Summary
Renal fibrosis is the common final manifestation of chronic kidney diseases and usually results in end‐stage renal failure. In this study, we evaluated the effect of fingolimod (FTY720), an analogue of sphingosine 1‐phosphate (S1P), as a treatment for the unilateral ureteral obstruction (UUO)‐induced renal fibrosis animal model. We treated mice with FTY720 at a dosage of 1 mg/kg/day by intragastric administration from day 1 until day 7. The control group received the same amount of saline. FTY720 reduced significantly the urine albumin/creatinine ratio (UACR) in treated UUO mice. FTY720 treatment also caused a significant decrease in interstitial expansion and collagen deposition in the kidney, accompanied by reduced mononuclear cell recruitment and inflammatory cytokine expression. In addition, the expression levels of the endothelial cell adhesion molecules P‐selectin and vascular cell adhesion protein 1 (VCAM‐1) were suppressed in the ligated kidney by FTY720 administration, suggesting reduced renal endothelial cell activation. Furthermore, in renal interstitial fibroblast normal rat kidney (NRK)‐49F cells, FTY720 significantly affected transforming growth factor (TGF)‐β‐induced α‐smooth muscle actin (SMA) expression and collagen synthesis by inhibiting both the Mothers against decapentaplegic homologue (Smad)2/3 and phosphatidylinositol 3‐kinase/protein kinase B/glycogen synthase kinase 3 beta (PI3K/AKT/GSK3β) signalling pathways. S1P1 knock‐down by siRNA reversed this effect significantly in our fibroblast cell culture model. Therefore, FTY720 attenuates renal fibrosis via two different mechanisms: first, FTY720 suppresses the synthesis of extracellular matrix in interstitial fibroblasts by interfering with TGF‐β signalling; and secondly, FTY720 affects endothelial cell activation and chemokine expression, thereby reducing immune cell recruitment into the kidney.
Keywords: endothelial activation, fibroblast, FTY720, leucocyte infiltration, renal fibrosis
Introduction
In the past 10 years, chronic kidney disease has become highly prevalent in developing countries and is accompanied by the increased prevalence of risk factors, such as diabetes and hypertension 1. Renal fibrosis is the common final manifestation of almost all types of chronic kidney diseases and is characterized by extracellular matrix deposition that usually results in end‐stage renal failure 2, 3. Renal fibrosis is a progressive process involving immune cell recruitment and intrinsic renal epithelial cell transition, accompanied by the production of inflammatory cytokines and extracellular matrix (ECM), driving the fibrotic process 3. Various injuries may initially promote renal fibrosis and induce inflammation in the kidney by recruiting inflammatory cells, particularly T cells and macrophages, through the secretion of chemokines 3, 4, 5. Infiltrating immune cells may further enhance renal inflammation by secreting more cytokines and chemokines 6. Sustained inflammation promotes fibrogenesis through activation of fibroblasts and epithelial to mesenchymal transition (EMT) to form myofibroblasts, which produce large amounts of ECM deposits in the kidney, resulting ultimately in renal fibrosis 3. Thus, the activation and transformation of fibroblasts may be the final committed step during the progression of renal fibrosis.
Sphingosine 1‐phosphate (S1P) is a bioactive sphingolipid that acts extracellularly via G protein‐coupled receptors to regulate numerous physiological processes, including vascular permeability regulation 7, angiogenesis 8, cell proliferation 9 and lymphocyte trafficking 10. S1P is formed by phosphorylation of the membrane lipid sphingosine catalyzed by sphingosine kinase 1 (SphK1) or sphingosine kinase 2 (SphK2). S1P elicits its biological effects through high‐affinity binding to G protein‐coupled receptors, particularly S1P receptors 1–5 (S1P1–5). S1P receptors are expressed in a wide variety of cell types and play an essential role in immune cell migration 11, 12, 13, 14. In maturing T cells, expression of S1P1 is a key regulator for emigration from the thymus 15. Moreover, in human monocytes, S1P induces thrombin receptor protease‐activated receptor‐4 (PAR‐4) expression to enhance chemotaxis 16. Fingolimod (FTY720) is a structural analogue of the sphingolipid S1P and is derived from myriocin, a fungal derivative used in Chinese medicine 17. Phosphorylated FTY720 binds to S1P1 with high affinity, induces receptor internalization and causes immune cells to become insensitive to S1P gradients 18. Interestingly, targeting S1P receptor signalling with FTY720 results in beneficial effects in many inflammatory conditions of the central nervous system (CNS). For example, FTY720 exhibits neuroprotective effects in CNS injury models of cerebral ischaemia 19 and spinal cord injury 20. FTY720 also elicits neuroprotective effects and significantly decreases oedema, neuronal apoptosis and brain atrophy in a mouse model of intracerebral haemorrhage 21. More importantly, FTY720 is a therapeutically efficacious, Food and Drug Administration (FDA)‐approved drug for the treatment of relapsing–remitting multiple sclerosis. The dominant action of FTY720 is attributed largely to autoreactive T cell sequestration in lymph nodes 22. Moreover, a series of studies have demonstrated therapeutic effects of FTY720 in animal models of other autoimmune diseases, such as psoriasis, polymyositis and lupus 23. Recent studies have also reported that FTY720 significantly prevents graft rejection in renal transplantation 24 and also has notable effects on kidney ischaemia/reperfusion injury 25.
In this study, we showed the beneficial effect of FTY720 on renal function and fibrogenesis in an animal model of unilateral ureteral obstruction (UUO)‐induced renal fibrosis. A dramatic reduction in immunocytes, especially macrophages and CD4+ T cells, was noted after FTY720 treatment in the ligated kidneys. We also found a significant down‐regulation of adhesion molecules in the ligated kidneys after FTY720 treatment. Transforming growth factor (TGF)‐β‐mediated ECM synthesis was also affected significantly by FTY720 via inhibition of both the Mothers against decapentaplegic homologue (Smad)2/3 and phosphatidylinositol 3‐kinase/protein kinase B/glycogen synthase kinase 3 beta (PI3K/AKT/GSK3β) signalling pathways. Overall, our data show that FTY720 can target S1P signals simultaneously in both renal fibroblasts and endothelial cells to reduce the progression of renal fibrosis.
Materials and methods
Animal preparation
C57BL/6 mice (male, 6–8 weeks old, 20–25 g weight) were purchased from the Model Animal Research Center of Nanjing University and cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee at Nanjing Medical University. Mice were divided randomly into three groups: (i) sham‐operated mice; (ii) UUO mice; and (iii) FTY720‐treated UUO mice. For the UUO operation, mice were anaesthetized with an intraperitoneal injection of ketamine (200 mg/kg) and xylazine (10 mg/kg) before the left ureter was ligated with a 4.0 silk suture. As normal controls, sham‐operated mice had their left ureters exposed without ligation. FTY720 (Cayman Chemical Company, Ann Arbor, MI, USA) was given at a dose of 1 mg/kg/day by intragastric administration immediately after surgery for 7 consecutive days. Mice were killed 7 days after sham or UUO operation, and tissues were collected for subsequent experiments.
Urine albumin assay
Urine was harvested after UUO or UUO/FTY720 challenge. The samples were then assayed directly as follows. Double‐distilled water (10 μl), standards (10 μl) and samples (10 μl) were transferred in duplicate into the wells of a clear‐bottomed 96‐well plate. Then, 2·5 ml working reagent was added, and the plate was tapped lightly to mix the solution. The plate was incubated for 10 min at room temperature, and the optical density (OD) was read at 628 nm. Urine albumin was measured using a mouse urine albumin kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer's instructions.
Urine creatinine assay
Urine was harvested after UUO or UUO/FTY720 challenge and was prepared for an assay with the following steps. Double‐distilled water (6 μl), standards (6 μl) and samples (6 μl) were transferred in duplicate into the wells of a clear‐bottomed 96‐well plate. Working Reagent A was prepared with 180 μl added to each well, and the OD was read at 546 nm after 5 min. Then, 60 μl Working Reagent B was added quickly to all wells, and the OD was read again at 546 nm after 5 min. Urine creatinine was measured using a mouse creatinine kit (Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's instructions.
Cell culture and treatment
The normal rat kidney (NRK)‐49F kidney fibroblast cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium/F12 (DMEM/F12; GE Healthcare HyClone, Logan, UT, USA) containing 10% fetal bovine serum (Gibco, Gaithersburg, MD, USA) and 1% 100 U/ml penicillin and 100 μg/ml streptomycin in 5% CO2 and at 37°C. For TGF‐β and FTY720 treatment, NRK‐49F cells were seeded in six‐well plates and pretreated with FTY720 (5 μM) for 30 min; these cells were then cultured with TGF‐β (2 μg/ml; R&D, Minneapolis, MN, USA) for another 12 h. To determine whether the effect of FTY720 was mediated by binding to S1P1, cells were transfected with either S1P1 siRNA (0·15 μM) or a negative control siRNA. The rat S1P1 siRNA sequences were as follows: forward, 5′‐AAGCACTATATTCTCTTCTGC‐3′; and reverse, 5′‐AAGCAGAAGAGAATATAGTGC‐3′).
Morphological analysis
Kidneys were isolated after UUO or UUO/FTY720 challenge, fixed in 10% formalin at 4°C for 24 h, embedded in paraffin and sliced into 4‐μm sections. These paraffin sections were processed for haematoxylin and eosin (H&E) and Masson staining according to standard methods and examined under a light microscope. Images were obtained at ×400 magnification for each sample.
Western blot analysis
Cells were lysed using radioimmunoprecipitation assay (RIPA) lysis buffer with 1 mM phenylmethylsulphonyl fluoride (PMSF) and 1 mM phosphatase inhibitor for 30 min on ice and centrifuged at 15000 g for 15 min at 4°C. The supernatant was then diluted in boiled 5 × sodium dodecyl sulphate–polyacryamide gel electrophoresis (SDS‐PAGE) loading buffer (10 min, 100°C). Proteins were extracted from both the ligated and contralateral kidneys. Protein concentrations were assessed using a BCA kit (ThermoFisher, Rockford, AL, USA). Equal amounts of protein (20 μg) were separated by SDS‐PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. Non‐specific antibody binding was blocked with 5% skimmed milk for 1 h. The membrane was incubated with primary antibodies, including anti‐α‐smooth muscle actin (SMA) (Sigma, St Louis, MO, USA), anti‐collagen IV (Abcam, Cambridge, UK),anti‐P‐selectin (Abcam), anti‐vascular cell adhesion molecule‐1 (VCAM)‐1 (Abcam), anti‐PI3K/phospho‐PI3K (Cell Signaling Technology, Danvers, MA, USA), anti‐Smad2/3 (Cell Signaling Technology), anti‐P‐Smad2/3 (Cell Signaling Technology), anti‐Smad2/P‐Smad2 (Cell Signaling Technology), anti‐AKT/P‐AKT (Cell Signaling Technology) and anti‐GSK3β/P‐GSK3β (Cell Signaling Technology), at 4°C overnight followed by incubation with a horseradish peroxidase (HRP)‐conjugated secondary antibody at room temperature for 2 h. The bands were visualized using enhanced chemiluminescence reagents according to the manufacturer's instructions.
Quantitative real‐time–polymerase chain reaction (qRT–PCR)
Total RNA was extracted from kidneys, and reverse transcription was performed to obtain single‐stranded cDNA. PCR was performed in a 20‐μl volume containing 4 μl cDNA, 2 μl of each primer (forward and reverse), 10 μl mix and 2 μl ddH2O. cDNA was prepared using random primers with purified total RNA (1 μg) from each sample, and quantitative PCR was carried out with the following primers: TGF‐β forward, 5′‐TGCTAATGGTGGACCGCAA‐3′; TGF‐β reverse, 5′‐CACTGCTTCCCGAATGTCTGA‐3′; tumour necrosis factor (TNF)‐α forward, 5′‐ACGGCATGGATCTCAAAGAC‐3′; TNF‐α reverse, 5′‐AGATAGCAAATCGGCTGACG‐3′; interleukin (IL)‐6 forward, 5′‐ACAACCACGGCCTTCCCTAC‐3′; IL‐6 reverse, 5′‐TCCACGATTTCCCAGAGAACA‐3′; IL‐1β forward, 5′‐TGTCTTGGCCGAGGACTAAGG‐3′; IL‐1β reverse, 5′‐TGGGCTGGACTGTTTCTAATGC‐3′; chemokine ligand (CCL)5 forward, 5′‐ATATGGCTCGGACACCACTC‐3′; CCL5 reverse, 5′‐GTGACAAACACGACTGCAAGA‐3′; macrophage inflammatory protein (MIP)‐1α forward, 5′‐ACCTGCTCAA‐CATCATGAAGG‐3′; MIP‐1α reverse, 5′‐AGATGGAGCTATGCAGGTGG‐3′; glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) forward, 5′‐TGCAGTGGCAAAGTGGAGATT‐3′; and GAPDH reverse, 5′‐TCGCTCCTGGAAGATGGTGAT‐3′. Quantitative PCR was carried out using the LightCycler with SYBR Green (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer's instructions. The amplification conditions were as follows: 32 cycles of 95°C (20 s), 57·2°C (30 s) and 72°C (30 s), followed by 95°C (2 min). Quantitative PCR assays were conducted in triplicate for each sample and were performed using the 2−ΔΔCt method.
Flow cytometry
Mice were anaesthetized and perfused with ice‐cold phosphate‐buffered saline (PBS) to remove the blood before the kidneys were isolated and chopped into 1 mm3 pieces. Cell suspensions were collected after the kidney pieces were digested by collagenase and DNase. Kidney immune cells were then isolated from the cell suspension. Briefly, the kidney cell suspensions were resuspended in 3 ml of 30% Percoll after washing with PBS and added slowly onto the surface of 3 ml of 70% Percoll. Then, 1 ml of PBS was added gently onto the surface of the 30% Percoll solution. Immune cells were collected at the interface between the 30% and 70% Percoll after centrifugation at 1000g for 30 min. Single immune cell suspensions were then incubated with mouse Fc‐receptor blocking reagent prior to antibody labelling to prevent unspecific binding. Different cell populations were identified by incubation with fluorescein isothiocyanate (FITC)‐anti‐CD45, phycoerythrin (PE)‐anti‐CD4, FITC‐anti‐CD3 and FITC‐anti‐F4/80 antibodies (all labelled antibodies were purchased from eBioscience, San Diego, CA, USA) for 30 min in the dark. Finally, the stained cells were analysed using a CytoFLEX (Beckman Coulter, Miami, FL, USA) flow cytometer. The acquired data were analysed using CytExpert software (Beckman Coulter). The gate for CD45+ cells was set on total live immune cells. The gate for F4/80+ cells was set on CD45+ cells, and the same gate was applied to CD3+ cells. The gate for CD4+ cells was set on CD3+ cells.
Statistical analysis
All data are presented as the means ± standard error of the mean (s.e.m.). The groups were compared using Student's t‐test and GraphPad Prism@ software (GraphPad Software, Inc., San Diego, CA, USA), with P < 0·05 considered to indicate a statistically significant difference.
Results
Renal function is improved by FTY720 after UUO operation
To investigate the effects of FTY720 on renal fibrosis induced by UUO operation, C57BL/6 mice were given FTY720 at a dose of 1 mg/kg/day by intragastric administration for 7 days starting on the operation date. The mice were killed on day 7 after the UUO operation. Both the ligated and contralateral kidneys were isolated and weighed. The ligated kidneys of the UUO mice were much larger than those of the sham mice (0·46 ± 0·06 g versus 0·15 ± 0·01 g), and FTY720 treatment reduced the weights of the ligated kidneys significantly (0·46 ± 0·06 g versus 0·33 ± 0·05 g, P < 0·05) (Fig. 1a).
Figure 1.
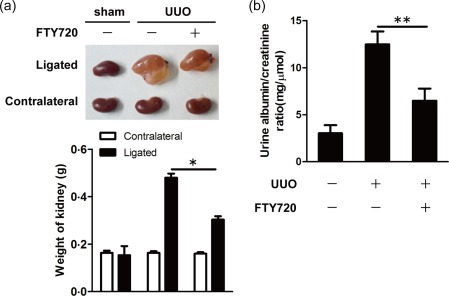
Oral administration of Fingolimod (FTY720) improved renal function in unilateral ureteral obstruction (UUO) mice. UUO mice were treated daily with FTY720 (1 mg/kg/day) or saline by intragastric administration immediately after surgery. All mice were euthanized 7 days after sham or UUO operation. (a) Seven days after the operation, the kidneys were weighed. (b) The urine albumin/creatinine ratio was measured by a urine albumin and creatinine assay. The data are presented as the mean ± standard error of the mean (s.e.m.). *P < 0·05 relative to the control or FTY720‐treated group. The data represent results from at least three independent experiments. Statistical analyses were carried out using two‐tailed paired t‐tests. [Colour figure can be viewed at wileyonlinelibrary.com]
Large plasma proteins such as albumin cannot pass through the glomerular filter. Increased urine albumin suggests injured renal function. Creatinine, a by‐product of muscle metabolism, is released normally into the urine at a constant rate, and its level in the urine is an indicator of urine concentration. The albumin/creatinine ratio (ACR) can determine more accurately how much albumin is escaping from the kidneys into the urine. Therefore, we performed a urine albumin/creatinine assay and found increased ACR in the urine of UUO mice, indicating impaired renal function after the UUO operation. Interestingly, FTY720 treatment reduced UACR effectively in the UUO mice (Fig. 1b).
Overall, these results indicate that FTY720 treatment restores significantly the impaired renal function induced by UUO.
FTY720 attenuates renal fibrosis in UUO mice
To determine the effects of FTY720 on renal fibrosis, Masson staining and H&E staining were performed on the ligated kidneys of UUO mice. As expected, kidneys from the UUO mice exhibited massive collagen deposition (the area in blue), a type of ECM produced by myofibroblasts. Interestingly, FTY720 reduced the production of collagen significantly (Fig. 2a). H&E staining also revealed improved integrity of renal parenchymal cells in the ligated kidneys from UUO mice after FTY720 treatment (Fig. 2a). Western blot analysis showed that α‐SMA, a specific marker of myofibroblasts and an indicator of fibrosis, was expressed highly in the ligated kidneys of UUO mice (Fig. 2b) but was suppressed significantly in FTY720‐treated UUO animals (Fig. 2b). Therefore, FTY720 affects the renal fibrotic process significantly in UUO‐operated mice.
Figure 2.
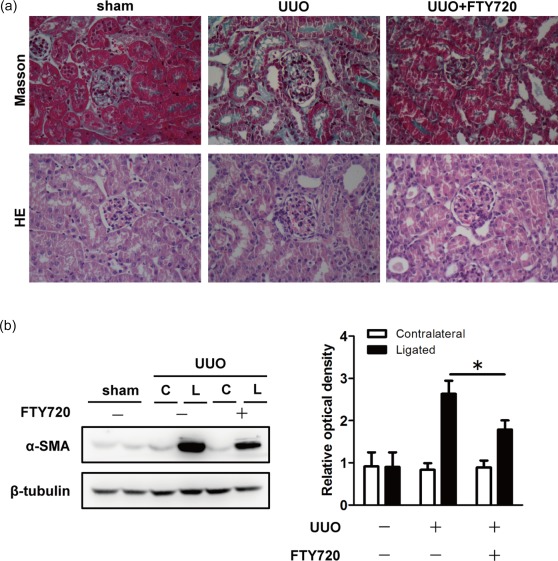
Fingolimod (FTY720) treatment attenuated renal fibrosis in ligated kidneys. Unilateral ureteral obstruction (UUO) mice were treated daily with FTY720 (1 mg/kg/day) or saline by intragastric administration immediately after surgery. Seven days after the operation, mice were euthanized, and kidney sections were prepared and stained with Masson and haematoxylin and eosin (H&E). (a) Masson staining showed massive collagen deposition (blue) in the ligated kidneys from UUO mice, and less collagen was observed after FTY720 treatment. H&E staining revealed improved integrity of the renal parenchymal cells in the ligated kidneys from unilateral ureteral obstruction (UUO) mice after FTY720 treatment. (b) Western blot analysis showed that FTY720 reduced α‐SMA expression in the ligated kidney. The data are presented as the mean ± standard error of the mean (s.e.m.). *P < 0·05 relative to the control or FTY720‐treated group. The data represent the results from at least three independent experiments. C = contralateral; L = ligated. [Colour figure can be viewed at wileyonlinelibrary.com]
FTY720 reduces renal inflammation
Renal fibrosis is a progressive process involving massive inflammation. To evaluate the effect of FTY720 on renal inflammation, we performed qRT–PCR to detect the mRNA levels of inflammatory cytokines and chemokines. As expected, the UUO operation caused significant increases in mRNA expression of many cytokines and chemokines. Interestingly, FTY720 treatment resulted in a significant reduction in mRNA levels of IL‐6 (Fig. 3a) and IL‐1β (Fig. 3b) but not TNF‐α (Fig. 3c). Additionally, FTY720 also down‐regulated the mRNA levels of chemokines, including CCL5 (Fig. 3d) and MIP‐1α (Fig. 3e). However, FTY720 had no significant effect on the mRNA levels of TGF‐β (Fig. 3f), a critical factor that triggers fibrogenesis.
Figure 3.
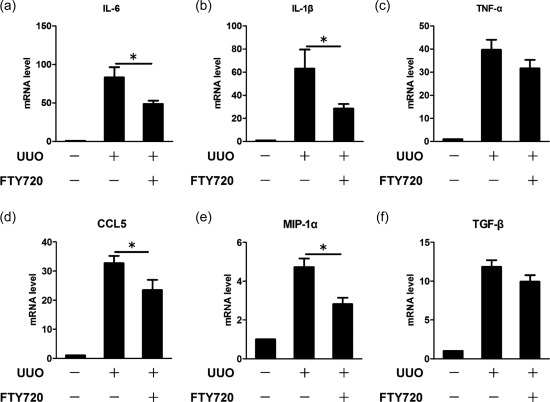
Fingolimod (FTY720) down‐regulates inflammatory cytokines in the ligated kidney. Unilateral ureteral obstruction (UUO) mice were treated with saline or FTY720 for 7 days. Total RNA was extracted, and the mRNA expression levels of inflammatory cytokines in the kidney were analysed by quantitative real‐time–polymerase chain reaction (RT–PCR). The results showed that FTY720 significantly reduced the mRNA levels of the inflammatory cytokines interleukin (IL‐6) (a) and IL‐1β (b) but not tumour necrosis factor (TNF)‐α (c), and the mRNA expression levels of chemokines such as chemokine ligand (CCL)‐5 (d) and macrophage inflammatory proteins (MIP)‐1α (e), were also down‐regulated. FTY720 tended to reduce the mRNA level of transforming growth factor (TGF)‐β, although not significantly (f). The data are presented as the mean ± standard error of the mean (s.e.m.). *P < 0·05 relative to the control or FTY720‐treated group. The data represent the results from at least three independent experiments.
Flow cytometry was performed to evaluate further the infiltration of immune cells. Seven days after UUO operation, a large amount of CD45+ leucocytes were detected in the ligated kidneys but not in the contralateral kidneys. Of these cells, 30·7% ± 17·7% stained positive for F4/80, while 36·3% ± 9·84% of the CD3+ cells were CD4+ T cells (Fig. 4a). After FTY720 treatment, the numbers of recruited macrophages and T cells detected in the kidney were decreased dramatically (Fig. 4a,b). Clearly, FTY720 affected significantly UUO‐induced leucocyte recruitment into the kidney.
Figure 4.
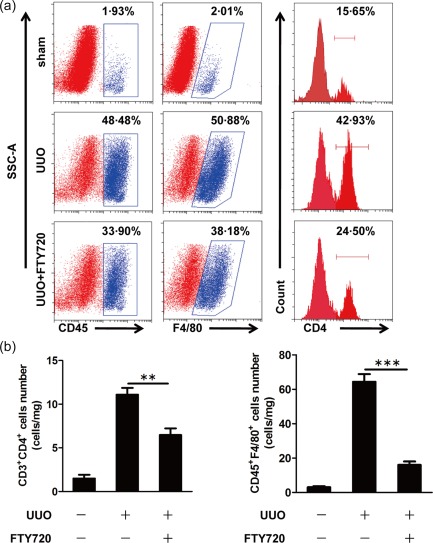
Fingolimod (FTY720) reduces immune cell infiltration in the ligated kidney. Mice were euthanized 7 days after sham or unilateral ureteral obstruction (UUO) operation, and flow cytometry analysis was performed to identify infiltrated immune cells (macrophages and CD4+ T cells) per mg kidney tissue (a). CD45‐positive leucocytes, especially F4/80‐positive macrophages and CD4‐positive T cells, were recruited into the ligated kidney after the UUO operation. FTY720 treatment reduced significantly the numbers of both macrophages and CD4+ T cells (b). The data are presented as the mean ± standard error of the mean (s.e.m.). **P < 0·01 relative to the control or FTY720‐treated groups. ***P < 0·001 relative to the control or FTY720‐treated groups. The data represent results from at least three independent experiments. [Colour figure can be viewed at wileyonlinelibrary.com]
FTY720 inhibits endothelial activation in vivo
Endothelial cells express high levels of adhesion molecules when they are activated in an inflammatory environment; as a result, leucocytes roll and adhere to the endothelium and are recruited into the tissue. The expression of adhesion molecules, such as P‐selectin and VCAM‐1, was increased significantly in the ligated kidney after the UUO operation (Fig. 5a,b). In addition, both the adhesion molecules measured were inhibited by FTY720 treatment (Fig. 5a,b), indicating that FTY720 can inhibit the activation of endothelial cells in vivo.
Figure 5.
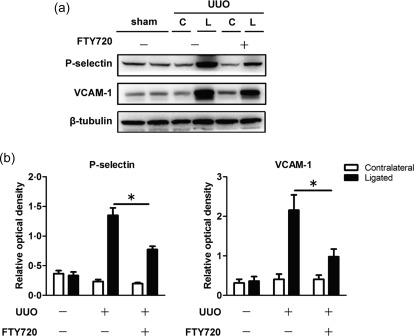
Endothelial cell adhesion molecules are down‐regulated in the ligated kidney after Fingolimod (FTY720) treatment. Unilateral ureteral obstruction (UUO) mice were treated with saline or FTY720 for 7 days. Proteins were extracted from kidney tissues. Western blot analysis showed that FTY720 suppressed significantly the expression of P‐selectin (a) and vascular endothelial cell VCAM‐1 (a) in the ligated kidneys. The optical densities of P‐selectin (b) and adhesion molecule protein 1 (VCAM‐1) (b) were normalized to that of β‐tubulin. The data are presented as the mean ± standard error of the mean (s.e.m.). *P < 0·05 relative to the control or FTY720‐treated groups. The data represent results from at least three independent experiments. C = contralateral; L = ligated.
FTY720 affects the synthesis of ECM by binding to S1P1 on renal fibroblasts and interfering with the Smad2/3 and PI3K/AKT/GSK3β signalling pathways.
Activation of fibroblasts is the final committed step of renal fibrosis. We cultured the renal fibroblast cell line NRK‐49F to investigate further the effect of FTY720 on fibroblasts in vitro and to explore the possible mechanisms. The expression of α‐SMA was up‐regulated after 12 h treatment with TGF‐β. A noticeable reduction in α‐SMA was observed after FTY720 treatment (Fig. 6a). Moreover, FTY720 also reduced the levels of type IV collagen in these cells (Fig. 6a). To determine whether FTY720 exerted its effect by binding to S1P1, siRNA was used to block the expression of S1P1 in NRK‐49F cells. After S1P1 was knocked down, the inhibitory effect of FTY720 on α‐SMA and fibronectin was reversed significantly (Fig. 6b), indicating that the effect of FTY720 on NRK‐49F was mediated by S1P1.
Figure 6.
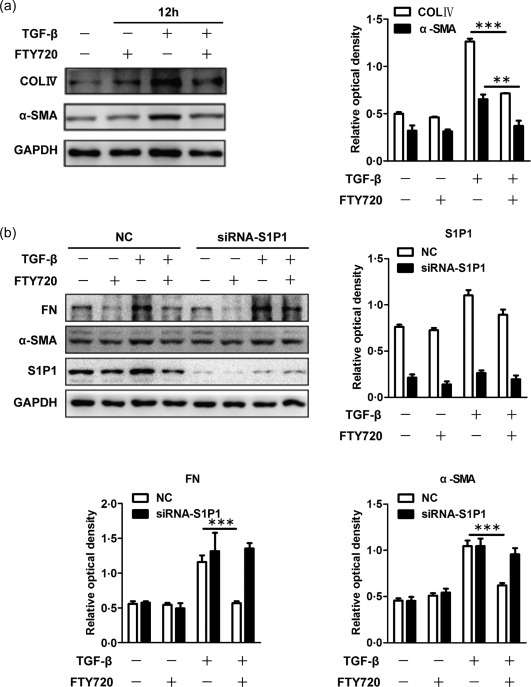
Fingolimod (FTY720) inhibited the synthesis of extracellular matrix in normal rat kidney (NRK)‐49F cells by binding with S1P1. Rat renal fibroblast NRK‐49F cells were incubated with FTY720 (5 μM), transforming growth factor (TGF)‐β (2 μg/ml) or TGF‐β plus FTY720 for 12 h. The cells were lysed, and total protein was extracted for Western blot analysis. The expression levels of α‐smooth muscle actin (SMA) and collagen IV were increased after 12 h of TGF‐β treatment, indicating activation of the NRK‐49F cells, and this activation was inhibited by FTY720 (a). The optical densities of α‐SMA and collagen IV were normalized to that of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (a). Cells were then pretreated with either S1P1‐siRNA or negative control siRNA before TGF‐β and FTY720 treatment. The inhibitory effect of FTY720 on fibronectin and α‐SMA was reversed significantly after S1P1 blockage (b). The optical densities of fibronectin, α‐SMA and S1P1 were normalized to that of GAPDH (b). The data are presented as the mean ± standard error of the mean (s.e.m.). **P < 0·01 relative to the control or FTY720‐treated groups. ***P < 0·001 relative to the control or FTY720‐treated groups. The data represent the results from at least three independent experiments.
TGF‐β has been shown to be a critical factor that activates fibroblasts to induce renal fibrosis. Because the mRNA level of TGF‐β in the ligated kidney was not reduced significantly after FTY720 treatment (Fig. 3f), it is likely that FTY720 may affect the downstream signalling of TGF‐β, including Smad or non‐Smad signalling pathways. Western blot analysis showed that FTY720 reduced significantly the levels of phospho‐Smad2/3 and phospho‐Smad2 in NRK‐49F cells (Fig. 7a). In addition, the expression of phospho‐AKT and phospho‐GSK3β was also down‐regulated, while phospho‐PI3K was unaffected (Fig. 7b). These results indicate that FTY720 interferes with renal fibrosis by affecting both the Smad2/3 and PI3K/AKT/GSK3β signalling pathways downstream of TGF‐β receptors.
Figure 7.
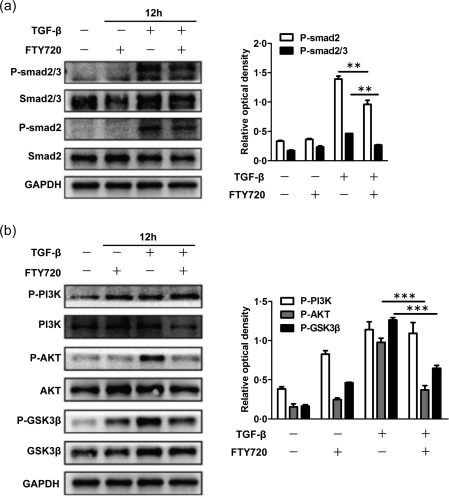
Fingolimod (FTY720) mediated its effects by inhibiting the activation of Mothers against decapentaplegic homologue (Smad)2/3 and phosphatidylinositol 3‐kinase/protein kinase B/glycogen synthase kinase 3 beta (PI3K/AKT/GSK3β). Rat renal fibroblast normal rat kidney (NRK)‐49F cells were deprived of serum in the culture medium for 12 h before incubation with FTY720 (5 μM), transforming growth factor (TGF)‐β (2 μg/ml) or TGF‐β plus FTY720 for 12 h. The cells were lysed, and total protein was extracted for Western blot analysis. The phosphorylation levels of Smad2/3 and Smad2 were increased significantly after TGF‐β treatment, and this effect was inhibited by FTY720 (a). The optical densities of P‐Smad2/3 and P‐Smad2 were normalized to that of GAPDH (a). FTY720 also reversed the up‐regulation of phospho‐PI3K signalling, including P‐AKT and P‐GSK3β, but not P‐PI3K (b). The optical densities of P‐PI3K, P‐AKT and P‐GSK3β were normalized to that of GAPDH (b). The data are presented as the mean ± standard error of the mean (s.e.m.). **P < 0·01 relative to the control or FTY720‐treated groups. ***P < 0·001 relative to the control or FTY720‐treated groups. The data represent the results from at least three independent experiments.
Discussion
Renal fibrosis is the common final pathway in almost all types of chronic kidney diseases and is increasing in prevalence worldwide. In this study, we demonstrated the beneficial effect of FTY720 on both renal fibrosis and inflammation in UUO‐operated mice. Our data showed that FTY720 was able to interfere simultaneously with chemokine expression and adhesion molecule expression on endothelial cells, therefore inhibiting leucocyte recruitment into the kidney effectively. In addition, FTY720 also affected inflammatory cytokine expression and ECM synthesis in renal parenchymal cells, which reduced fibrogenesis further. The results of our study reveal novel mechanisms that possibly contribute to the therapeutic effects of FTY720 in chronic kidney diseases.
Sustained inflammation after injury has been shown to promote fibrogenesis. The recruitment of CD4+ T cells is an important early event that precedes the infiltration of macrophages 3, 5. Classically activated M1 macrophages are also considered to have pathogenic functions that lead to renal fibrosis 26, 27, 28. Infiltration of immune cells may produce inflammatory cytokines and chemokines that recruit more immune cells, thereby amplifying the inflammatory responses in the kidney 6. The anti‐inflammatory function of FTY720 has been well documented, especially in multiple sclerosis, due mainly to its regulatory effect of accelerating autoreactive lymphocyte homing into the lymph node 29. In our study, we observed a notable reduction in CD4+ T cells and macrophages in the ligated kidneys of FTY720‐treated UUO mice, possibly caused by reduced endothelial cell activation and chemokine expression. Meanwhile, the mRNA levels of inflammatory cytokines were also decreased. Inflammatory cytokines contribute to vascular endothelial activation by inducing the expression of adhesion molecules, including VCAM‐1, intercellular adhesion molecule‐1 (ICAM‐1) and P‐selectin. Therefore, it is likely that the suppression of renal parenchymal cell activation might also have contributed to reductions in renal endothelial activation (decreased expression of adhesion molecules), ultimately decreasing infiltration of immune cells into the kidney. Nonetheless, reduced homing of immune cells may also have caused reduced levels of inflammatory cytokines in the ligated kidneys. The inactivation of endothelial cells may also reduce the adhesion and transmigration of leucocytes, thus attenuating local inflammation in the ligated kidney. Overall, FTY720 affected the activation of both renal endothelial cells and epithelial cells simultaneously, which is the primary advantage of this drug in renal fibrosis therapy.
Sustained inflammation activates renal fibroblasts to form myofibroblasts, the final critical step of renal fibrosis. In our study, FTY720 reduced α‐SMA and collagen expression, suggesting that FTY720 prevents the genesis of myofibroblasts and reduces the synthesis of ECM protein in vivo. Furthermore, FTY720 inactivated NRK‐49F cells by reducing the phosphorylation of Smad2/3 molecules and inhibiting the activation of PI3K/AKT/GSK3β molecules in the non‐Smad signalling pathway, indicating a direct effect of FTY720 on renal fibroblasts. This effective interference with multiple signalling pathways in TGF‐β‐induced fibrogenesis would constitute the second advantage of this drug in the treatment of renal fibrosis. Regarding its detailed mechanisms, other studies have discovered that FTY720 can promote PP2A phosphatase activities in many types of cells 30, 31, 32 and dephosphorylate activated signal transducer and activator of transcription‐1 (STAT‐3) 33, 34 and AKT 35, 36. In addition, it is now clear that FTY720 targets G protein‐coupled S1P receptors as a potent agonist 18, thereby regulating a variety of intracellular signalling pathways. The possibilities of the above‐mentioned mechanisms require further investigation in future studies.
Conclusion
FTY720 simultaneously affected multiple pathways in TGF‐β‐induced signalling to suppress fibrogenesis. In addition, it decreased the inflammatory response by reducing inflammatory cytokine expression and immune cell recruitment. Therefore, FTY720 is a potential effective treatment for renal fibrosis.
Author contributions
Z. H. and S. D. designed the experiments and supervised the project. T. T. performed the in‐vivo experiments and participated in the study design. Z. J. performed the in‐vitro experiments and contributed to the data analysis. Z. X. performed the flow cytometry and helped to treat the mice. W. S. performed the qRT–PCR. All authors read and approved the final paper.
Disclosure
None.
Acknowledgements
Financial support for this study was provided by the National Natural Science Foundation of China (Grant nos 81571614 and 81501349), the Jiangsu Provincial Innovation Team Program Foundation and the Research Fund for the Doctoral Program of Higher Education (20133234110005).
Contributor Information
D. Shi, Email: shidongyan@njmu.edu.cn
H. Zhou, Email: hzhou@njmu.edu.cn.
References
- 1. Zhang L, Wang F, Wang L et al Prevalence of chronic kidney disease in China: a cross‐sectional survey. Lancet 2012; 379:815–22. [DOI] [PubMed] [Google Scholar]
- 2. Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 2010; 6:643–56. [DOI] [PubMed] [Google Scholar]
- 3. Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 2011; 7:684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin SL, Castaño AP, Nowlin BT, Lupher ML Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 2009; 183:6733–43. [DOI] [PubMed] [Google Scholar]
- 5. Tapmeier TT, Feam A, Brown K et al Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int 2010; 78:351–62. [DOI] [PubMed] [Google Scholar]
- 6. Vielhauer V, Anders HJ, Mack M et al Obstructive nephropathy in the mouse: progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2‐ and 5‐positive leukocytes. J Am Soc Nephrol 2001; 12:1173–87. [DOI] [PubMed] [Google Scholar]
- 7. Christensen PM, Liu CH, Swendeman SL et al Impaired endothelial barrier function in apolipoprotein M‐deficient mice is dependent on sphingosine‐1‐phosphate receptor 1. FASEB J 2016; 30:2351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang L, Yue S, Yang L et al Sphingosine kinase/sphingosine 1‐phosphate (S1P)/S1P receptor axis is involved in liver fibrosis‐associated angiogenesis. J Hepatol 2013; 59:114–23. [DOI] [PubMed] [Google Scholar]
- 9. Jin Y, Knudsen E, Wang L et al Sphingosine 1‐phosphate is a novel inhibitor of T‐cell proliferation. Blood 2003; 101:4909–15. [DOI] [PubMed] [Google Scholar]
- 10. Matloubian M, Lo CG, Cinamon G et al Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004; 427:355–60. [DOI] [PubMed] [Google Scholar]
- 11. Sic H, Kraus H, Madl J et al Sphingosine‐1‐phosphate receptors control B‐cell migration through signalling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J Allergy Clin Immunol 2014; 134:420–8. [DOI] [PubMed] [Google Scholar]
- 12. Weichand B, Weis N, Weigert A, Grossmann N, Levkau B, Brüne B. Apoptotic cells enhance sphingosine‐1‐phosphate receptor 1 dependent macrophage migration. Eur J Immunol 2013; 43:3306–13. [DOI] [PubMed] [Google Scholar]
- 13. Rathinasamy A, Czeloth N, Pabst O, Förster R, Bernhardt G. The origin and maturity of dendritic cells determine the pattern of sphingosine 1‐phosphate receptors expressed and required for efficient migration. J Immunol 2010; 185:4072–81. [DOI] [PubMed] [Google Scholar]
- 14. Walzer T, Chiossone L, Chaix J et al Natural killer cell trafficking in vivo requires a dedicated sphingosine 1‐phosphate receptor. Nat Immunol 2007; 8:1337–44. [DOI] [PubMed] [Google Scholar]
- 15. Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1‐phosphate receptor, S1P1, on T‐cells controls thymic emigration. J Biol Chem 2004; 279:15396–401. [DOI] [PubMed] [Google Scholar]
- 16. Mahajan‐Thakur S, Sostmann BD, Fender AC et al Sphingosine‐1‐phosphate induces thrombin receptor PAR‐4 expression to enhance cell migration and COX‐2 formation in human monocytes. J Leukoc Biol 2014; 96:611–8. [DOI] [PubMed] [Google Scholar]
- 17. Fujita T, Inoue K, Yamamoto S et al Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot (Tokyo) 1994; 47:208–15. [DOI] [PubMed] [Google Scholar]
- 18. Brinkmann V, Davis MD, Heise CE et al The immune modulator FTY720 targets sphingosine 1‐phosphate receptors. J Biol Chem 2002; 277:21453–7. [DOI] [PubMed] [Google Scholar]
- 19. Wei Y, Yemisci M, Kim HH et al Fingolimod provides long‐term protection in rodent models of cerebral ischemia. Ann Neurol 2011; 69:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee KD, Chow WN, Sato‐Bigbee C et al FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J Neurotrauma 2009; 26:2335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu L, Barfejani AH, Qin T, Dong Q, Ayata C, Waeber C. Fingolimod exerts neuroprotective effects in a mouse model of intracerebral hemorrhage. Brain Res 2014; 1555:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiba K, Yanagawa Y, Masubuchi Y et al FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol 1998; 160:5037–44. [PubMed] [Google Scholar]
- 23. Mao‐Draayer Y, Sarazin J, Fox D, Schiopu E. The sphingosine‐1‐phosphate receptor: a novel therapeutic target for multiple sclerosis and other autoimmune diseases. Clin Immunol 2016; 175:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tedesco‐Silva H, Szakaly P, Shoker A et al FTY720 versus mycophenolate mofetil in de novo renal transplantation: six‐month results of a double‐blind study. Transplantation 2007; 84:885–92. [DOI] [PubMed] [Google Scholar]
- 25. Suleiman M, Cury PM, Pestana JO, Burdmann EA, Bueno V. FTY720 prevents renal T‐cell infiltration after ischemia/reperfusion injury. Transplant Proc 2005; 37:373–4. [DOI] [PubMed] [Google Scholar]
- 26. Tian S, Zhang L, Tang J, Guo X, Dong K, Chen SY. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Renal Physiol 2015; 308:F69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen B, Liu X, Fan Y, Qiu J. Macrophages regulate renal fibrosis through modulating TGFβ superfamily signalling. Inflammation 2014; 37:2076–84. [DOI] [PubMed] [Google Scholar]
- 28. Li C, Ding XY, Xiang DM et al Enhanced M1 and impaired M2 macrophage polarization and reduced mitochondrial biogenesis via inhibition of AMP kinase in chronic kidney disease. Cell Physiol Biochem 2015; 36:358–72. [DOI] [PubMed] [Google Scholar]
- 29. Henning G, Ohl L, Junt T et al CC chemokine receptor 7‐dependent and ‐independent pathways for lymphocyte homing: modulation by FTY720. J Exp Med 2001; 194:1875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ottenlinger F, Schwiebs A, Pfarr K et al Fingolimod targeting protein phosphatase 2A differently affects IL‐33 induced IL‐2 and IFN‐γ production in CD8(+) lymphocytes. Eur J Immunol 2016; 46:941–51. [DOI] [PubMed] [Google Scholar]
- 31. Rahman MM, Rumzhum NN, Hansbro PM et al Activating protein phosphatase 2A (PP2A) enhances tristetraprolin (TTP) anti‐inflammatory function in A549 lung epithelial cells. Cell Signal 2016; 28:325–34. [DOI] [PubMed] [Google Scholar]
- 32. Oaks JJ, Santhanam R, Walker CJ et al Antagonistic activities of the immunomodulator and PP2A‐activating drug FTY720 (Fingolimod, Gilenya) in Jak2‐driven hematologic malignancies. Blood 2013; 122:1923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang J, Nagahashi M, Kim EY et al Sphingosine‐1‐phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis‐associated cancer. Cancer Cell 2013; 23:107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Deng J, Wang L et al S1PR1 is an effective target to block STAT3 signalling in activated B cell‐like diffuse large B‐cell lymphoma. Blood 2012; 120:1458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L, Wang H, Zhu J, Ding K, Xu J. FTY720 reduces migration and invasion of human glioblastoma cell lines via inhibiting the PI3K/AKT/mTOR/p70S6K signalling pathway. Tumour Biol 2014; 35:10707–14. [DOI] [PubMed] [Google Scholar]
- 36. Hou H, Cao R, Miao J et al Fingolimod ameliorates the development of experimental autoimmune encephalomyelitis by inhibiting Akt‐mTOR axis in mice. Int Immunopharmacol 2016; 30:171–8. [DOI] [PubMed] [Google Scholar]


