | ||
| III | 22 | 4 (18.2) | 18 (81.8) | ||
| Pathology grading | 4.64 | 0.03 | |||
| I – II | 18 | 3(16.7) | 15(83.3) | ||
| II – III | 46 | 21 (45.7) | 25(54.3) | ||
| Breast cancer family history | 0.28 | 0.59 | |||
| Yes | 4 | 1(25.0) | 3(75.0) | ||
| No | 60 | 23(38.3) | 37(61.7) | ||
3.3. Expression of PD-L1 mRNA ininvasive ductal carcinoma
Real-time quantitative PCR results showed that PD-L1 was highly expressed in breast invasive ductal carcinoma with an average ΔCt of 7.45±1.82. PD-L1 was lowly expressed in normal peritumorial breast tissues with an average ΔCt of 14.17±3.53. The difference of PD-L1 mRNA expression was statistically different (P<0.05), Figure 2.
Figure 2.
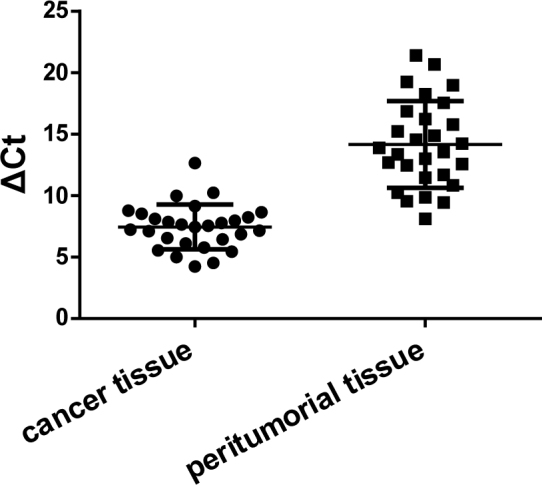
Expression of PD-L1 mRNA in cancer tissue and peritumorial tissue
4. Discussion
Several studies [8, 9] have investigated PD-L1 expression in breast cancer specimens via immunohistochemical methods and found that PD-L1 expression is correlated with ER-negative, PR-negative and histopathological grade III in patients of breast cancer. Studies on breast cancer with lymph node metastasis found that PD-L1 expression is positively correlated with a large lymph node volume and tumors that express positive human epidermal growth factor receptor 2 (HER2) [10,11]. Moreover, our study showed that PD-L1 was highly expressed in invasive ductal carcinoma tissues and that PD-L1 was positively correlated with clinical stages and histopathological tumor grade. In a tumor-bearing mouse model, treatment with anti-PD-L1 monoclonal antibodies significantly inhibited tumor growth and cleared tumor cells; some cases even showed complete remission [12]. Compared with the single treatment of cytotoxic T lymphocyte therapy, the combined use of cytotoxic T lymphocytes can effectively improve the survival rate of tumor-bearing mice [13]. Therefore, the PD-1/PD-L1 pathway may be involved in the recurrence and metastasis of breast cancer subtypes, such as triple negative (with negative ER, PR, and HER2 expression) breast cancer [8].
Chemotherapy is generally used clinically to inhibit cancer cells proliferation, invasion, and metastasis. Breast cancer is commonly treated with anthracycline chemotherapy drugs. Studies have shown that adriamycin reduces PD-L1 expression on the surface of breast cancer cells, promotes the specific killing of tumor cells by T cells, and inhibits the binding of PD-L1 and PD-1 on the T-cell surface [14]. Studies have proved that although adriamycin effectively reduces PD-L1 expression on the surface of breast cancer cells, PD-L1 expression is up-regulated in the nuclei of breast cancer cells[8, 15]. PD-L1 promotes the nuclear migration of PD-L1 on the tumor cell membrane to increase PD-L1 expression in the cell nucleus; this mechanism is a self-protective mechanism of tumor cells to prevent apoptosis during cancer chemotherapy[16]. Nimesulide selectively inhibits scyclooxygenase II and induces PD-L1 expression on the surface of breast cancer cells through interferons, thereby promoting the apoptosis of cancer cells while simultaneously inhibiting metastasis and progression [17]. However, given that chemotherapy drugs are not highly selective, chemotherapy will inevitably damage the body’s normal cells, resulting in adverse drug reactions. CD8+ T-cell can inhibit PD-L1 expression on the surfaces of tumor cells and promote the apoptosis of tumor cells. CD8+T-cell can be activated by transfecting the costimulatory molecule CD80 in human tumor cell lines via RNA interference technology. Therefore, CD80 is a potential drug target for anti-tumor therapy. Using costimulatory molecules as therapeutic drugs can not only facilitate targeted tumor treatment, but also reduce the adverse reactions caused by traditional chemotherapy drugs [18].
In our present study, we found that PD-L1 positive expression was associated with clinical pathological characteristics of TNM stage and pathology grading, which indicated that PD-L1 may play an important role in invasive ductal carcinoma of breast cancer patients. However, because of small sample size and short term follow-up period, the correlation between PD-L1 expression and prognosis of breast cancer patients was not evaluated. So, more patients and long term follow-up are need for further evaluation PD-L1 expression and patients’s survival.
In recent years, the wide clinical applications of radiotherapy, chemotherapy, and medical therapy have significantly improved the prognosis of breast cancer patients. However, triple-negative breast cancer and metastatic breast cancer are still difficult to management. The detection of PD-L1 can provide guidance for the selection of appropriate chemotherapeutic drugs. PD-L1 can also be used as an independent factor to determine prognosis, and specific anti-PD-L1 monoclonal antibodies are novel concepts to consider in the immune therapy of breast cancer.
Footnotes
Conflict of interest statement: Authors state no conflict of interest.
References
- [1].DeSantis C, Siegel R, Bandi P. et al. Breast cancer statistics. CA Cancer J Clin. 2011;2011;61(6):409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- [2].Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer[J] Oncologist. 2004;9(6):606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- [3].Ezzat AA, Ibrahim hEM, Raja MA. et al. Locally advanced breast cancer in Saudi Arabia: high frequency of stage III in a young population[J] Medical oncology (Northwood, London, England) 1999;16(2):95–103. doi: 10.1007/BF02785842. [DOI] [PubMed] [Google Scholar]
- [4].Newman LA.. Epidemiology of locally advanced breast cancer[J] Semin Radiat Oncol. 2009;19(4):195–203. doi: 10.1016/j.semradonc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- [5].Gianchecchi E, Delfino DV, Fierabracci A.. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity[J] Autoimmun Rev. 2013;12(11):1091–1100. doi: 10.1016/j.autrev.2013.05.003. [DOI] [PubMed] [Google Scholar]
- [6].Pedoeem A, Azoulay-Alfaguter I, Strazza M. et al. Programmed death-1 pathway in cancer and autoimmunity. [J]. Clinical immunology (Orlando, Fla.) 2014;153(1):145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- [7].Mu CY, Huang JA, Chen Y. et al. High expression of PD-L1 lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltr; dendritic cells maturation. [J] Medical oncology (Northwood, London, England) 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- [8].Mittendorf EA, Philips AV, Meric-Bernstam F. et al. PD-L1 expression in triple-negative breast cancer[J] Cancer Immunol Res. 2014;2(4):361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Soliman H, Khalil F, Antonia S.. PD-L1 expression is increased in a subset of basal type breast cancer cells[J] PLoS One. 2014;9(2):e88557. doi: 10.1371/journal.pone.0088557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Janakiram M, Abadi YM, Sparano JA. et al. T cell coinhibition and immunotherapy in human breast cancer[J] Discov Med. 2012;14(77):229–236. [PMC free article] [PubMed] [Google Scholar]
- [11].Sendur MA, Aksoy S, Demirci S. et al. Can targeted programmed death-1 antibody be a new treatment approach in breast cancer[J] J BUON. 2014;19(2):584. [PubMed] [Google Scholar]
- [12].Wang J, Jensen M, Lin Y. et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. [J] Hum Gene Ther. 2007;18(8):712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- [13].Charo J, Finkelstein SE, Grewal N. et al. Bcl-2 overexpression: enhances tumor-specific T-cell survival[J] Cancer Res. 2005;65(5):2001–2008. doi: 10.1158/0008-5472.CAN-04-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hasan A, Ghebeh H, Lehe C. et al. Therapeutic targeting of B7-H1 in breast cancer. [J] Expert Opin Ther Targets. 2011;15(10):1211–1225. doi: 10.1517/14728222.2011.613826. [DOI] [PubMed] [Google Scholar]
- [15].Muenst S, Schaerli AR, Gao F. et al. Expression of programmed; death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. [J] Breast Cancer Res Treat. 2014;146(1):15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ghebeh H, Lehe C, Barhoush E. et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule[J] Breast Cancer Res. 2010;12(4):R48. doi: 10.1186/bcr2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liang M, Yang H, Fu J.. Nimesulide inhibits IFN-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells by COX-2 and PGE2 independent mechanisms[J] Cancer Lett. 2009;276(1):47–52. doi: 10.1016/j.canlet.2008.10.028. [DOI] [PubMed] [Google Scholar]
- [18].Haile ST, Bosch JJ, Agu NI. et al. Tumor cell programmed death ligand 1-mediated T cell suppression is overcome by coexpression of CD80[J] Journal of immunology (Baltimore, Md.: 1950) 2011;186(12):6822–6829. doi: 10.4049/jimmunol.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]


