Summary
Blomia tropicalis is the major asthma allergen in the tropics comparable to Dermatophagoides pteronyssinus. However, little is known about the B. tropicalis epitopes recognized by T cells. Our aim was to identify the T‐cell epitopes in the major B. tropicalis allergen, Blo t 5, and investigate the potential of the corresponding peptides to inhibit the allergic inflammatory lung response. C57BL/6 mice were immunized with plasmid DNA encoding Blo t 5 and T‐cell epitopes identified using the interferon‐γ ELISPOT assay with 15‐mer overlapping peptides. C57BL/6 mice were sensitized with bone‐marrow‐derived dendritic cells (BMDC) pulsed with Blo t 5 allergen followed by intranasal Blo t 5 challenge. Two H‐2b restricted epitopes (Bt576–90 and Bt5106–115) were recognized by CD4 T cells specific for Blo t 5, but no CD8 epitopes were identified. In mice sensitized with Blo t 5‐pulsed BMDC and challenged with intranasal Blo t 5 Bt576–90 and Bt5106–115, peptide‐specific CD4 T cells were found to secrete the T helper type 2 cytokines interleukin‐5 and interleukin‐13. Intradermal administration of synthetic peptides encoding the identified T‐cell epitopes suppressed allergic airway inflammation to further allergen challenges. Hence, we have identified novel CD4 T‐cell epitopes specific for Blo t 5 and demonstrated that these peptides could be employed therapeutically to suppress the T‐cell response in a murine model of allergic airway inflammation.
Keywords: Blomia tropicalis, DNA vaccine, Allergy, mouse model
Introduction
Mite allergens play an important role in allergic asthma. Dermatophagoides mites (particularly the house dust mite, Dermatophagoides pteronyssinus) are well studied. However, in the tropics, sensitization to allergens from the storage mite Blomia tropicalis is at frequencies comparable to Dermatophagoides pteronyssinus.1, 2 Allergens from B. tropicalis have only low to moderate cross‐reactivity with antibodies against D. pteronyssinus.3, 4, 5, 6 Group 1 and 2 (Der p 1 and Der p 2) comprise the major house dust mite allergens that appear to interact directly with immune cells as well as lung epithelium and play a major role in allergic sensitization to house dust mite allergens [reviewed in refs 7, 8, 9]. However, these allergens only play a minor role in the allergic responses to B. tropicalis. Instead, IgE responses against this mite appear to be dominated by Blo t 5 and its paralogue Blo t 21.6, 10, 11, 12, 13, 14 These observations suggest that B. tropicalis and D. pteronyssinus mites represent very different allergens that should be considered separately. Unfortunately, our understanding of the role played by B. tropicalis allergens in asthma pathogenesis is limited.
T helper type 2 (Th2) cells are central to the allergic airway response, secreting a number of critical cytokines, including interleukin‐4 (IL‐4), IL‐5 and IL‐13 that mediate key effects on B‐cell class switching, eosinophil differentiation and recruitment and direct effects on the airways.15, 16, 17, 18, 19 CD4 T cells recognize peptide epitopes presented in the peptide‐binding groove of the class II MHC molecules present on antigen‐presenting cells. Hypoallergenic proteins are currently being explored as a possible therapeutic approach for the treatment of allergic asthma. In most cases, the hypoallergenic proteins retained the epitopes recognized by T cells but were modified to omit the IgE binding regions.20, 21, 22 In other approaches, allergen‐specific T‐cell epitopes were also replaced so as to avoid T‐cell‐mediated effects.23 Peptide immunotherapy, administration of synthetic peptides containing known allergen T‐cell epitopes that inhibit T cells, has been demonstrated in animal and clinical studies.24, 25, 26, 27, 28 Most studies on mite allergens have focused on D. pteronyssinus and T‐cell epitopes identified for Der p 1 and Der p 2 proteins29, 30, 31, 32, 33, 34 with the aim of targeting these T cells for therapy.35, 36, 37, 38, 39, 40 However, epitopes recognized by B. tropicalis‐specific T cells remain unknown and current understanding of the role of T cells in response to B. tropicalis allergens is unstudied.
The aim of our study was to identify the epitopes recognized by T cells responding to the major B. tropicalis allergen, Blo t 5. Mice were immunized using a DNA immunization strategy and the T‐cell response was screened using the ELISPOT assay. Allergen‐specific T cells were recruited to the lung following intranasal Blo t 5 exposure and produced Th2 cytokines such as IL‐5 and IL‐13. Administration of Blo t 5 peptides were tested in a murine Blo t 5‐induced allergic asthma model and resulted in the suppression of the allergic inflammatory responses. We observed reduced cytokine production by allergen‐specific T cells as well as a reduction in eosinophilic infiltration into the lung following allergen exposure. These data show that human paralogues of murine Blo t 5 peptides have the potential to treat asthma.
Materials and methods
Animals
Age‐ and sex‐matched C57BL/6 and BALB/c mice (8–10 weeks old) were purchased from the animal breeding centre of the National University of Singapore. Mice were maintained under specific pathogen‐free conditions. All experiments were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee under the protocol number 029/09.
DNA immunization constructs
Gene sequences encoding the allergen protein Blo t 5 (GenBank accession number U59102) and Der p 2 (GenBank AF276329) were codon‐optimized for murine expression and synthesized by GenScript, Biomed Diagnostics Pte, Singapore. We also produced another plasmid with the Blo t 5 gene and an ovalbumin(257–264) (OVA257–264) epitope cloned in‐frame at the C‐terminus. A Kozak initiation sequence was inserted upstream of the N‐terminus of the protein. Restriction sites were inserted to facilitate downstream cloning processes. The gene sequence encoding each allergen protein was cloned into multiple cloning sites of the pVAX‐1 plasmid (Thermofisher, Life Technologies Holdings, Singapore Pte). These constructs are named pVAX‐Blo t 5, pVAX‐Der p 2 and pVAX‐Blo t 5‐SIINFEKL, respectively.
Immunization of mice
Mice were anaesthetized by intraperitoneal injection with a cocktail containing ketamine (7·5% v/v) and medetomidine (10% v/v). The left leg of each anaesthetized mouse was shaved and 10 μg of plasmid DNA encoding the allergen was applied to the skin and the skin was tattooed with an 11RL needle at 100 Hz for 20 seconds as described previously.41
Peptides
Peptides used in this study were synthesized and isolated to >75–85% purity by GenScript.
Interferon‐γ ELISPOT assay
ELISPOT assays were performed using BD Mouse IFN‐γ ESLIPOT Pair (BD Biosciences, Singapore) and Millipore Multiscreen HTS plates (Merck Pte, Singapore) in accordance with the manufacturer's instructions. The assay was developed using streptavidin‐alkaline phosphatase and BCIP/NBT substrate (Mabtech, iDNA Biotechnology Pte Ltd, Singapore). Spleen and draining lymph nodes of mice were harvested and homogenized into a single‐cell suspension. Red blood cell lysis was performed and cells were re‐suspended in complete RPMI‐1640 (Life Technologies, Sigma‐Aldrich, Singapore) supplemented with 10% v/v fetal bovine serum (FBS), 1% non‐essential amino acids, 1 mm sodium pyruvate, 100 IU/ml penicillin and 0·1 mg/ml streptomycin as well as 50 μm β‐mercaptoethanol. Then, 2 × 105 cells (cRPMI) were loaded into each well and 1 μm of the test peptides was added. Each condition was tested in triplicate. Positive control wells were supplemented with 10 ng/ml PMA and 400 ng/ml ionomycin. Background control wells contained cells, medium and peptide diluent (DMSO). The plates were incubated for 18 hr at 37° with 5% CO2. The plates were developed and then spots were enumerated using the CTL‐Immunospot Analyzer (Cellular Technologies Limited, Biomed Diagnostics, Singapore).
Magnetic depletion of cells
CD4 or CD8 T cells were depleted using MACS magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocols. PBS with 2 mm EDTA and 2% FBS (MACS) buffer were used throughout and the cells were finally re‐suspended in cRPMI before use.
Expression and purification of recombinant Blo t 5
Escherichia coli BL21 cells transformed with pET28 plasmid encoding Blo t 5 were grown to log‐phase and protein expression was induced by the addition of 1 mm isopropyl β‐d‐1‐thiogalactopyranoside (IPTG). The bacterial pellet was collected and lysed by a combination of lysozyme and DNase followed by sonication. The bacterial lysate containing the Blo t 5 protein was initially enriched for the Blo t 5‐containing fractions at 50% and 60% saturated ammonium sulphate precipitation. The Blo t 5 protein was subsequently purified by anion‐exchange followed by size‐exclusion chromatography using a GE Healthcare Life Sciences Äkta FPLC system with MonoQ 5/50 GL and HiLoad 16/60 Superdex75 columns (GE Healthcare Pte, Singapore).
Culture and antigen pulsed bone‐marrow‐derived dendritic cells
Bone marrow cells were isolated from the femur and cultured with 20 ng/ml granulocyte–macrophage colony‐stimulating factor for 6 days with media changes every 2 days. The non‐adherent cells were collected and washed before being cultured in a fresh six‐well plate at a density of 4 × 106 cells/well. 10 μg of recombinant Blo t 5 was added to each well and the cells were cultured for 18–20 hr. The antigen‐pulsed bone‐marrow‐derived dendritic cells (BMDC) were washed and re‐suspended in Hanks’ balanced salt solution before inoculation into mice.
Allergen sensitization and challenge of mice
For initial sensitization, mice were anaesthetized by intraperitoneal injection with a cocktail containing ketamine (7·5% v/v) and medetomidine (10% v/v). Then, 1 × 106 antigen‐pulsed BMDC were introduced intranasally into each mouse. For subsequent allergen challenges, mice were anaesthetized with 2·5% isoflurane and challenged with 10 μg of recombinant Blo t 5.
Bronchoalveolar lavage analysis
Mice were killed, the trachea was intubated and the lungs were lavaged by two consecutive washes with 0·5 ml of MACS buffer each. Red blood cell lysis was performed and the bronchoalveolar lavage (BAL) cells were stained and analysed by flow cytometry.
Isolation of lung and draining lymph node cells
Lungs from euthanized mice were homogenized in RPMI‐1640 with 1% fetal bovine serum and digested with Liberase Blendzyme (Roche Pte, Singapore) for 45 min before mechanical disruption through a 70‐micron cell strainer to obtain a single‐cell suspension. For draining lymph nodes, mechanical disruption was immediately carried out without enzyme digestion. Red blood cell lysis using lysis buffer was performed and the cells were re‐suspended in cRPMI.
Culture of draining lymph node or lung
Cells from the lung or draining lymph nodes were cultured at density of 2 × 106 cells per well in cRPMI supplemented with 10 μg recombinant Blo t 5 or 10 μm of peptide. Control cells were cultured in cRPMI only. The cells were cultured at 37° and 5% CO2 for 3–5 days and harvested for analysis.
Homogenization of lungs for cytokine analysis
The weight of the lungs was measured before homogenization. Lungs were homogenized by mechanical disruption using the Miltenyi gentleMACS (Miltenyi) dissociator without previous enzymatic digestion. Cells and cell debris were pelleted by centrifugation and the homogenate was filtered through a 41‐µm membrane before storage at 80°.
Measurement of cytokines and total IgE
Cytokine levels were measured using the Mouse Duoset ELISA kits (R&D Systems, Singlab Technologies Pte, Singapore) in accordance with the manufacturer's instructions. Total IgE was measured with the OptEIA mouse IgE ELISA kit (BD Biosciences).
Flow cytometry
The following antibodies were used for staining cells: CD11c Peridinin chlorophyll protein (PerCP) ‐Cy5.5 (N418; eBioscience, Thermofisher Pte, Singapore), Ly‐6G allophycocyanin (APC) (1A8; BioLegend, Genomax Technologies Pte, Singapore), SiglecF phycoerythrin (PE) (E50‐2440; BD Pharmingen, Biomed Diagnostics Pte), CD3e eFluor450 (17A2; eBioscience) or PerCP‐Cy5.5 (145‐2c11; eBioscience), CD4 PB or PE‐Cy7 (RM4‐5; BD Pharmingen) or APC (RM4‐5; eBioscience), CD62L FITC (MEL‐14; BioLegend), CD44 APC (IM7; BD Pharmingen), CD25 PE (PC61; BioLegend), programmed cell death‐1 (PD‐1) FITC (J43; eBioscience), IL‐10 PE or BV711 (JES5‐16E3; BD Pharmingen) IL‐4 BV421 (11B11; BD Pharmingen), T1/ST2 APC (D1H9; BD Pharmingen), KLRG‐1 (2F1; eBioscience), interferon‐γ (IFN‐γ) PE or APC (XMG1.2; BD Pharmingen), CD45 FITC (30‐F11; Bio‐Legend). Fc blocking antibody (2.4G2; BD Pharmingen) was used during all FACS staining. Cells were incubated for 30 min on ice with the appropriate antibodies. Live/Dead cells were differentiated by violet or blue live/dead fixable dye staining, according to the manufacturer's instructions (Life Technologies).
To perform intracellular staining of lung‐draining lymph node cells after re‐stimulation, cells were further stimulated with PMA and ionomycin (Sigma‐Aldrich) in the presence of Golgiplug (BD Pharmingen) and Golgistop (BD Pharmingen) for 6 hr. Cells were stained for surface markers before addition of fixation/permeabilization buffer (eBioscience) and thereafter were stained using the appropriate antibodies. Flow cytometric analysis was performed using CyAn ADP (Beckman Coulter Pte, Singapore), BD Fortessa, or BD Fortessa X‐20 (BD Pharmingen) and the data were analysed using flowjo software (Tree Star, OR).
Statistics
Statistical analyses were performed using graphpad prism (La Jolla, CA, USA). Paired comparisons were performed using the Mann–Whitney U‐test. Data are expressed as means ± standard errors of the means (SEM) (P values of < 0·05, < 0·01 and < 0·001 are indicated as *, ** or ***). Unless specified, bar charts are shown as the mean value and error bars indicate the standard error of mean. Flow cytometric profiles and histograms are representative of repeated experiments.
Results
Optimization of DNA vaccine immunization
To validate the use of the DNA immunization approach for epitope mapping, we first assessed T‐cell responses in mice immunized with a construct encoding the house dust mite allergen Der p 2. Der p 2 is a relatively well‐studied allergen compared with Blo t 5.29, 33 CD4 T‐cell responses were assessed using the IFN‐γ ELISPOT assay and a panel of 15‐mer peptides (each overlapping by 10 amino acids) comprising the entire Der p 2 protein sequence. T‐cell IFN‐γ production from BALB/c immunized mice was observed with peptide 13 (see Supplementary material, Fig. S1a) while T‐cell IFN‐γ responses against peptides 21, 22 and 27 were detected in C57BL/6 immunized mice (see Supplementary material, Fig. S1b). The peptide sequences are provided in Table 1. The T‐cell epitopes identified closely matched those previously described.29 Interferon‐γ release was abrogated following the depletion of CD4 T cells through negative selection using magnetic beads [see Supplementary material, Fig. S1c (BALB/c mice) and Fig. S1d (C57BL/6 mice)]. Depletion of CD8 T cells in the same manner did not alter the production of IFN‐γ, confirming that CD4 T cells are the population responding to the Der p 2 peptides identified.
Table 1.
Sequences of 15‐mer peptides containing epitopes recognized by T cells specific for (a) Blo t 5 and (b) Der p 2 in C57BL/6 and BALB/c mice
| Strain | Name | Position | Sequence |
|---|---|---|---|
| Blo t 5 (a) | |||
| C57BL/6 | Bt5#16 | 76–90 | LDVVCAMIEGAQGAL |
| C57BL/6 | Bt5#21 | 101–115 | ILERFNYEEAQTLSK |
| C57BL/6 | Bt5#22 | 106–120 | NYEEAQTLSKILLKD |
| BALB/c | Bt5#15 | 71–85 | KIIRELDVVCAMIEG |
| BALB/c | Bt5#16 | 76–90 | LDVVCAMIEGAQGAL |
| Der p 2 (b) | |||
| C57BL/6 | Dp2#21 | 101–115 | QQYDIKYTWNVPKIA |
| C57BL/6 | Dp2#22 | 106–120 | KYTWNVPKIAPKSEN |
| C57BL/6 | Dp2#27 | 131–146 | DGVLACAIATHAKIRD |
| BALB/c | Dp2#13 | 61–75 | NQNTKTAKIEIKASI |
The potential of the DNA immunization approach to generate a CD8 T‐cell response was subsequently validated through immunization of C57BL/6 mice with pVAX‐Blo t 5 SIINFEKL (see Supplementary material, Fig. S2a). Immunization of mice using this construct induced a CD8 T‐cell response against SIINFEKL in 1·4% of CD8 T cells (see Supplementary material, Fig. S1b). Using IFN‐γ ELISPOT assay we determined the optimum time to harvest these cells as 14 days after DNA immunization (see SUPplementary material, Fig. S1c).
Identification of Blo t 5 T‐cell epitopes
C57BL/6 mice were primed with pVAX‐Blo t 5 and splenocytes were screened using the IFN‐γ ELISPOT with 15‐mer peptides that overlapped with adjacent peptides by 10 amino acids spanning the entire Blo t 5‐gene sequence. T‐cell responses were observed for 15‐mers #16, #21 and #22 (Fig. 1a). To determine if the responding T cells were CD4 or CD8, we depleted CD4‐ or CD8‐positive cells using anti‐CD4 or anti‐CD8 magnetic beads before the ELISPOT assay. Depletion of CD4 cells completely abrogated detection of any peptide‐specific IFN‐γ + cells whereas depletion of CD8 cells failed to do so, indicating that the Blo t 5 peptides were recognized by CD4 and not CD8 T cells (Fig. 1b). Next, we used incrementally truncated peptides to identify the minimal epitopes recognized by CD4 T cells from C57BL/6 mice (Fig. 1c,d). The peptides were truncated from both the N‐terminus and C‐terminus and cultured with cells from pVAX‐Blo t 5 immunized mice and T‐cell responses to the peptides were measured using the IFN‐γ ELISPOT. For peptides #21 and #22, we hypothesized that the minimal epitope lay within the region of overlap between the peptides and this did indeed prove to be the case. We identified the minimal epitopes as LDVVCAMIEGAQG (within peptide #16) and NYEEAQTLSK (between peptide #21 and #22), respectively. We also examined the response to pVAX‐Blo t 5 in BALB/c mice (Fig. 1e) and identified two epitopes, 15 and 16 that again were CD4 epitopes (Fig. 1f).
Figure 1.
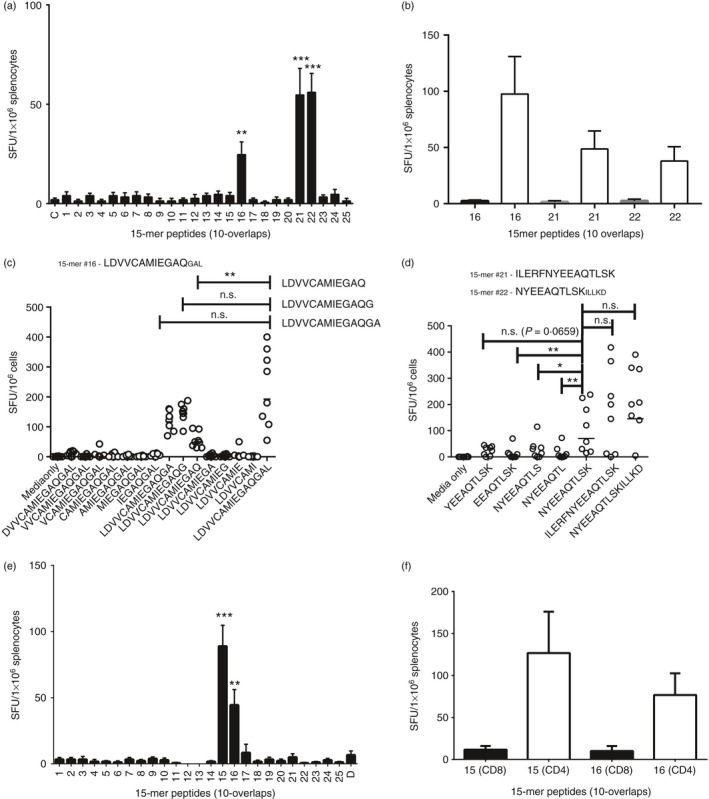
Mapping of epitopes recognized by Blo t 5‐specific T cells from C57BL/6 and BALB/c mice. Splenocytes from C57BL/6 mice immunized with pVAX‐Blo t 5 were screened using interferon‐γ (IFN‐γ) ELISPOT. (a) The T‐cell response to Blo t 5 peptides was measured by culture of Blo t 5‐specific T cells with 15‐mer peptides (10‐overlap) mean ± SEM, n = 6. (b) Splenocytes from immunized mice were depleted of CD4 T cells (open columns) or CD8 T cells (closed columns) before the IFN‐γ ELISPOT with the epitope containing peptides previously identified. Each column depicts the mean ± SEM of the pooled results of each group (n = 4). Identified peptides were truncated from both N‐ and C‐termini and the minimal epitope capable of eliciting a T‐cell response was measured (n = 8). Screening of peptides #16 (c), #21 and #22 (d), only amino acids present in the overlap region between the two peptides were tested. (e, f) Blo t 5 epitopes peptides identified in BALB/c mice immunized as above using the same approach. *P < 0·05, **P < 0·01, ***P = 0·001.
Respiratory response of Blo t 5 DNA vaccinated mice to recombinant Blo t 5 protein
To verify that Blo t 5‐specific CD4 T cells identified in our study can be induced in the lung immune response to Blo t 5, we primed mice with pVAX‐Blo t 5 and subsequently challenged them intranasally with recombinant Blo t 5 as shown in the schema (Fig. 2a). Control mice were primed with the native pVAX1 plasmid and subsequently challenged with Blo t 5 protein in the same manner. BAL eosinophilia was observed in the mice that had been primed with pVAX‐Blo t 5 before intranasal allergen exposure (Fig. 2b). This was not observed in control mice. Culture of cells from the draining lymph nodes of these mice with either recombinant Blo t 5 or antigenic peptides identified in the earlier screening predominantly produced the Th2 cytokines IL‐5 and IL‐13 and a smaller amount of IL‐10 or very little IFN‐γ and no IL‐4 (Fig. 2c). Comparable amounts of these cytokines were induced by culture with either Blo t 5 and or peptide 16 but much less with peptides 21 and 22 (Fig. 2c). Intracellular cytokine staining of ex vivo stimulated CD4 T cells identified 5·5% IL‐5‐positive and 3·3% IL‐13‐positive CD4 T cells from the lymph nodes of pVAX‐Blo t 5 of an individual primed mouse (Fig. 2d). Again peptide 16 generated as many of these cells as whole Blo t 5 (Fig. 2d). The collated data from the groups of mice are shown in Figure 2(e).
Figure 2.
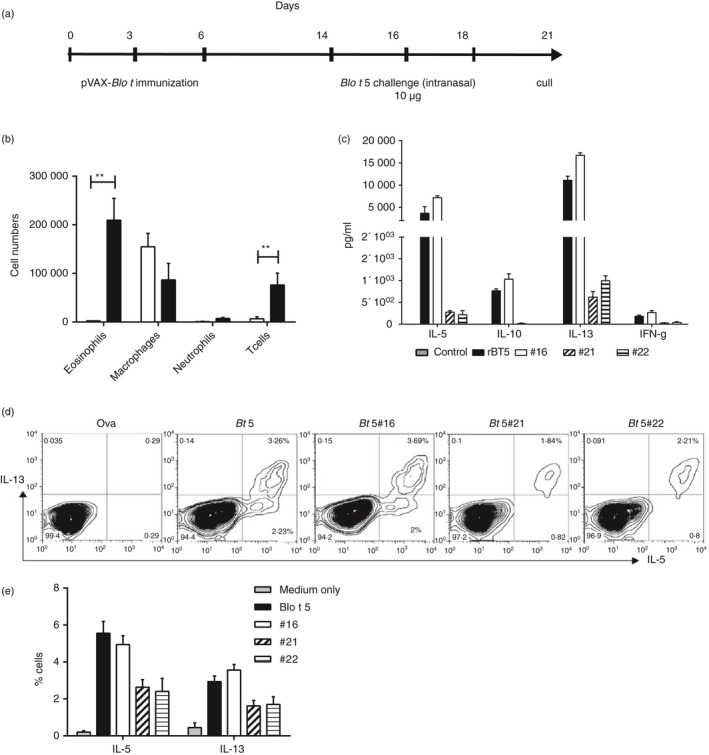
Blo t 5‐specific CD4 T cells are recruited to the lung following allergen challenge and participate in the allergic inflammatory response. (a) DNA immunization with Blo t 5 pVAX on days 0, 3 and 6 and intranasal challenge with Blo t 5 protein on days 14, 16 and 18. (b) Immune cell infiltration into the bronchoalveolar lavage (BAL) following allergen challenge analysed by flow cytometry (n = 6). (c) Cytokine production from cells in the draining lymph nodes after 3 days of culture with Blo t 5 protein. Two replicates with pooled lymph node cells from three mice per experiment). (d) Representative intracellular staining for interleukin‐4 (IL‐4), IL‐5 and IL‐13 of lymph node cells after 3 days of culture with different antigens. Cells were gated on live CD3+ CD4+ cells. (e) Tabulated intracellular cytokine staining results from two different experiments (three mice per experiment). Blo t 5 sensitization and challenge model. Mice were sensitized by Blo t 5‐pulsed bone‐marrow‐derived dendritic cells (BMDC) and challenged with Blo t 5 intranasally. **P < 0·01.
Respiratory response of Blo t 5‐pulsed BMDC inoculated mice to recombinant Blo t 5 protein
We established an alternative Blo t 5 sensitization and challenge model as we found that the skin tattoo method was rather variable. Mice were sensitized to Blo t 5 by the intranasal administration of 1 × 106 Blo t 5‐pulsed BMDC followed by intranasal Blo t 5‐challenge on Days 14, 16 and 18 (Fig. 3a). Mice were culled on Day 21. Marked eosinophilia were observed from mice sensitized and challenged using this protocol (Fig. 3b). Draining lymph node cells cultured with Blo t 5 or peptides #16, #21 and #22 secreted IL‐5 and IL‐13, determined using both intracellular cytokine staining (Fig. 3c) and ELISA (Fig. 3d). This demonstrated that the T cells recognizing the epitopes identified following plasmid DNA immunization were indeed generated following exposure to Blo t 5 and contribute to Th2 lung inflammation and that T cells responding to peptide #16 dominate the airway response to Blo t 5.
Figure 3.
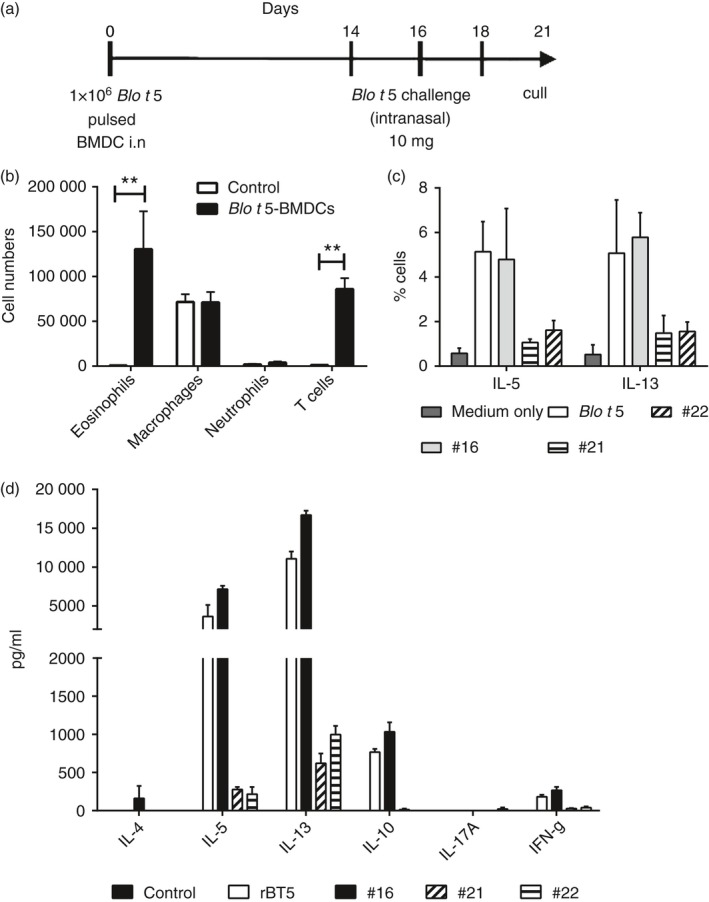
T cells recognizing Blo t 5 epitopes were induced by allergen sensitization and challenge through intranasal exposure. (a) Mice were sensitized through the intranasal administration of bone‐marrow‐derived dendritic cells (BMDC) pulsed with recombinant Blo t 5 allergen and subsequently challenged with the recombinant protein. (b) Mice showed increased bronchoalveolar lavage (BAL) eosinophilia compared with control mice treated with PBS. (c) Percentage of cells secreting interleukin‐5 (IL‐5) and IL‐13 in lymph node cultures from mice sensitized and challenged with Blo t 5 (n = 3). Cells were cultured with medium only (control), Blo t 5 protein or 15‐mer peptides containing Blo t 5 epitopes. Cells were gated on live CD3+ CD4+ cells for analysis. (d) Cytokine productions from cells isolated from the draining lymph nodes following 3 days of culture with Blo t 5 protein (n = 3). **P < 0·01.
Treatment of mice with allergen peptide inhibited the inflammatory airway response to Blo t 5 challenge
Peptide immunotherapy using synthetic peptides encoding known T‐cell epitopes has been demonstrated to be effective in suppressing the allergic immune responses to allergens Fel d 1 and Der p 1 in mice.26, 27 We determined whether synthetic peptides encoding the epitopes identified in this study could function in a similar manner. Mice were sensitized by the intranasal instillation of 1 × 106 BMDC pulsed with Blo t 5 followed by intranasal challenges with Blo t 5. Mice were then inoculated intradermally with a mixture of synthetic peptides containing the T‐cell epitopes before a second series of intranasal Blo t 5 challenges (Fig. 4a). Mice were culled 3 days following the last allergen challenge and BAL was analysed for immune cell infiltration. In mice that had received the Blo t 5 peptides, there was a marked reduction in BAL (Fig. 4b) and lung (Fig. 4c) eosinophilia compared with mice given the control peptide (OVA323–339). However, in Blo t 5 peptide‐treated mice, production of the Th2 cytokines IL‐5 and IL‐13 was not significantly decreased in cultures of BAL cells (Fig. 4d), but IL‐13 was reduced in the cultures of lung cells (Fig. 4e) and both IL‐5 and IL‐13 were reduced in cultures of mesenteric lymph node cells (Fig. 4f) each cultured with Blo t 5. We did not observe a significant effect of peptide treatment on serum IgE (Fig. 4g).
Figure 4.
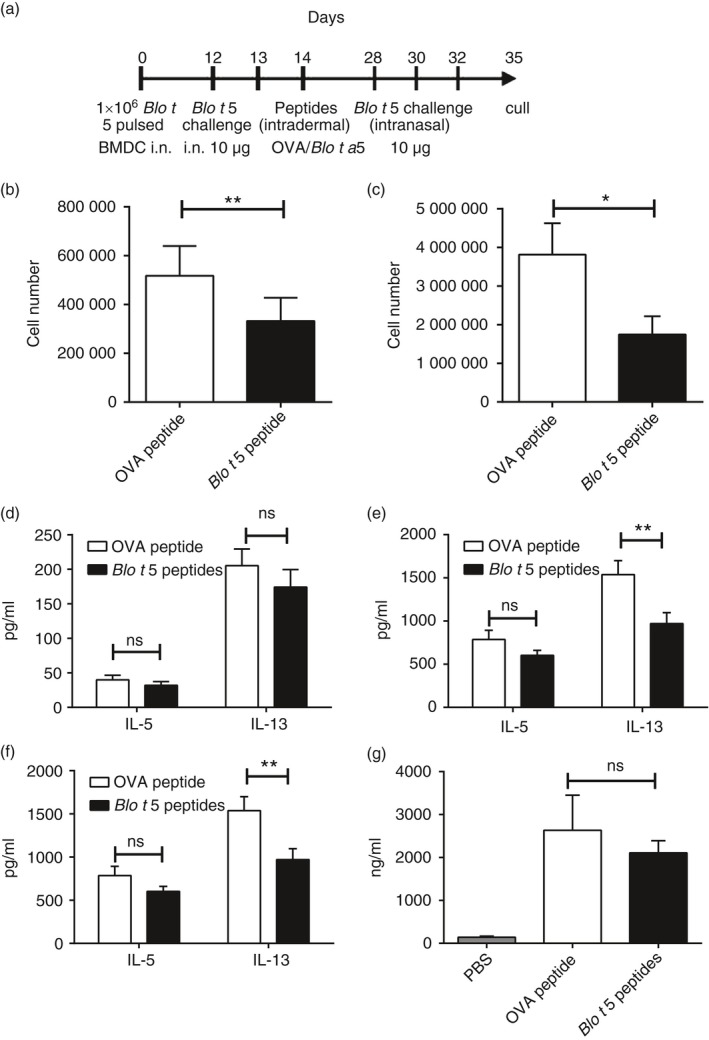
Application of Blo t 5 peptides for peptide immunotherapy of allergic asthma. (a) Experimental protocol for peptide immunotherapy studies. Eosinophilia in the bronchoalveolar lavage (BAL) (b) and lungs (c) were assessed by flow cytometry. Eosinophils were identified by flow cytometry as Siglec‐F+ CD11c– Ly6G– cells (n = 38 and n = 37 for BAL and n = 15 for lung). Interleukin‐5 (IL‐5) and IL‐13 levels in the BAL (d) and lungs (e) were assessed by ELISA. (f) Draining lymph node cells were cultured for 3 days and the cytokine production was assayed by ELISA (n = 18). (g) Total serum IgE levels were not affected by peptide immunotherapy (n = 24). **P < 0·05, **P < 0·01.
Mechanism of peptide inhibition of the allergic lung response
To investigate the mechanism of peptide inhibition of eosinophilia and Th2 cytokines we determined the number of CD3+ CD4+ FoxP3+ regulatory T cells in peptide‐treated mice. We did not find any difference in the number of FoxP3+ cells (Fig. 5a) or the intensity of the FoxP3 staining (Fig. 5b). Nor did we observe any difference in IL‐10 production in lymph node cultures (Fig. 5c) or in the lung homogenate (Fig. 5d) or in the Th1 cytokine IFN‐γ (Fig. 5e).
Figure 5.
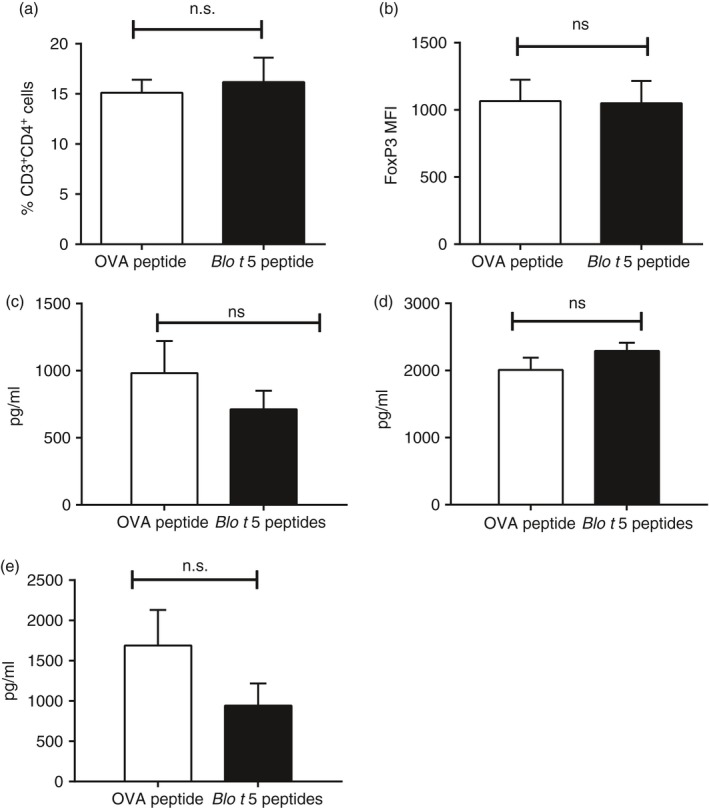
Role of regulatory T cells and interleukin‐10 (IL‐10) in Blo t 5 peptide immunotherapy. (a) Infiltration of CD3+ CD4+ CD25+ FoxP3+ cells in lungs of mice that had received either Blo t 5 peptides immunotherapy or ovalbumin (OVA) peptide (mock) were assessed by flow cytometry (n = 14). (b) FoxP3 expression as measured by the MFI of FoxP3 staining in CD3+ CD4+ CD25+ FoxP3+ cells (n = 14). ELISA measurement of IL‐10 levels in (c) lymph node culture (n = 18) and (d) lung homogenate (n = 8) of mice that received peptide immunotherapy compared with control mice. (e) Levels of the T helper type 1 cytokine interferon‐γ as measured by ELISA (n = 18).
Peptide therapy reduced the number of Th2 cells
The percentage of IL‐5‐producing lung T cells was reduced in mice that had received the Blo t 5 peptides before allergen challenge compared with the mice that received control peptides (Fig. 6a). However, the proportion of cells producing IL‐13 was not significantly affected. We observed a reduction in the number of T1/ST2 expressing CD4 T cells, generally recognized as the population of Th2‐polarized T cells (Fig. 6b). This suggested that the strength of the Th2 T‐cell response against the allergen was reduced in the peptide‐treated mice. We also assessed the expression level of PD‐1 on the lung‐infiltrating T cells and observed a small but significant increase in PD‐1 expression in T cells from mice that had received the Blo t 5 peptide compared with the mice that received the control peptides (Fig. 6c).
Figure 6.
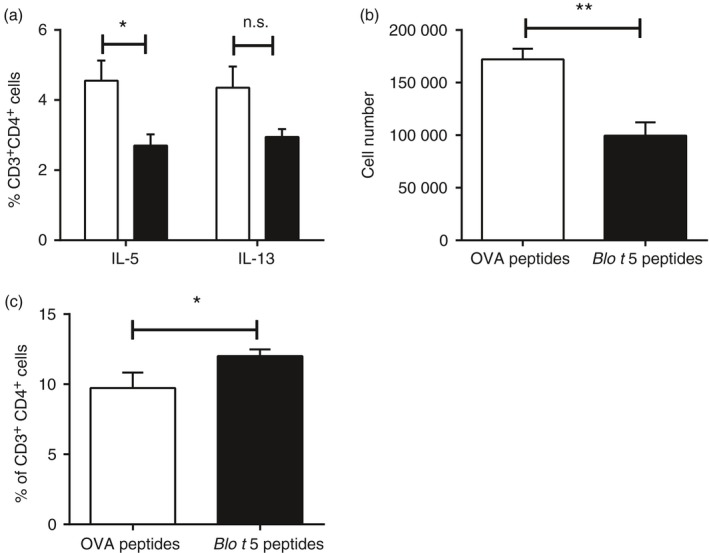
Response of T helper type 2 (Th2) cells suppressed by peptide immunotherapy. (a) Percentage of CD3+ CD4+ cells secreting interleukin‐5 (IL‐5) or IL‐13 measured by intracellular cytokine staining (n = 5). (b) The number of IL‐33 receptor (T1/ST2) expressing cells gated on CD3+ cells. (c) Programmed death 1 (PD‐1) expression in CD3+ cells in lungs of peptide‐treated mice compared with control mice (n = 10). **P < 0·05, **P < 0·01.
Discussion
T cells play a critical role in the pathogenesis of asthma. In this study, we identified two epitopes recognized by Blo t 5‐specific CD4 T cells using DNA immunization. This approach was chosen because it allows for rapid screening of various allergen epitopes. This approach was validated using Der p 2 as a model allergen. Der p 2 is a relatively well‐studied allergen protein compared with Blo t 5 and T‐cell epitopes recognized by mouse T cells were previously described.29, 33 We identified Der p 2 epitopes that generally agreed with those described in Hoyne et al.29 We also demonstrated that immunization using this approach can induce a strong CD8 T‐cell response through the inclusion of a known class I MHC epitope within the immunization construct. This showed that DNA immunization resulted in the expression of the allergen protein (with the included SIINFEKL epitope) that was subsequently processed through the MHC class I as well as the MHC class II pathway. We further demonstrated that T cells recognizing Blo t 5 epitopes identified in this study were recruited to the lungs following allergen exposure and induced asthma‐like allergic airway inflammation characterized by BAL and lung eosinophilia and production of Th2 cytokines. We also showed that these T cells could be targeted using peptide immunotherapy to reduce the allergic airway inflammatory response to the Blo t 5.
Interleukin‐10 and regulatory T cells have been reported to suppress Th2 allergic inflammation following allergen‐specific immunotherapy in several studies, including peptide immunotherapy.26, 27, 38, 39, 42, 43 An increase in FoxP3‐positive cells following peptide immunotherapy has been described26, 27 whereas increased FoxP3 expression by T cells has also been observed.26 However, we were unable to see a change in regulatory T‐cell numbers or IL‐10 production following peptide treatment. We did observe a decline in the Blo t 5‐specific T‐cell response compared with control and the number of T cells expressing the Th2 marker T1/ST2 was significantly lower in the lung of mice that had received peptide immunotherapy before antigen challenge compared with the control mice. Furthermore, T cells producing the Th2 cytokine IL‐5 were significantly reduced in the peptide immunotherapy group.
Another suggested mechanism in the suppression of the T‐cell function by peptides was through the induction of T‐cell anergy or deletion.44, 45 This suggests that a portion of the allergic T‐cell population may have been rendered anergic or even deleted following peptide treatment. PD‐1 has been demonstrated to have an inhibitory effect on T‐cell function and is expressed at higher levels on exhausted T cells.46, 47 We examined the levels of PD‐1 on T cells from these mice, following peptide administration. We found that PD‐1 expression was slightly increased in the treated mice. PD‐1 is recognized as a marker for T‐cell exhaustion and the interaction between PD‐1 and its ligand PDL‐2 appears to negatively regulate allergic inflammation and airway hyperresponsiveness (reviewed in ref. 48). We believe that this indicates that peptide administration may have induced a state of anergy (unresponsiveness) in allergen‐specific T cells and reduced their response to subsequent allergen exposure. To confirm this in vivo neutralization of PD‐1 and PD‐L1/L2 would have helped answer this question. Induction of a Th1 response has also been proposed as a possible mechanism to counter a Th2 response;49, 50 however, we did not see any change in IFN‐γ, indicating that our approach did not induce a Th1 response.
We have identified for the first time the CD4 T‐cell‐restricted peptide of Blo t 5 using a novel approach to allergen immunization. We have shown that airway sensitization to Blo t 5 induces T cells that recognize these peptides and that administration of peptides after sensitization attenuated the ongoing inflammatory response.
Disclosure
The authors declare no financial conflict of interest.
Supporting information
Figure S1. Validation of DNA immunization protocol. Splenocytes from C57BL/6 (a) and BALB/c (b) mice immunized with the pVAX‐Der p 2 construct were screened using the interferon‐γ (IFN‐γ) ELISPOT assay with 15‐mer overlapping peptides (10‐overlaps) spanning the sequence of the Der p 2 protein. Subsequent screening of CD4 or CD8 T‐cell‐depleted splenocytes using the IFN‐γ ELISPOT with the peptides containing the identified epitopes were conducted for C57BL/6 (c) and BALB/c mice (d) immunized with the pVAX‐Der p 2 construct.
Figure S2. Validation and optimization of DNA immunization protocol. (a) pVAX Blo t 5 SIINFEKL DNA construct used for immunization. (b) Kb‐SIINFEKL tetramer staining of T cells specific for OVA257–264 following immunization with pVAX‐Blo t 5‐SIINFEKL. Results shown are gated on live CD3+ CD8+ cells against SIINFEKL tetramer. (c) Mice were immunized with pVAX‐Blo t 5‐SIINFEKL and SIINFEKL‐specific responses measured by culture with SIINFEKL peptide using interferon‐γ ELISPOT. Results shown are the mean ± SEM, n = 6.
Acknowledgements
We are grateful to Dr Paul Hutchinson and Mr Teoh Guo Hui of the NUS Immunology Programme Flow Cytometry Facility for their help and advice on the flow cytometry assays used in this study. KHW, YLC, NP, KF and QZ performed the experimental work, analysed the data and drafted the manuscript. DMK and GMG, the principal investigators, conceived the study design, wrote the grant, analysed data and edited the manuscript. This work was funded by the National Medical Research Council of Singapore (NMRC) (Grant No. NMRC/1321/2012) and by the National Research Foundation (NRF) National University of Singapore‐Hebrew University of Jerusalem programme on inflammation. We would also like to express our gratitude to Dr Paul MacAry for his advice and suggestions. None of the authors has any conflict of interest.
References
- 1. Yeoh SM, Kuo IC, Wang DY, Liam CK, Sam CK, de Bruyne JA et al Sensitization profiles of Malaysian and Singaporean subjects to allergens from Dermatophagoides pteronyssinus and Blomia tropicalis . Int Arch Allergy Immunol 2003; 132:215–20. [DOI] [PubMed] [Google Scholar]
- 2. Arruda LK, Vailes LD, Platts‐Mills TA, Fernandez‐Caldas E, Montealegre F, Lin KL et al Sensitization to Blomia tropicalis in patients with asthma and identification of allergen Blo t 5. Am J Respir Crit Care Med 1997; 155:343–50. [DOI] [PubMed] [Google Scholar]
- 3. Yi FC, Cheong N, Shek LP, Wang DY, Chua KY, Lee BW. Identification of shared and unique immunoglobulin E epitopes of the highly conserved tropomyosins in Blomia tropicalis and Dermatophagoides pteronyssinus . Clin Exp Allergy 2002; 32:1203–10. [DOI] [PubMed] [Google Scholar]
- 4. Simpson A, Green R, Custovic A, Woodcock A, Arruda LK, Chapman MD. Skin test reactivity to natural and recombinant Blomia and Dermatophagoides spp. allergens among mite allergic patients in the UK. Allergy 2003; 58:53–6. [DOI] [PubMed] [Google Scholar]
- 5. Kuo IC, Cheong N, Trakultivakorn M, Lee BW, Chua KY. An extensive study of human IgE cross‐reactivity of Blo t 5 and Der p 5. J Allergy Clin Immunol 2003; 111:603–9. [DOI] [PubMed] [Google Scholar]
- 6. Chew FT, Yi FC, Chua KY, Fernandez‐Caldas E, Arruda LK, Chapman MD et al Allergenic differences between the domestic mites Blomia tropicalis and Dermatophagoides pteronyssinus . Clin Exp Allergy 1999; 29:982–8. [DOI] [PubMed] [Google Scholar]
- 7. Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med 2010; 16:321–8. [DOI] [PubMed] [Google Scholar]
- 8. Gregory LG, Lloyd CM. Orchestrating house dust mite‐associated allergy in the lung. Trends Immunol 2011; 32:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bessot JC, Pauli G. Mite allergens: an overview. Eur Ann Allergy Clin Immunol 2011; 43:141–56. [PubMed] [Google Scholar]
- 10. Carvalho Kdos A, de Melo‐Neto OP, Magalhaes FB, Ponte JC, Felipe FA, dos Santos MC et al Blomia tropicalis Blo t 5 and Blo t 21 recombinant allergens might confer higher specificity to serodiagnostic assays than whole mite extract. BMC Immunol 2013; 14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao YF, Wang de Y, Ong TC, Tay SL, Yap KH, Chew FT. Identification and characterization of a novel allergen from Blomia tropicalis: Blo t 21. J Allergy Clin Immunol 2007; 120:105–12. [DOI] [PubMed] [Google Scholar]
- 12. Chua KY, Cheong N, Kuo IC, Lee BW, Yi FC, Huang C‐H et al The Blomia tropicalis allergens. Protein Pept Lett 2007; 14:325–33. [DOI] [PubMed] [Google Scholar]
- 13. Yi FC, Shek LP, Cheong N, Chua KY, Lee BW. Molecular cloning of Blomia tropicalis allergens – a major source of dust mite allergens in the tropics and subtropics. Inflamm Allergy Drug Targets 2006; 5:261–6. [DOI] [PubMed] [Google Scholar]
- 14. Tan KW, Ong TC, Gao YF, Tiong YS, Wong KN, Chew FT et al NMR structure and IgE epitopes of Blo t 21, a major dust mite allergen from Blomia tropicalis . J Biol Chem 2012; 287:34776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Busse WW, Coffman RL, Gelfand EW, Kay AB, Rosenwasser LJ. Mechanisms of persistent airway inflammation in asthma. A role for T cells and T‐cell products. Am J Respir Crit Care Med 1995; 152:388–93. [DOI] [PubMed] [Google Scholar]
- 16. Lewis CC, Aronow B, Hutton J, Santeliz J, Dienger K, Herman N et al Unique and overlapping gene expression patterns driven by IL‐4 and IL‐13 in the mouse lung. J Allergy Clin Immunol 2009; 123:e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wills‐Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL et al Interleukin‐13: central mediator of allergic asthma. Science 1998; 282:2258–61. [DOI] [PubMed] [Google Scholar]
- 18. Webb DC, McKenzie AN, Koskinen AM, Yang M, Mattes J, Foster PS. Integrated signals between IL‐13, IL‐4, and IL‐5 regulate airways hyperreactivity. J Immunol 2000; 165:108–13. [DOI] [PubMed] [Google Scholar]
- 19. Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills‐Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol 2010; 184:1663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiedermann U, Herz U, Vrtala S, Neuhaus‐Steinmetz U, Renz H, Ebner C et al Mucosal tolerance induction with hypoallergenic molecules in a murine model of allergic asthma. Int Arch Allergy Immunol 2001; 124:391–4. [DOI] [PubMed] [Google Scholar]
- 21. Wiedermann U, Herz U, Baier K, Vrtala S, Neuhaus‐Steinmetz U, Bohle B et al Intranasal treatment with a recombinant hypoallergenic derivative of the major birch pollen allergen Bet v 1 prevents allergic sensitization and airway inflammation in mice. Int Arch Allergy Immunol 2001; 126:68–77. [DOI] [PubMed] [Google Scholar]
- 22. Vrtala S, Akdis CA, Budak F, Akdis M, Blaser K, Kraft D et al T cell epitope‐containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v 1, induce blocking antibodies. J Immunol 2000; 165:6653–9. [DOI] [PubMed] [Google Scholar]
- 23. Niespodziana K, Focke‐Tejkl M, Linhart B, Civaj V, Blatt K, Valent P et al A hypoallergenic cat vaccine based on Fel d 1‐derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol 2011; 127:e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tarzi M, Klunker S, Texier C, Verhoef A, Stapel SO, Akdis CA et al Induction of interleukin‐10 and suppressor of cytokine signalling‐3 gene expression following peptide immunotherapy. Clin Exp Allergy 2006; 36:465–74. [DOI] [PubMed] [Google Scholar]
- 25. Patel D, Couroux P, Hickey P, Salapatek AM, Laidler P, Larché M et al Fel d 1‐derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo‐controlled study. J Allergy Clin Immunol 2013; 131:e107. [DOI] [PubMed] [Google Scholar]
- 26. Moldaver DM, Bharhani MS, Wattie JN, Ellis R, Neighbour H, Lloyd CM et al Amelioration of ovalbumin‐induced allergic airway disease following Der p 1 peptide immunotherapy is not associated with induction of IL‐35. Mucosal Immunol 2013; 7:379–90. [DOI] [PubMed] [Google Scholar]
- 27. Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WLG, Stern LJ et al Peptide immunotherapy in allergic asthma generates IL‐10‐dependent immunological tolerance associated with linked epitope suppression. J Exp Med 2009; 206:1535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oldfield WL, Larche M, Kay AB. Effect of T‐cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet 2002; 360:47–53. [DOI] [PubMed] [Google Scholar]
- 29. Hoyne GF, Callow MG, Kuo MC, Thomas WR. Characterization of T‐cell responses to the house dust mite allergen Der p II in mice. Evidence for major and cryptic epitopes. Immunology 1993; 78:65–73. [PMC free article] [PubMed] [Google Scholar]
- 30. Draghi M, Jarman ER, Grifantini R, Galli‐Stampino L, Lamb JR, Valiante NM et al Different profile of CD8+ effector T cells induced in Der p 1‐allergic and naive mice by DNA vaccination. Eur J Immunol 2002; 32:3720–8. [DOI] [PubMed] [Google Scholar]
- 31. Kristensen NM, Hoyne GF, Hayball JD, Hetzel C, Bourne T, Lamb JR. Induction of T cell responses to the invariant chain derived peptide CLIP in mice immunized with the group 1 allergen of house dust mite. Int Immunol 1996; 8:1091–8. [DOI] [PubMed] [Google Scholar]
- 32. Wu B, Toussaint G, Vander Elst L, Granier C, Jacquemin MG, Saint‐Remy JM. Major T cell epitope‐containing peptides can elicit strong antibody responses. Eur J Immunol 2000; 30:291–9. [DOI] [PubMed] [Google Scholar]
- 33. Wu B, Elst LV, Carlier V, Jacquemin MG, Saint‐Remy J‐MR. The Dermatophagoides pteronyssinus Group 2 allergen contains a universally immunogenic T cell epitope. J Immunol 2002; 169:2430–5. [DOI] [PubMed] [Google Scholar]
- 34. Harris SJ, Roth JF, Savage N, Woodrow SA, Hemingway IK, Hoyne GF et al Prediction of murine MHC class I epitopes in a major house dust mite allergen and induction of T1‐type CD8+ T cell responses. Int Immunol 1997; 9:273–80. [DOI] [PubMed] [Google Scholar]
- 35. Walgraffe D, Matteotti C, El Bakkoury M, Garcia L, Marchand C, Bullens D et al A hypoallergenic variant of Der p 1 as a candidate for mite allergy vaccines. J Allergy Clin Immunol 2009; 123:1150–6. [DOI] [PubMed] [Google Scholar]
- 36. Hoyne GF, Dallman MJ, Lamb JR. Linked suppression in peripheral T cell tolerance to the house dust mite derived allergen Der p 1. Int Arch Allergy Immunol 1999; 118: 122–4. [DOI] [PubMed] [Google Scholar]
- 37. Higgins JA, Lamb JR, Marsh SG, Tonks S, Hayball JD, Rosen‐Bronson S et al Peptide‐induced nonresponsiveness of HLA‐DP restricted human T cells reactive with Dermatophagoides spp. (house dust mite). J Allergy Clin Immunol 1992; 90:749–56. [DOI] [PubMed] [Google Scholar]
- 38. Zuleger CL, Gao X, Burger MS, Chu Q, Payne LG, Chen D. Peptide induces CD4+CD25+ and IL‐10+ T cells and protection in airway allergy models. Vaccine 2005; 23:3181–6. [DOI] [PubMed] [Google Scholar]
- 39. Hall G, Houghton CG, Rahbek JU, Lamb JR, Jarman ER. Suppression of allergen reactive Th2 mediated responses and pulmonary eosinophilia by intranasal administration of an immunodominant peptide is linked to IL‐10 production. Vaccine 2003; 21:549–61. [DOI] [PubMed] [Google Scholar]
- 40. Hoyne GF, O'Hehir RE, Wraith DC, Thomas WR, Lamb JR. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med 1993; 178:1783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bins AD, Jorritsma A, Wolkers MC, Hung CF, Wu TC, Schumacher TN et al A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med 2005; 11:899–904. [DOI] [PubMed] [Google Scholar]
- 42. Jutel M, Akdis M, Budak F, Aebischer‐Casaulta C, Wrzyszcz M, Blaser K et al IL‐10 and TGF‐β cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol 2003; 33:1205–14. [DOI] [PubMed] [Google Scholar]
- 43. Verhoef A, Alexander C, Kay AB, Larche M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med 2005; 2:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wambre E, DeLong JH, James EA, Torres‐Chinn N, Pfutzner W, Mobs C et al Specific immunotherapy modifies allergen‐specific CD4+ T‐cell responses in an epitope‐dependent manner. J Allergy Clin Immunol 2014; 133:e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Korb LC, Mirshahidi S, Ramyar K, Sadighi Akha AA, Sadegh‐Nasseri S. Induction of T cell anergy by low numbers of agonist ligands. J Immunol 1999; 162:6401–9. [PubMed] [Google Scholar]
- 46. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH et al Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682–7. [DOI] [PubMed] [Google Scholar]
- 47. Rosshart S, Hofmann M, Schweier O, Pfaff AK, Yoshimoto K, Takeuchi T et al Interaction of KLRG1 with E‐cadherin: new functional and structural insights. Eur J Immunol 2008; 38:3354–64. [DOI] [PubMed] [Google Scholar]
- 48. Singh AK, Stock P, Akbari O. Role of PD‐L1 and PD‐L2 in allergic diseases and asthma. Allergy 2011; 66:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang TJ, MacAry PA, Eynott P, Moussavi A, Daniel KC, Askenase PW et al Allergen‐specific Th1 cells counteract efferent Th2 cell‐dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN‐γ . J Immunol 2001; 166:207–17. [DOI] [PubMed] [Google Scholar]
- 50. Nakagome K, Okunishi K, Imamura M, Harada H, Matsumoto T, Tanaka R et al IFN‐γ attenuates antigen‐induced overall immune response in the airway as a Th1‐type immune regulatory cytokine. J Immunol 2009; 183:209–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Validation of DNA immunization protocol. Splenocytes from C57BL/6 (a) and BALB/c (b) mice immunized with the pVAX‐Der p 2 construct were screened using the interferon‐γ (IFN‐γ) ELISPOT assay with 15‐mer overlapping peptides (10‐overlaps) spanning the sequence of the Der p 2 protein. Subsequent screening of CD4 or CD8 T‐cell‐depleted splenocytes using the IFN‐γ ELISPOT with the peptides containing the identified epitopes were conducted for C57BL/6 (c) and BALB/c mice (d) immunized with the pVAX‐Der p 2 construct.
Figure S2. Validation and optimization of DNA immunization protocol. (a) pVAX Blo t 5 SIINFEKL DNA construct used for immunization. (b) Kb‐SIINFEKL tetramer staining of T cells specific for OVA257–264 following immunization with pVAX‐Blo t 5‐SIINFEKL. Results shown are gated on live CD3+ CD8+ cells against SIINFEKL tetramer. (c) Mice were immunized with pVAX‐Blo t 5‐SIINFEKL and SIINFEKL‐specific responses measured by culture with SIINFEKL peptide using interferon‐γ ELISPOT. Results shown are the mean ± SEM, n = 6.


