Summary
Induction of tolerance is a key mechanism to maintain or to restore immunological homeostasis. Here we show that Foxp3+ regulatory T (Treg) cells use Dickkopf‐1 (DKK‐1) to regulate T‐cell‐mediated tolerance in the T‐cell‐mediated autoimmune colitis model. Treg cells from DKK‐1 hypomorphic doubleridge mice failed to control CD4+ T‐cell proliferation, resulting in CD4 T‐cell‐mediated autoimmune colitis. Thymus‐derived Treg cells showed a robust expression of DKK‐1 but not in naive or effector CD4 T cells. DKK‐1 expression in Foxp3+ Treg cells was further increased upon T‐cell receptor stimulation in vitro and in vivo. Interestingly, Foxp3+ Treg cells expressed DKK‐1 in the cell membrane and the functional inhibition of DKK‐1 using DKK‐1 monoclonal antibody abrogated the suppressor function of Foxp3+ Treg cells. DKK‐1 expression was dependent on de novo protein synthesis and regulated by the mitogen‐activated protein kinase pathway but not by the canonical Wnt pathway. Taken together, our results highlight membrane‐bound DKK‐1 as a novel Treg‐derived mediator to maintain immunological tolerance in T‐cell‐mediated autoimmune colitis.
Keywords: autoimmune colitis, Dickkopf‐1, regulatory T cells
Introduction
Induction of immunological tolerance is a key mechanism to maintain homeostasis in the host.1 The failure in maintaining or restoring immunological tolerance after immune responses often leads to chronic or autoimmune inflammation.2 Several studies demonstrated that Foxp3+ regulatory T (Treg) cells play a crucial role as a central coordinator of immune suppression in a variety of inflammatory diseases.3
The importance of the canonical Wnt pathway has been implicated in tissue repair, wound healing and cell differentiation processes.4, 5 It has been suggested that T cells undergo active Wnt signalling, implicating the importance of Wnt ligands in regulating T‐cell responses.6, 7 Dickkopf‐1 (DKK‐1) is a quintessential Wnt antagonist and was initially found to affect head development in Xenopus laevis.8 DKK family members DKK‐2, DKK‐3 and DKK‐4, were identified subsequently.9 DKK‐1 is a competitive inhibitory ligand that has markedly higher binding affinity than agonistic Wnt ligands such as Wnt3a to its receptor low‐density lipoprotein receptor‐related protein (LRP‐6).9 Mice that were homozygous for the total Dkk1 knockout died at birth due to defects in the cranium and structures formed by the neural crest.10 Several studies have reported that elevated levels of DKK‐1 were associated with disease severity or a poor prognosis, which provided a rationale to regulate the canonical Wnt pathway in cancer and bone diseases for therapeutic purposes.11, 12, 13, 14 It has been shown that DKK‐1 might also use cell‐to‐cell contact to bind to LRP‐6.15
The immunomodulatory role of DKK‐1 in cancer immune surveillance and its pro‐tumorigenic role were also shown in its effect on myeloid‐derived suppressor cells.16, 17 Our recent study reported a novel role of DKK‐1 to promote pathological chronic type 2 inflammation.18 Given the potential of DKK family member proteins to be involved in tolerance and immunomodulation, we decided to investigate whether DKK‐1 may be present in immune cells and play a crucial role in tolerance induction and maintenance.
In this study, we demonstrate that DKK‐1 is uniquely expressed in Foxp3+ Treg cells to inhibit T‐cell‐mediated autoimmune colitis as a membrane‐bound form. Foxp3+ Treg cells showed a robust expression of DKK‐1 but not any other DKK family member genes. T‐cell receptor (TCR) stimulation induced membrane‐bound DKK‐1 expression via the mitogen‐activated protein kinase (MAPK) pathways.
Materials and methods
Mice
C57BL/6J and Rag2‐deficient knockout mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and had been bred in our mouse facility. The animals were kept under normal light/dark cycle (12 hr/12 hr). The doubleridge mice (Dkk‐1d/d) were kindly provided by Asma Nusrat (Emory University, Atlanta, GA). Foxp3‐IRES‐RFP mice were bred in our mouse facility. Thy1‐IRES‐IL‐10 reporter mice were provided by Casey Weaver (University of Alabama) and bred with Foxp3‐IRES‐RFP mice. T‐cell factor 1 (TCF‐1) ‐deficient mice were kindly provided by Hai‐Hue Xue (University of Iowa) and bred with Foxp3‐IRES‐RFP mice. All mouse protocols were approved by the Yale University Institutional Animal Care and Use Committee in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International.
Adoptive transfer: inflammatory bowel disease model
For inflammatory bowel disease experiments using DKK‐1 inhibitor (WAY‐262611), 4 × 105 splenic naive CD4 T cells and 1·5 × 105 Treg cells were co‐transferred into 8 week‐old Rag2‐deficient mice. Body weight was monitored on a weekly basis. For the assessment of T‐cell proliferation in vivo, 4 × 105 splenic wild‐type naive CD4 T cells and 1·5 × 105 wild‐type or doubleridge Treg cells were co‐transferred into 8‐week‐old Rag2‐deficient mice. One week later, spleens from these mice were harvested. Cells were counted and analysed by flow cytometry for their TCR‐β chain expression.
Histopathology
Mouse colons were fixed in 10% neutral buffered formalin for 24 hr and embedded in paraffin. Haematoxylin & eosin staining of paraffin‐embedded 5‐µm tissue sections was performed according to standard protocols. Image acquisition was made using an Olympus microscope with Spot RT camera and acquisition software. Histology scores of colon were generated using the following phenotypes in a blinded fashion by a certified pathologist. Chronicity: 0, no increased inflammation; 1, low level of inflammation with mildly increased inflammatory cells in the lamina propria; 2, moderately increased inflammation in the lamina propria; 3, high level of inflammation with evidence of wall thickening by inflammation; 4, maximal severity of inflammation with transmural leucocyte infiltration and/or architectural distortion. Activity (observed epithelial injury): 0, normal, no inflammation by neutrophils; 1, occasional epithelial lesion (focal and superficial or rare cryptitis); 2, foci of cryptitis, including rare crypt abscess; 3, multiple crypt abscess and/or focal ulceration; 4, extensive ulceration and multiple crypt abscess. An average of five fields of view per colon was evaluated in a blinded fashion.
Antibodies and reagents
Anti‐mouse CD4 (clone RM4‐5), anti‐mouse CD8β (clone 53‐6.7), anti‐mouse TCR‐β (clone H57‐597), anti‐CD45RB (clone C363‐16A), anti‐CD3 (clone 145‐2C11), anti‐CD28 (clone 37.51), anti‐mouse CD45 (clone 30‐F11), anti‐CD25 (clone 7D4), anti‐CD62L (clone MEL‐14), anti‐CD44 (clone IM7), anti‐mouse LAP (transforming growth factor‐β 1; TGF‐β 1), anti‐Nrp‐1 (clone 3E12), anti‐Thy1.1 (clone OX‐7) and Rag IgG2a isotype control (clone eBR2a) were purchased from eBioscience (San Diego, CA) or BioLegend (San Diego, CA)). Polyclonal anti‐DKK‐1 and isotype control antibody were purchased (BioSS). DKK‐1 antibody and isotype antibody were purchased from R&D Systems (Minneapolis, MN). Serum glucocorticoid kinase 1 (SGK‐1) inhibitor (GSK650394) was purchased from GSK (GlaxoSmithKline, Brentford, UK). DKK‐1 inhibitor (EMD Millipore, Billerica, MA, USA), p38 inhibitor SB203528 (Tocris), GSK3β inhibitor BIO (Calbiochem, San Diego, CA) and atorvastatin (Sigma, St Louis, MO, USA) were purchased from the indicated vendors. Porcupine inhibitor IWP‐2 was purchased from ApexBio (Houston, TX). Jun N‐terminal kinase inhibitor SP12560001 and extracellular signal‐regulated kinase inhibitors U0126 and PD98059 were kindly provided by Dr Bing Su (Yale University). Cyclohexamide (Sigma) was kindly gifted by Dr Peter Cresswell (Yale University). Human and mouse DKK‐1 ELISA kits were purchased from R&D Systems. CellVue cell membrane staining dye and eFluor 670 cell proliferation dye were purchased from eBioscience. Experimental procedures for each dye followed the manufacturers’ protocols. Interleukin‐17A (IL‐17A), IL‐1β, IL‐23 and IL‐6 were purchased from Peprotech (Rocky Hill, NJ, USA). Anti‐CD3ε F(ab’)2 fragment was purchased from BioXcell (West Lebanon, NH).
Cell lines and plasmids
DKK‐1 cDNA was cloned into the pFRSV‐SRα expression vector. Briefly, Chinese Hamster Ovary cells were transfected with DKK‐1‐pFRSV‐SRα and then DKK‐1 expression was amplified by methotrexate treatment. Before harvest, methotrexate was removed, and cells were washed. As a control, pFRSV‐SRα expression vector was transfected and then the supernatant was also harvested and used in the experiment as a control. Amounts of DKK‐1 were determined by DKK‐1 ELISA (R&D Systems).
Real‐time quantitative PCR
RNA was extracted from FACS‐sorted cells using the RNeasy Micro Kit (Qiagen, Germantown, MD, USA). Complementary DNA was generated using an iScript cDNA synthesis kit (Bio‐Rad, Hercules, CA, USA), and real‐time reactions were performed in triplicate using SYBR Green master mix (Bio‐Rad). Data were acquired on an iCycler iQ Real‐time PCR Detection System (Bio‐Rad). Expression was normalized to GAPDH. Primer sequences were DKK‐1 forward: 5′‐GCG GCA AGA CCT ACA CCA AGA G‐3′; DKK‐1 reverse: 5′‐CTT TCG GTA GTG GCG GGT AAG C‐3′; Gapdh forward: 5′‐GCC TTC CGT GTT CCT ACC‐3′; Gapdh reverse: 5′‐GCC TGC TTC ACC ACC TTC‐3′; DKK‐2 forward: 5′‐TGG ATC ATG ACT TCC GTG ACG CTT‐3′; DKK‐2 reverse: 5′‐GCC ATC TTG CCA TGG TCT TGG TTT‐3′; DKK‐3 forward: 5′‐AGA ACT CCA AGA GGC ACA GAG CAA‐3′; DKK‐3 reverse: 5′‐TGT TGT GAG GGA TCT GAC AAG CCA‐3′; DKK‐4 forward: 5′‐TGG TGG TGG CTC TCC TTG‐3′; DKK‐4 reverse: 5′‐AGT TCC GCA CAT CCT TCT TG‐3′; Wnt3a forward: 5′‐TCG GAG ATG GTG GTA GAG AAA CAC‐3′; Wnt3a reverse: 5′‐AAG TTG GGT GAG GCC TCG TAG TAG‐3′; Wnt5a forward: 5′‐ACA ACC TGG CAG ATG TAG CCT GTA‐3′; Wnt5a reverse: 5′‐CGC GCT ATC ATA CTT CTC CTT GAG‐3′; Wnt7b forward: 5′‐CCC AAT GGA GAC AAA TCC CTT TAC‐3′; Wnt7b reverse: 5′‐GAA ACC GTT TTG AGT GTG ACT GGT‐3′; Wnt10b forward: 5′‐GTT TCC GTG AGA GTG CTT TCT CCT‐3′; Wnt10b reverse: 5′‐TCT TGC TCA CCA CTA CCC TTC CAT‐3′; Wnt11 forward: 5′‐GGG CTT CAA AGG AAA CTG ATA GGA‐3′; Wnt11 reverse: 5′‐CCT TGA AAG GTC AAA TGC ACA GTC‐3′.
Cell culture and in vitro Treg cell suppression assay
Unless specified, Treg cells and naive CD4 T cells or effector CD4 T cells were activated with anti‐CD3 (2 μg/ml) and anti‐CD28 (2 μg/ml) antibodies for 72 hr. Cells were cultured in RPMI‐1640 media supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 50 μm β2‐mercaptoethanol for 72 hr.
Immunofluorescence
CD4+ TcRβ + Foxp3+ cells were sorted after 6 weeks of adoptive transfer in Rag2‐deficient mice. Sorted Treg cells were either stained with DKK‐1 isotype antibody or DKK‐1 antibody. Also, these Treg cells were briefly stained with CellVue lipophilic dye to visualize cell membrane. Cells were then centrifuged briefly with a Cytospin (3 min, 200 g). To visualize the nucleus, cells were mounted with VECTASHIELD anti‐fade medium containing DAPI. A Zeiss Axio Observer 7 immunofluorescence microscope was used to acquire images of stained cells. Zeiss ZEN imaging software and photoshop were used for image processing and analysis.
T‐cell differentiation
For CD4 T‐cell differentiation, naive CD4 T cells (CD4+ CD62Lhi CD44lo Foxp3(RFP)–) were isolated from 6‐ to 7‐week‐old Foxp3‐IRES‐RFP mice. For T helper type 1 (Th1) differentiation, cells were activated with plate‐bound anti‐CD3 (2 μg/ml), anti‐CD28 (2 μg/ml) in the presence of IL‐2 (25 U/ml) and IL‐12 (20 ng/ml) for 96 hr in RPMI‐1640 medium (5% fetal bovine serum, 1% penicillin/streptomycin and 10 mm HEPES). For Th2 differentiation, cells were activated with plate‐bound anti‐CD3 (2 μg/ml), anti‐CD28 (2 μg/ml) in the presence of IL‐2 (25 U/ml), anti‐interferon‐γ (XMG1.2) monoclonal antibody (10 μg/ml) and IL‐4 (100 U/ml) for 96 hr. For Th17 cell differentiation, TGF‐β 1 (1 ng/ml) and IL‐6 (25 ng/ml) were used with anti‐CD3 and anti‐CD28 antibodies. For invariant Treg cell differentiation TGF‐β 1 (2 ng/ml) and IL‐2 (100 U/ml) were used with anti‐CD3 and anti‐CD28 antibodies. For the isolation of CD4 T cells, spleens from 6‐ to 7‐week‐old male Foxp3‐IRES‐RFP mice were prepared as single‐cell suspensions. After red blood cell lysis, cells were stained with CD16/CD32 FcR blocking antibody (clone 2.4G2). A Stratedigm S1000EX analyser was used for flow cytometry, and data were analysed using flowjo software (Treestar, Ashland, OR, USA).
Statistical analysis
Statistical significance was determined by one‐way analysis of variance with Dunnet's post‐hoc test or Student's t‐test (P < 0·05 was taken as significant) with graph pad prism software (GraphPad Software, San Diego, CA, USA).
Results
Foxp3+ Treg cell‐derived DKK‐1 prevents autoimmune T‐cell‐mediated colitis
The dysregulation of Wnt signalling components or the presence of Wnt agonists showed the loss of suppressor function of Treg cells in autoimmune disease or the intestinal tumorigenesis microenvironment.19, 20 Among Wnt ligands and their antagonists, DKK‐1 was expressed in Foxp3+ Treg cells under homeostatic conditions (Fig. 1a). Next, we decided to test whether the reduction of DKK‐1 expression in Treg cells may impair their suppressor function. To this end, we used Treg cells from DKK‐1 hypomorphic doubleridge (Dkk‐1 d/d) mice in an autoimmune T‐cell‐mediated colitis model. We monitored whether doubleridge Treg cells could prevent T‐cell‐mediated colitis. In contrast to wild‐type Treg cells, doubleridge Treg cells failed to prevent T‐cell‐mediated colitis, as shown by the mean body weight loss (Fig. 1b). Histopathological scores also showed that both severity and chronicity were exacerbated in the group that received doubleridge Treg cells (Fig. 1c–e). CD4 T‐cell proliferation was increased when doubleridge Treg cells were co‐transferred whereas Treg cell numbers did not decrease when compared with the wild‐type Treg cell group (Fig. 1f,g). Previously we did not observe any marked autoimmune phenotypes in DKK‐1 hypomorphic doubleridge mice and their Foxp3+ Treg cell numbers were comparable to wild‐type littermate controls.18 We examined the expression of Treg cell markers in doubleridge Treg cells, and there were no notable differences when compared with wild‐type Treg cells (see Supplementary material, Fig. S1a). We further tested whether the loss of suppressor function occurs readily after adoptive transfer. One week after adoptive transfer, CD4 T‐cell numbers were quantified in spleens. The doubleridge Treg cells failed to control CD4 T‐cell proliferation in Rag2‐deficient mice after 7 days (see Supplementary material, Fig. S1b). Finally, we tested the functional significance of DKK‐1 in Treg cells in vitro. DKK‐1 monoclonal antibody or its isotype control monoclonal antibody was added in a conventional suppressor assay. Treg‐mediated suppression was abrogated, and effector T‐cell proliferation was increased (Fig. 1h). To see whether an exogenous or membrane‐bound form of DKK‐1 may exert similar biological activity, we tested the exogenous DKK‐1 in a suppressor assay. The DKK‐1‐treated Treg cells still maintained suppressor activity whereas Wnt3a abrogated Treg cell‐mediated suppression, indicating that exogenous DKK‐1 did not have additional bioactivity on Treg cells (see Supplementary material, Fig. S2a). Taken together, our data demonstrated that Treg cells express high levels of DKK‐1 mRNA, and the genetic reduction of DKK‐1 in Treg cells results in the loss of control of CD4 T‐cell proliferation, leading to T‐cell‐mediated colitis.
Figure 1.
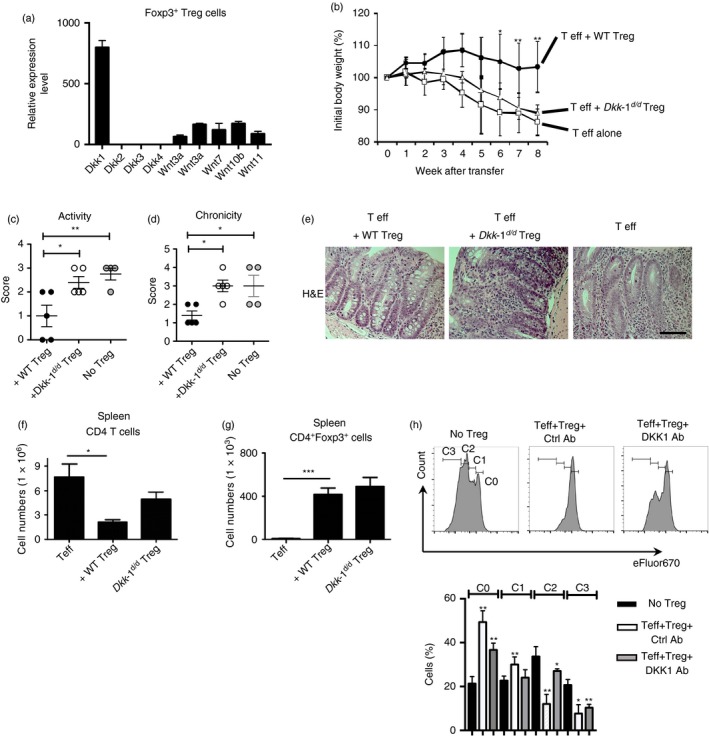
DKK‐1 in Foxp3+ regulatory T (Treg) cells controls T‐cell‐mediated colitis. (a) Wnt ligands mRNA expressions in Treg cells (CD4+ Foxp3+) were analysed by quantitative RT‐PCR. (b–g) Naive CD4 T cells and wild‐type or doubleridge (Dkk‐1 d/d) CD4+ CD25+ CD45RB lo Treg cells were co‐transferred into Rag2‐deficient mice. Mean body weight was measured on a weekly basis (b). Colon tissues were assessed for disease chronicity and severity (c,d), and representative images are displayed. Scale bar in the right panel represents 10 μm. Original magnification is 40× (e). CD4 T cells and Treg cell numbers in spleen were counted (f,g). (h) Naive CD4 T cells and Treg cells were co‐cultured in the presence of anti‐CD3 with anti‐CD28 monoclonal antibody (1 μg/ml) for 90 hr. Naive CD4 T cells were labelled with eFluor670 to measure proliferation. Then 50 μg of DKK‐1 or Isotype antibody was added to the culture. C0–C3 indicates the number of cycles of proliferation. A representative of two independent experiments is shown. One‐way analysis of variance with Dunnett's post‐hoc test was performed. Small horizontal lines indicate the mean (± SEM).
Membrane‐bound expression of DKK‐1 is unique to thymus‐derived Foxp3+ Treg cells
As we observed that splenic Foxp3+ Treg cells expressed DKK‐1 mRNA, we further investigated DKK‐1 mRNA expression in various conditions. We found that Foxp3+ Treg cells expressed high levels of DKK‐1 mRNA compared with naive CD4 T cells or effector CD4 T cells (Fig. 2a). Upon activation, Treg cells expressed more DKK‐1, yet the activated naive/effector CD4 T cells showed marginal induction DKK‐1 expression compared with Treg cells (Fig. 2a). The mRNA expression of DKK‐1 was measured with various types of in vitro differentiated CD4 T cells including TGF‐β 1‐induced Treg cells. Little DKK‐1 expression was detected in the TGF‐β 1‐induced Treg cells (Fig. 2b). As the isolated splenic Foxp3+ Treg cells expressed DKK‐1 mRNA, we examined whether thymic Foxp3+ Treg cells express DKK‐1. Thymus‐derived Foxp3+ Treg cells expressed higher levels of DKK‐1 mRNA (Fig. 2c). The transcription factor Foxp3 programmes Treg cells, so we tested whether the forced expression of Foxp3 would induce DKK‐1 mRNA expression. To this end, we observed no detectable signals of DKK‐1 in retrovirally transduced Foxp3‐expressing CD4 T cells (Fig. 2d). We also tested other pro‐inflammatory cytokines, but they had little effect on DKK‐1 mRNA expression (Fig. 2e). We attempted to detect DKK‐1 protein in splenic Foxp3+ Treg cells. Approximately 6–8% of splenic Foxp3+ Treg cells expressed DKK‐1 on the cell surface (Fig. 2f). We attempted to detect DKK‐1 protein after stimulating Treg cells from DKK‐1 hypomorphic doubleridge mice or wild‐type mice in vitro, but high levels of DKK‐1 were not observed in wild‐type Treg cells in a soluble form in culture supernatants (see Supplementary material, Fig. S2b). We then questioned whether DKK‐1 could be present in a membrane‐bound form. Interestingly, DKK‐1 was readily detected in the membrane of Foxp3+ Treg cells after CD3 and CD28 stimulation but not in Foxp3− effector CD4 T cells (Fig. 2g,h). This membrane‐bound DKK‐1 was not prominently expressed in effector CD4 T cells or other in vitro differentiated helper T cells, including TGF‐β 1‐induced Treg cells (see Supplementary material, Fig. S3a).
Figure 2.
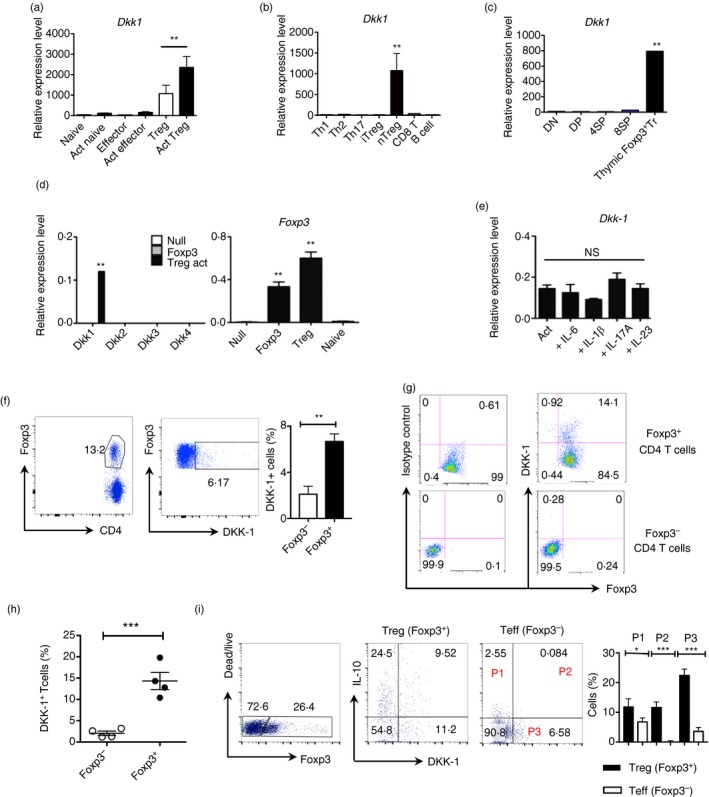
DKK‐1 expression in thymus‐derived Foxp3+ regulatory T (Treg) cells. (a) Splenic naive (CD4+ CD25− CD62Lhi Foxp3−), Effector (CD4+ CD25− CD62Llo Foxp3−) and Treg cells (CD4+ Foxp3+) were isolated from 8‐ to 10‐week‐old C57BL/6 mice (for naive and effector T cells) or Foxp3‐IRES‐RFP (FIR) mice (for Treg cells). Cells were activated with anti‐CD3 and anti‐CD28 antibodies for 72 hr and analysed by quantitative PCR. (b) CD8 T cells, B cells and splenic naive CD4 T cells were differentiated under each condition (Th1, Th2, Th17 and iTreg) for 96 hr. (c) Each thymocyte population was isolated from 8‐ to 10 week‐old FIR mice. (d) Foxp3‐GFP‐positive cells or Null‐GFP cells were sorted 72 hr after retroviral transduction, and analysed by quantitative PCR. (e) Treg cells were activated in the presence of interleukin‐6 (IL‐6) (50 ng/ml), IL‐1β (25 ng/ml), IL‐17A (50 ng/ml) and IL‐23 (50 ng/ml). (f) CD4+ TCR β + cells were isolated from FIR mice, and DKK‐1 and Foxp3 expression were analysed by flow cytometry. (g,h) Splenic CD4+ T cells were activated for 72 hr. (i) Splenic CD4+ Foxp3− T cells and Treg cells from 8‐ to 10‐week‐old BiT‐FIR (IL‐10‐Foxp3 reporter) mice were co‐cultured and activated for 72 hr. A representative of two experiments is shown. Student's t‐test or one‐way analysis of variance with Dunnett's post‐hoc test was performed. Small horizontal lines indicate the mean (± SEM).
Finally, we checked whether DKK‐1 is expressed in suppressor assays that have both effector CD4 T cells and Foxp3+ Treg cells, and observed that high percentages of Foxp3+ Treg cells do express DKK‐1 (Fig. 2i). Approximately 50% of DKK‐1+ Foxp3+ Treg cells co‐express IL‐10, and this co‐expression of DKK‐1 and IL‐10 was detected minimally in effector CD4 T cells (Fig. 2i). Taken together, our results showed that DKK‐1 is highly expressed in Foxp3+ Treg cells and DKK‐1 expression is increased following T‐cell receptor‐mediated stimulation of Foxp3+ Treg cells.
Robust expression of DKK‐1 in Foxp3+ Treg cells in vivo
Next, we tested whether Foxp3+ Treg cells do express DKK‐1 as a membrane‐bound form in vivo. Interestingly, DKK‐1 was highly expressed in Foxp3+ Treg cells compared with CD4+ Foxp3− effector T cells when DKK‐1 was detected at the cell surface in a standard T‐cell‐mediated colitis model (Fig. 3a). We found that DKK‐1+ Foxp3+ Treg cells in these mice co‐expressed other Treg markers such as LAP, NRP‐1 and CD25 (Fig. 3b). Further analyses using splenic DKK‐1+ Foxp3+ Treg cells by confocal microscopy showed that DKK‐1 was co‐localized with the lipophilic cell membrane‐staining dye CellVue, suggesting that DKK‐1 is located on the membrane of Treg cells in vivo (Fig. 3c). We decided to test whether Treg cells would express DKK‐1 upon direct stimulation through the TCR in vivo. To this end, we used a non‐Fc‐binding anti‐CD3ε F(ab’)2 that is often used to activate T cells in vivo producing a much‐reduced cytokine storm compared with an Fc‐binding anti‐CD3 antibody. It has been shown that anti‐CD3 administration elicits transient T‐cell depletion both in preclinical and clinical studies.21, 22, 23 Anti‐CD3 administration primarily impacts T effector cells but spares Foxp3+ Treg cells from apoptosis, anergy and antigenic modulation while giving the Treg cell population an advantage to proliferate and induce immunological tolerance.24 After 3 days of consecutive treatment of non‐Fc‐binding anti‐CD3ε F(ab’)2, > 90% of peripheral T cells were depleted at day 4. T cells gradually repopulated until day 14 (Fig. 3d). Two weeks after anti‐CD3ε F(ab’)2 treatment, we observed that Foxp3+ Treg cells were still expressing DKK‐1 in the spleen (Fig. 3e,f). Taken together, our data suggest that DKK‐1 is expressed in Foxp3+ Treg cells as a membrane‐bound form in vivo, and anti‐CD3ε F(ab’)2 treatment expands a pool of DKK‐1+ Treg cells through TCR stimulation in vivo.
Figure 3.
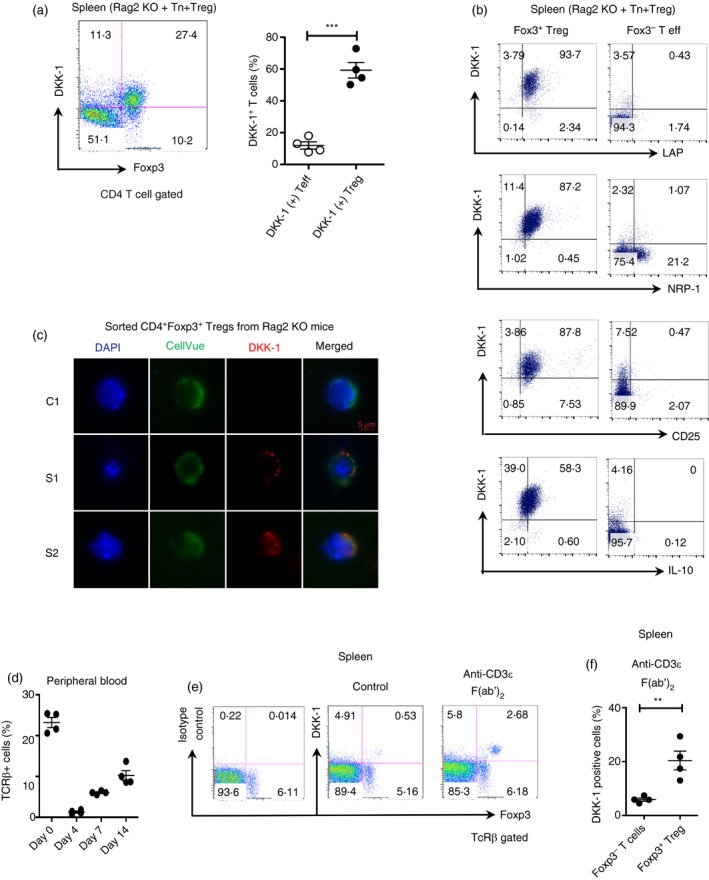
T cell receptor (TCR) stimulation induces a membrane‐bound form of DKK‐1 expression in regulatory T (Treg) cells. (a) Six weeks after adoptive transfer, Foxp3 and DKK‐1 expressions were analysed by flow cytometry. (b) Isolated CD4 T cells from (a) were analysed by flow cytometry. (c) Isolated Treg cells from (a) were analysed by immunofluorescence microscopy. C1, Treg cells stained with isotype control antibody for DKK‐1; S1, S2, individual images of Treg cells stained with DKK‐1 antibody. (d–f) 8‐ to 10‐week‐old FIR mice were treated with 80 μg of anti‐CD3ε F(ab’)2 fragment intravenously for three consecutive days (n = 4). TCR‐β + cells in the peripheral blood were measured by flow cytometry (d). TCR‐β + cells in the spleen were measured by flow cytometry for DKK‐1 and Foxp3. (e,f) A representative of two experiments is shown. In (f), each data point was calculated by the following equation: (DKK‐1+ Foxp3+ Treg cells (%) / (DKK‐1–Foxp3+ Treg cells (%) + DKK‐1+ Foxp3+ Treg cells (%)) × 100. Student's t‐test was performed. Small horizontal lines indicate the mean (± SEM).
De novo expression of DKK‐1 is regulated by the MAPK pathway in Treg cells
We questioned whether DKK‐1 expression required de novo protein synthesis upon TCR stimulation. Cycloheximide was used to test this question. Protein synthesis inhibition by cycloheximide markedly decreased DKK‐1 expression (Fig. 4a). As stimulation of Treg cells through the TCR and the co‐stimulatory molecule (CD28) induced DKK‐1, we investigated downstream signalling pathways that could regulate DKK‐1 expression. Interestingly, we found that the inhibition of p38 MAPK and Jun N‐terminal kinase activity, and MAPK kinase activity, markedly decreased DKK‐1 expression (Fig. 4b). We tested whether other important pathways in Treg cell function were affected such as the mechanistic or mammalian Target of Rapamycin (mTOR) pathway or the mevalonate pathway using rapamycin or atorvastatin. However, rapamycin or atorvastatin treatment did not alter DKK‐1 expression when Treg cells were activated, suggesting that these pathways are not involved (Fig. 4c). In addition, we tested whether mTORC2 inhibition using SGK‐1 inhibitor. We found that SGK‐1 inhibition has a marginal inhibitory effect on DKK‐1 expression (Fig. 4c). As DKK‐1 is known as a Wnt target gene, we tested whether activation of the canonical Wnt signalling pathway would enhance DKK‐1 expression. We first tested whether the lack of the canonical Wnt pathway transcription factor TCF‐1 may regulate DKK‐1 expression. DKK‐1 expression in TCF‐1−/− Treg cells was not markedly changed (Fig. 4d). We also tested two different Wnt mimetics to determine whether the canonical Wnt pathway is involved in DKK‐1 expression in Treg cells. GSK3‐β inhibitor (BIO) and porcupine inhibitor IWP‐2 did not change DKK‐1 expression in Treg cells (Fig. 4e). This suggested that the canonical Wnt pathway components are not involved in regulating DKK‐1 expression in Treg cells. In addition, we tested whether cytokines that stimulate homeostatic proliferation of T cells (IL‐7 or IL‐15) may enhance DKK‐1 expression. It has been reported that Wnt signalling up‐regulates IL‐7R and IL‐2Rβ, enhancing the survival of T cells.25 However, these homeostatic cytokines decreased DKK‐1 expression in Treg cells, suggesting that IL‐7 and IL‐15‐mediated signalling events might play even an inhibitory role in DKK‐1 expression (see Supplementary material, Fig. S3b). Very recently, it has been shown that the inhibition of PI3K/Akt signalling pathways induces DKK‐1 to inhibit cell proliferation.26 As IL‐7/IL‐15‐mediated signalling uses PI3K/Akt signalling pathways, it might be possible that the activation of PI3K/Akt signalling pathway activation might have led to the inhibition of DKK‐1 expression. Further investigation on this point is warranted.
Figure 4.
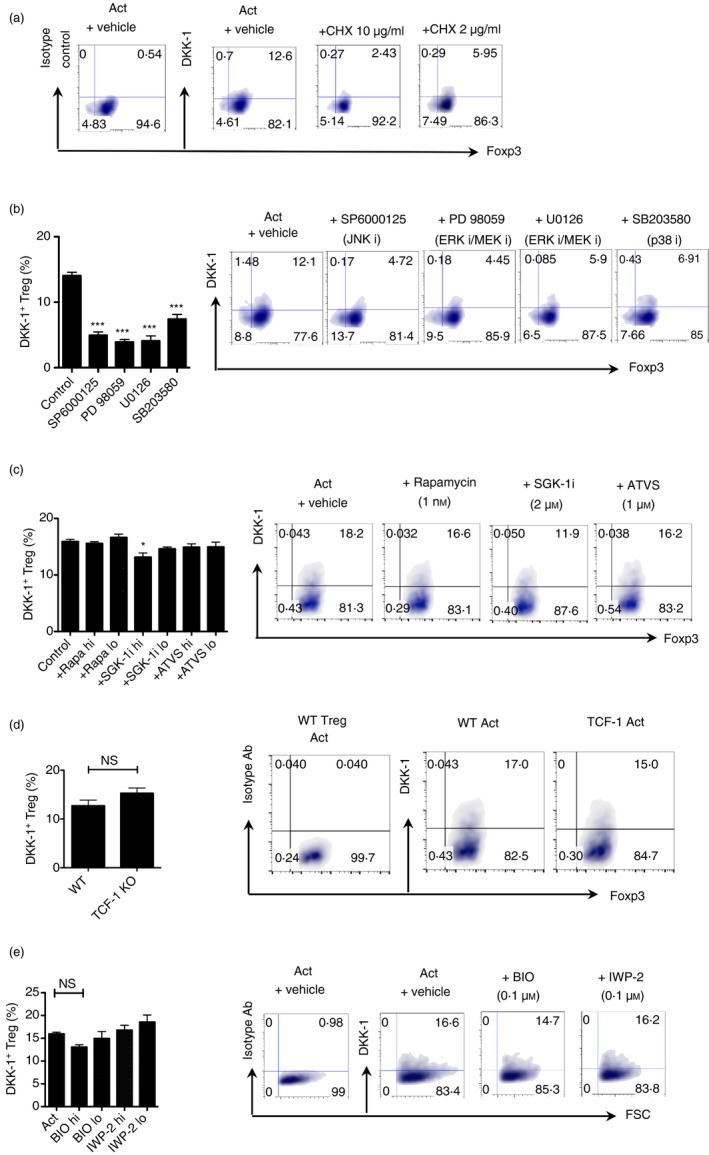
De novo expressions of DKK‐1 in regulatory T (Treg) cells is regulated by the mitogen‐activated protein kinase (MAPK) pathway. (a) Splenic Treg cells were isolated from 8‐week‐old FIR mice and activated for 72 hr. Cyclohexamide was added for the last 24 hr of culture at the indicated doses (10 μg/ml and 2 μg/ml). DKK‐1 expression was measured by flow cytometry. (b) Treg cells were activated in the presence of Jun N‐terminal kinase inhibitor SP6000125 (2 μm), MAPK kinase inhibitor PD98059 and U0126 (1 μm), and p38 MAPK inhibitor SB203580 (5 μm). (c) Treg cells were activated in the presence of rapamycin (1 and 0·1 nm), SGK‐1 inhibitor GSK653094 (2 and 0·4 μm), and atorvastatin (10 and 1 μm). (d) Treg cells from 8‐week‐old wild‐type or TCF‐1 knockout mice were activated for 72 hr. (e) Treg cells were activated in the presence of GSK3β inhibitor BIO (100 nm), and its negative control MeBIO (100 nm), and porcupine inhibitor IWP‐2 (100 nm) for 72 hr. A representative of two independent experiments is shown. For (b) and (c), One‐way analysis of variance with Dunnet's post‐hoc test was performed. For (d), Student's t‐test was performed.
Taken together, our data show that DKK‐1 expression in TCR‐activated Treg cells requires the MAPK pathway, but is independent of the canonical Wnt pathway, the mTOR or mevalonate pathway.
Discussion
We demonstrate that thymus‐derived Foxp3+ Treg cells express DKK‐1 in a membrane‐bound form to suppress T‐cell‐mediated colitis. The unexpected localization and function of DKK‐1 in Foxp3+ Treg cells indicates that DKK‐1 function is an important regulator of peripheral tolerance by controlling T‐cell proliferation in a cell–cell contact‐dependent manner as well as a paracrine manner.
Our study raises an important cell biological question regarding whether DKK‐1 can be present as a membrane‐bound form as well as a soluble ligand. So far DKK‐1 has been mostly considered to be a soluble ligand that would bind to its receptor LRP‐6, competing with a Wnt agonist such as Wnt3a. Notably, a possibility has been raised in which DKK‐1 might be present in the membrane while it competes with mesodermin to bind LRP‐6.15 It is not uncommon that soluble ligands such as TGF‐β or fractalkines are present as membrane‐bound forms. In fact, the genecards database also predicted the presence of DKK‐1 in the cellular membrane with the same confidence level as the extracellular milieu (http://www.genecards.org/cgi-bin/carddisp.pl?gene=DKK1).
There are multiple possible mechanisms regarding DKK‐1‐mediated suppression of naive CD4 T‐cell activation and differentiation. First, DKK‐1 has been well known as a quintessential Wnt antagonist to inhibit cell proliferation. Hence, it is possible that DKK‐1 in Treg cells decrease the proliferation of naive CD4 T cells by inhibiting the canonical Wnt pathway. Alternatively, DKK‐1 may up‐regulate gene expression of inhibitory proteins for naive CD4 T‐cell proliferation. We observed that the gene expression of IL‐2‐mediated signalling inhibitory protein Suppressor of Cytokine Signalling‐1 was increased by DKK‐1 (data not shown). DKK‐1 may inhibit Th1 cell differentiation since our previous study showed that DKK‐1 antagonizes the expression of interferon‐γ expression by increasing Gata‐3.18
The dependence of DKK‐1 expression on the MAPK pathway in Foxp3+ Treg cells is in line with its enhanced expression upon TCR stimulation in vivo and in vitro. Given that DKK‐1 is a Wnt target gene, an increase in DKK‐1 expression would have been expected by the treatment of Wnt mimetics or the elevation of TCF‐1, breaking Treg cell‐mediated suppression by the activated canonical Wnt pathway. Our experimental findings using the Wnt mimetics or TCF‐1‐deficient Treg cells suggest that DKK‐1 expression in Foxp3+ Treg cells is independent of the canonical Wnt pathway activation.
Previous studies demonstrated thrombocytes (or platelets) or cancer cells as sources of DKK‐1 upon exposure to external stimuli (e.g. parasites or allergens) or alterations in cancerous cells.18, 27, 28, 29 Notably, membrane‐bound TGF‐β is also highly expressed in thrombocytes and Treg cells similar to DKK‐1.30 Although the biological importance of platelet‐derived DKK‐1 and Treg cell‐derived DKK‐1 is now being addressed by our studies, how the mammalian immune system has evolved to share expression patterns of these two seemingly important immune regulators such as DKK‐1 and TGF‐β in thrombocytes and Treg cells awaits further studies.
Very recent reports including ours in the field showed pro‐inflammatory roles of DKK‐1 in various diseases such as asthma, parasitic or viral infection and cancer.16, 17, 18, 31 The role of DKK‐1 in Treg cells is to control unwanted inflammation that is very different from its previously known role as a pro‐inflammatory ligand. A possible explanation of this context‐dependent role of DKK‐1 is that the role of DKK‐1 could be multifunctional in a given inflammatory milieu. This microenvironment‐dependent expression of DKK‐1 has been well‐documented in a very recent study describing the role of DKK‐1 to promote, not to inhibit, haematopoiesis in bone marrow after radiation injury through p38 MAPK in OSX+ cells.32 Alternatively, DKK‐1 may use different types of receptors to trigger signalling pathways other than the canonical Wnt pathways. Recent findings and expectations in the field predict that there are other unknown or novel receptors than LRP‐6 that transmit DKK‐1‐mediated signalling events such as cytoskeleton‐associated protein‐4 (CKAP‐4).33, 34 The signalling pathways through CKAP‐4 were independent of the canonical Wnt pathway, suggesting that more studies regarding the DKK‐1‐mediated signalling pathway will ensue.
Taken together, our findings identify the novel immunoregulatory role of DKK‐1 in Foxp3+ Treg cells to control peripheral tolerance, highlighting DKK‐1 as a compelling molecular target to control autoimmune diseases.
Author contributions
W‐JC, J‐HP, SY performed experiments. LH scored histopathology score; W‐JC, OH, SY, JHC and ALB discussed and analysed data; OH contributed intellectual discussion. W‐JC conceptualized experimental designs and wrote the paper; ALB supervised the study.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1. Doubleridge Tregs are impaired to control T cell proliferation but express conventional Treg markers.
Figure S2. Soluble DKK1 is not detected in Treg and DKK‐1 does not impair suppressor function of Tregs.
Figure S3. DKK‐1 expression is not regulated by T cell differentiation or homeostatic cytokines.
Acknowledgements
We thank Gouzel Tokmoulina for cell sorting. This work is supported by NIH 1R21AI107957‐01 and NIH 1R01CA168670‐01 (awarded to ALMB). This research was supported in part by a Pilot Grant from the Yale Cancer Center.
References
- 1. Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol 2007; 19:176–85. [DOI] [PubMed] [Google Scholar]
- 2. Pesenacker AM, Cook L, Levings MK. The role of FOXP3 in autoimmunity. Curr Opin Immunol 2016; 43:16–23. [DOI] [PubMed] [Google Scholar]
- 3. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133:775–87. [DOI] [PubMed] [Google Scholar]
- 4. Whyte JL, Smith AA, Helms JA. Wnt signaling and injury repair. Cold Spring Harb Perspect Biol 2012; 4:a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J 2012; 31:2670–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Staal FJ, Arens R. Wnt signaling as master regulator of T lymphocyte responses: implications for transplant therapy. Transplantation 2016; 100:2584–92. [DOI] [PubMed] [Google Scholar]
- 7. Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M et al Canonical wnt signaling regulates hematopoiesis in a dosage‐dependent fashion. Cell Stem Cell 2011; 9:345–56. [DOI] [PubMed] [Google Scholar]
- 8. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf‐1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998; 391:357–62. [DOI] [PubMed] [Google Scholar]
- 9. Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol 2013; 5:a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mukhopadhyay M, Shtrom S, Rodriguez‐Esteban C, Chen L, Tsukui T, Gomer L et al Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 2001; 1:423–34. [DOI] [PubMed] [Google Scholar]
- 11. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D et al Dickkopf‐1 is a master regulator of joint remodeling. Nat Med 2007; 13:156–63. [DOI] [PubMed] [Google Scholar]
- 12. Goldstein SD, Trucco M, Guzman WB, Hayashi M, Loeb DM. A monoclonal antibody against the Wnt signaling inhibitor dickkopf‐1 inhibits osteosarcoma metastasis in a preclinical model. Oncotarget 2016; 7:21114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aguilera O, Gonzalez‐Sancho JM, Zazo S, Rincon R, Fernandez AF, Tapia O et al Nuclear DICKKOPF‐1 as a biomarker of chemoresistance and poor clinical outcome in colorectal cancer. Oncotarget 2015; 6:5903–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heider U, Kaiser M, Mieth M, Lamottke B, Rademacher J, Jakob C et al Serum concentrations of DKK‐1 decrease in patients with multiple myeloma responding to anti‐myeloma treatment. Eur J Haematol 2009; 82:31–8. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Chen J, Lu W, McCormick LM, Wang J, Bu G. Mesd binds to mature LDL‐receptor‐related protein‐6 and antagonizes ligand binding. J Cell Sci 2005; 118:5305–14. [DOI] [PubMed] [Google Scholar]
- 16. D'Amico L, Mahajan S, Capietto AH, Yang Z, Zamani A, Ricci B et al Dickkopf‐related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J Exp Med 2016; 213:827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y et al Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 2016; 165:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chae WJ, Ehrlich AK, Chan PY, Teixeira AM, Henegariu O, Hao L et al The Wnt antagonist Dickkopf‐1 promotes pathological type 2 cell‐mediated inflammation. Immunity 2016; 44:246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Loosdregt J, Fleskens V, Tiemessen MM, Mokry M, van Boxtel R, Meerding J et al Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity 2013; 39:298–310. [DOI] [PubMed] [Google Scholar]
- 20. Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL‐17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci USA 2010; 107:5540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valle A, Barbagiovanni G, Jofra T, Stabilini A, Perol L, Baeyens A et al Heterogeneous CD3 expression levels in differing T cell subsets correlate with the in vivo anti‐CD3‐mediated T cell modulation. J Immunol 2015; 194:2117–27. [DOI] [PubMed] [Google Scholar]
- 22. Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G et al Insulin needs after CD3‐antibody therapy in new‐onset type 1 diabetes. N Engl J Med 2005; 352:2598–608. [DOI] [PubMed] [Google Scholar]
- 23. Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti‐CD3 therapy permits regulatory T cells to surmount T cell receptor‐specified peripheral niche constraints. J Exp Med 2010; 207:1879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chatenoud L. Immune therapy for type 1 diabetes mellitus‐what is unique about anti‐CD3 antibodies? Nat Rev Endocrinol 2010; 6:149–57. [DOI] [PubMed] [Google Scholar]
- 25. Li L, Kim HT, Nellore A, Patsoukis N, Petkova V, McDonough S et al Prostaglandin E2 promotes survival of naive UCB T cells via the Wnt/β‐catenin pathway and alters immune reconstitution after UCBT. Blood Cancer J 2014; 4:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niessner H, Kosnopfel C, Sinnberg T, Beck D, Krieg K, Wanke I et al Combined activity of temozolomide and the mTOR inhibitor temsirolimus in metastatic melanoma involves DKK1. Exp Dermatol 2017; doi: 10.1111/exd.13372. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27. Lattanzio S, Santilli F, Liani R, Vazzana N, Ueland T, Di Fulvio P et al Circulating dickkopf‐1 in diabetes mellitus: association with platelet activation and effects of improved metabolic control and low‐dose aspirin. J Am Heart Assoc 2014; 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Y, Chen B, George SK, Liu B. Downregulation of MicroRNA‐152 contributes to high expression of DKK1 in multiple myeloma. RNA Biol 2015; 12:1314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Voorzanger‐Rousselot N, Goehrig D, Facon T, Clezardin P, Garnero P. Platelet is a major contributor to circulating levels of Dickkopf‐1: clinical implications in patients with multiple myeloma. Br J Haematol 2009; 145:264–6. [DOI] [PubMed] [Google Scholar]
- 30. Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF‐β on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci USA 2009; 106:13445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo Y, Mishra A, Howland E, Zhao C, Shukla D, Weng T et al Platelet‐derived Wnt antagonist Dickkopf‐1 is implicated in ICAM‐1/VCAM‐1‐mediated neutrophilic acute lung inflammation. Blood 2015; 126:2220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Himburg HA, Doan PL, Quarmyne M, Yan X, Sasine J, Zhao L et al Dickkopf‐1 promotes hematopoietic regeneration via direct and niche‐mediated mechanisms. Nat Med 2017; 23:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kimura H, Fumoto K, Shojima K, Nojima S, Osugi Y, Tomihara H et al CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J Clin Invest 2016; 126:2689–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moon RT, Gough NR. Beyond canonical: the Wnt and β‐catenin story. Sci Signal 2016; 9:eg5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Doubleridge Tregs are impaired to control T cell proliferation but express conventional Treg markers.
Figure S2. Soluble DKK1 is not detected in Treg and DKK‐1 does not impair suppressor function of Tregs.
Figure S3. DKK‐1 expression is not regulated by T cell differentiation or homeostatic cytokines.


