Summary
T follicular helper (Tfh) cells are a distinct type of CD4+ T cell specialized in providing help to B cells during the germinal centre (GC) reaction. As such, they are critical determinants of the quality of an antibody response following antigen challenge. Excessive production of Tfh cells can result in autoimmunity whereas too few can result in inadequate protection from infection. Hence, their differentiation and maintenance must be tightly regulated to ensure appropriate but limited help to B cells. Unlike the majority of other CD4+ T‐cell subsets, Tfh cell differentiation occurs in three phases defined by their anatomical location. During each phase of differentiation the emerging Tfh cells express distinct patterns of co‐receptors, which work together with the T‐cell receptor (TCR) to drive Tfh differentiation. These signals provided by both TCR and co‐receptors during Tfh differentiation alter proliferation, survival, metabolism, cytokine production and transcription factor expression. This review will discuss how engagement of TCR and co‐receptors work together to shape the formation and function of Tfh cells.
Keywords: activation, co‐stimulation, inhibitory/activating receptors, signal transduction, T follicular helper cell
Abbreviations
- Bcl6
B‐cell lymphoma 6
- CTLA‐4
cytotoxic T‐lymphocyte antigen 4
- DC
dendritic cell
- GC
germinal centre
- IL‐21
interleukin‐21
- ICOS
inducible T‐cell co‐stimulator
- NFAT
nuclear factor of activated T cells
- NF‐κB
nuclear factor‐κB
- PD‐1
programmed cell death 1
- SAP
SLAM‐associated protein
- SFR
SLAM family receptor
- SLAM
signalling lymphocytic activation molecule
- TCR
T‐cell receptor
- Th
T helper
- Tfh
T follicular helper
Introduction
The induction of long‐lasting antibody‐mediated immunity depends upon the formation of a productive germinal centre (GC) where B cells can differentiate into memory B cells and antibody‐secreting cells.1, 2 During the GC reaction the B cells undergo affinity maturation and antibody class switching. GC formation is dependent upon the ability of T follicular helper (Tfh) cells to interact with B cells and provide them with ‘help’ in the form of cytokine secretion and co‐receptor expression (reviewed in refs 3 and 4). Tfh cells differentiate from naive CD4+ precursors, a process that must be tightly controlled to ensure optimal B‐cell help. Too many Tfh cells can lead to autoimmunity whereas too few result in inadequate protection from infection. Hence, Tfh cells are an attractive target for therapeutic intervention. This requires an in‐depth understanding of the mechanisms regulating their differentiation and function. A plethora of cytokines, signalling molecules and transcription factors are reported as critical for Tfh cell identity and function. Yet the dominating intracellular signalling pathways that drive Tfh cell differentiation are less well understood. The role of cytokines in driving Tfh cell differentiation has been recently reviewed,5 so here we will discuss how antigenic and co‐receptor signals shape Tfh cell differentiation.
Phases of Tfh cell differentiation
Tfh cell differentiation is a multistage process, occurring over a period of days.3, 4 It can be divided into three phases, broadly defined by the anatomical location of the T cell as illustrated in Fig. 1. In the first phase, naive CD4+ T cells are antigenically stimulated by dendritic cells (DC) in the T‐cell zone of secondary lymphoid organs. If the delivery of antigen occurs in combination with delivery of specific cytokine and co‐receptor signals, primed T cells can enter into the Tfh differentiation programme and become pre‐Tfh cells that express high levels of programmed cell death protein 1 (PD‐1) and inducible T‐cell co‐stimulator (ICOS). This is accompanied by increased expression of the transcription factors TCF‐1, cMAF and B‐cell lymphoma 6 (Bcl6), with concurrent repression of Blimp‐1. In addition, T cells lose expression of CCR7 and EBI2, and gain expression of CXCR5, CXCR4 and S1PR2 allowing them to respond to chemotactic signals and migrate to the areas of the secondary lymphoid organs where T and B cells meet, such as the interfollicular area and the T–B‐cell border.6 Here the second phase of their differentiation occurs. In this phase the T–B‐cell interaction is symbiotic, providing both cell types with signals required to support the differentiation that enables both cell types to participate in the GC reaction. The activated B cells provide further antigenic stimulation and co‐stimulatory and co‐inhibitory signals (including; CD80, CD86, ICOSL, OX40L, PD‐L1, PD‐L2), enabling the pre‐Tfh cells to complete differentiation and move into the GC. In turn, the pre‐Tfh cell provides help to B cells by secreting cytokines [interleukin‐21 (IL‐21) and IL‐4] and expressing CD40L, that can prompt B‐cell follicular entry. The third phase occurs in the GC where Tfh cells engage with GC B cells providing them with help to ensure their proliferation, and subsequent differentiation into plasma and memory B cells.3, 4, 7 The antigenic and co‐stimulatory signals that direct each of the three phases of Tfh cell differentiation will be outlined in this review.
Figure 1.
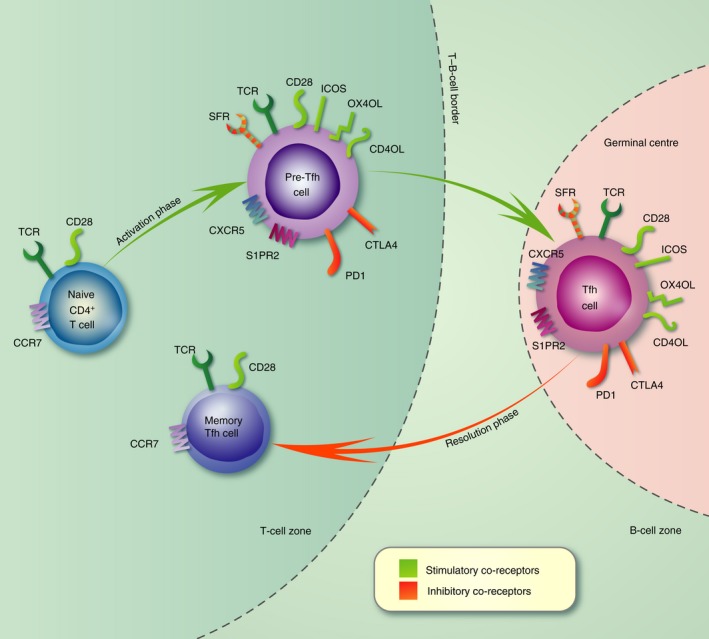
Expression of co‐receptors during the three phases of T follicular helper (Tfh) cell differentiation. Schematic depicting the multistage and location‐specific nature of Tfh cell differentiation. The expression of co‐receptors is shown at each stage with stimulatory receptors coloured green and inhibitory receptors coloured red. During the first phase of differentiation (days 0–3) naive CD4+ T cells are activated by dendritic cells in the T‐cell zone where they proliferate and alter expression of co‐receptors. This allows the resulting pre‐Tfh cell to migrate towards the T–B‐cell border where it engages with antigen‐specific B cells. Co‐receptors expressed by Tfh cells also modulate the signals transduced through the T‐cell receptor, allowing their differentiation. In the second phase (days 4–5) antigen is presented by B cells and the co‐receptors expressed by pre‐Tfh cells are used symbiotically to both induce Tfh cell differentiation and provide co‐stimulation to B cells. In the third phase (days 6–10), Tfh cells engage with germinal centre (GC) B cells within the GC. Tfh cells provide a limiting source of help to ensure that appropriate B cells differentiate into antibody‐secreting cells and memory B cells. During the resolution phase the Tfh cell can leave the GC and in the absence of further antigenic stimulation resides as a long‐lived memory Tfh cell in the periphery.
The role of antigen presentation in Tfh cell differentiation
Strength and duration of T‐cell receptor signal
Although high‐affinity CD4+ T‐cell clones and T‐cell receptors (TCR) with the strongest peptide–MHCII binding show skewed differentiation into Tfh cells, this is most likely due to increased clonal expansion in response to high‐affinity antigens.8, 9 It has been suggested that although antigen affinity does not correlate with partitioning to Tfh cell fate, different peptide epitopes may dictate T helper type 1 (Th1) versus Tfh cell differentiation.10 Intriguingly, single cell transfer demonstrated that individual polyclonal T cells specific for a single peptide tend to produce progeny with a specific balance of Th1 and Tfh cells.11 This divergence was due to enhanced Tfh differentiation with increased aggregate dwell time (the half‐life for which a TCR productively binds to its cognate pMHCII ligand).11 Work using a synthetic system where TCR and peptide–MHC were replaced with hybridizing DNA strands showed that signalling is initiated when single bound TCR are converted into clusters of bound TCRs.12 Longer dwell times and higher ligand densities synergize to promote TCR clustering. This increases the probability of TCR phosphorylation and ZAP‐70 recruitment, resulting in appropriate downstream TCR signalling.
Unlike other T helper subsets, Tfh cells require continuous antigenic stimulation for their maintenance. Experimental strategies to prolong antigen presentation by DC leads to increased numbers of Tfh cells in mice.13, 14 In humans, use of high antigen dose vaccines also results in increased antibody levels and circulating Tfh‐like cells.15, 16, 17 In autoimmune settings, chronic antigen exposure correlates with increased numbers of Tfh cells.18, 19, 20, 21 Likewise, increased proximal TCR signalling (e.g. in PTPN22‐deficient mice) results in increased proliferation and accumulation of Tfh cells.22 In our own studies we find that continuous antigenic stimulation is necessary for human Tfh differentiation in vitro (Webb and Linterman unpublished observation), demonstrating the dependence of Tfh cells on continuous antigen stimulation.
Presentation of antigen by DC
Antigen is presented to naive CD4+ T cells by DC. This initial T–DC interaction results in the induction of Bcl6, the transcriptional repressor required for Tfh formation.23, 24, 25 DCs are essential for Tfh induction, with B cells becoming the major antigen‐presenting cell type for Tfh cells in the second and third phases of their differentiation.26, 27 In comparison to signals that regulate the B–Tfh cell interaction relatively little is known about the signals required to generate Tfh cells during the first DC–T‐cell interaction. However, in conditions of high antigen dose such as viral infection, DC are dispensable for the generation of Tfh cells, suggesting that they are only essential when the amounts of antigen are limiting.27, 28 The mode of antigen presentation, the co‐receptors and the cytokines expressed by DC are key determinants of Tfh cell differentiation. Further rounds of antigenic stimulation in the second phase of Tfh cell differentiation, usually mediated by B cells, are required to stabilize Bcl6 expression and complete Tfh cell differentiation.29
Presentation of antigen by B cells
B cells play an essential role in supporting Tfh differentiation. Depletion of B cells or disruption of their ability to present antigen results in a substantial reduction in Tfh cell numbers.23, 29, 30, 31 This is not due to a unique B‐cell signal because the defect can be overcome by boosting with antigen and/or prolonged antigen presentation by DC.32 Recent work has shown that B cells produce Ephrin B1 to repulse Tfh cells from the GC, thereby restricting their access to B cells and ensuring clonal competition.33 In the absence of Ephrin B1, the Tfh cell production of IL‐21 is reduced and fewer plasma cells are generated. The TCR signalling triggered in pre‐Tfh cells by B cells results in prolonged calcium signalling, inducing the cytokines IL‐4 and IL‐21.34 Qualitatively, this is a different response to that elicited during antigen presentation by DC, probably due to the increased size and duration of the synapses formed between pre‐Tfh and B cells. Calcium signalling downstream of the TCR is essential for Tfh cell development; T cells that have a reduced ability to release Ca2+ (due to deficiency in both Stim1 and Stim2) do not form Tfh cells.35 Nuclear factor of activated T cells (NFAT) transcription factors are activated by TCR‐induced Ca2+ signalling and pre‐Tfh cells have enhanced NFAT nuclear localization.36 Genetic ablation of both NFAT1 and NFAT2 results in a T‐cell intrinsic defect in Tfh cell generation.37 This is not due to a general defect in T‐cell activation as Th1 cell generation was elevated in the absence of NFAT1 and NFAT2. In humans, nearly half of genes differentially expressed in Tfh cells possess NFAT binding sites near their transcriptional start sites (including ICOS, CXCR5 and SLAMF1), suggesting that NFAT is a global regulator of Tfh cell differentiation, induced by antigen presentation during T–B‐cell interactions.37
The importance of co‐receptor signals in Tfh cell differentiation
Antigen presentation provides the antigen‐presenting cells with an opportunity to further regulate Tfh cell differentiation through co‐receptor interactions. Many co‐receptors act as rheostats, tuning the magnitude of antigenic responses. Tfh cells express high levels of many co‐receptors, a reflection of the sustained multi‐signal pathways necessary for their generation and function.3, 4 In particular, CD28, cytotoxic T‐lymphocyte antigen 4 (CTLA‐4) and ICOS are essential for Tfh cell biology. They work sequentially along with OX40, PD‐1 and signalling lymphocytic activation molecule (SLAM) family receptors (SFR) to regulate TCR signalling events, and initiate specific signalling pathways essential for Tfh differentiation.
CD28, CTLA‐4 and ICOS receptor family
CD28, CTLA‐4 and ICOS arose through a tandem duplication of an ancestral gene.38 Despite their common evolutionary origin, they perform distinct roles in T‐cell biology; CD28 and ICOS are positive regulators of T‐cell activation, whereas CTLA‐4 negatively regulates T‐cell expansion.39 CD28 and ICOS share 39% of their amino acid identity, and have overlapping capacity to activate the phosphoinositide 3 kinase (PI3K) signalling pathway; however, they play distinct roles in Tfh cell differentiation.40 CD28 is highly expressed on naive and resting cells, whereas expression of ICOS and CTLA‐4 depends upon T‐cell activation.39 The distinct patterns of their expression partly define their individual roles, but there are also differences in the signalling motifs in their cytoplasmic tails.41 CD28 is the main inducer of IL‐2, critical for the early growth phase of recently activated T cells, and is also an inhibitor of late Tfh cell differentiation.42, 43 In contrast, ICOS is a poor inducer of IL‐2.44, 45 Neither CD28 nor ICOS co‐stimulation alone are sufficient but rather but work sequentially to drive Tfh cell development.46, 47
The strength of CD28 signalling in vivo translates directly into the level of ICOS expression on the T cells.46 CD28 co‐stimulation also induces expression of PD‐1, OX40 and CXCR5.46 Expression of CXCR5 allows pre‐Tfh cells to respond to CXCL13 and migrate into B‐cell follicles.48 When CD28 signalling is blocked at the time of T‐cell priming, T‐cell activation is suppressed and this prevents Tfh cell differentiation in vivo.49 CD28 co‐stimulation depends upon the RLTPR, a scaffold protein that links CD28 to the CARD11/CARMA1 cytosolic adapter and to the nuclear factor‐κB (NF‐κB) pathway.50, 51 Mice and humans deficient in RLTPR have very few Tfh cells and this is associated with defects in activation‐induced expression of CD40L, ICOS and RelA phosphorylation (indicative of impaired NF‐κB signalling).50, 51 Once T cells have acquired CXCR5 expression and migrated to B‐cell follicles the role of CD28 is less clear. Tfh cell differentiation can occur when CD28 co‐stimulation is inhibited after priming in vivo by administration of CTLA‐4–immunoglobulin, a treatment that would also prevent CTLA‐4 signalling.46, 52 However, deletion of CD28 expression after T‐cell priming results in fewer Tfh cells and increased Tfh cell death following influenza virus infection suggesting that CD28 is required up until the third phase of Tfh cell differentiation.53 Importantly, ICOS expression in Cd28 −/− T cells does not rescue the decrease in Tfh cell numbers, suggesting that CD28 stimulation provides unique signals essential for Tfh cells.53
CTLA‐4 is expressed at high levels on Tfh cells where it imparts a negative signal to restrain their numbers.46, 52 CTLA‐4‐deficient mice show a skewing towards Tfh differentiation, with induction of IL‐21 production and spontaneous GC formation.46, 54 CTLA‐4 exerts its suppressive effects through cell extrinsic and cell intrinsic mechanisms. It reduces the expression of CD80 and CD86 (co‐stimulatory ligands for CD28) on antigen‐presenting cells through transendocytosis and its ligation inhibits T‐cell proliferation and IL‐2 transcription.55 The cytoplasmic tail of CTLA‐4 interacts with the Src homology domain‐containing tyrosine phosphatases SHP1, SHP2 and PPS2, which dephosphorylate key TCR signalling kinases (Fyn, Lck and ZAP‐70) and members of the Ras pathway.56 By preventing CD28 co‐stimulation, limiting ICOS expression and directly suppressing TCR signalling, CTLA‐4 acts as a brake for Tfh cell differentiation.52, 56, 57
ICOS is critical for the GC response and is highly expressed on Tfh cells.58 In the absence of ICOS, reduced Tfh cell numbers are seen in both mice and humans.59, 60, 61, 62, 63 ICOS signalling is required for the maintenance of many characteristics of Tfh cell identity, including Bcl6 expression and IL‐21 production. Impaired negative regulation of ICOS by the E3 ubiquitin ligase Roquin leads to abberant accumulation of Tfh cells.64, 65, 66 A limited GC response can be mounted in Icos −/− mice; however, once GC are established the ICOS–ICOSL interaction becomes critical and Icos −/− mice have no GC following secondary challenge.60, 61, 62, 67 ICOS‐deficient T cells are unable to enter B‐cell follicles and this cannot be overcome by transgenic expression of CXCR5, suggesting that ICOS signalling imparts some CXCR5‐independent motility to Tfh cells.68 Within the GC, ICOS–ICOSL interactions promote extensive cell surface engagement of Tfh cells with B cells, resulting in Tfh cell calcium spikes and B‐cell acquisition of CD40 signals, a feed‐forward loop that promotes further ICOS expression and provision of help to B cells.69
PI3K signalling is crucial for ICOS function in Tfh cells.70 A summary of the role of PI3K signalling following ICOS co‐stimulation is depicted in Fig. 2. Mutations in the cytoplasmic tail of ICOS that abrogate recruitment of PI3K impair the generation of Tfh cells.71 There is also a direct correlation between the magnitude of PI3K signalling and Tfh cell numbers.72 PI3K signalling can be mediated through a number of different catalytic subunits. The p110δ catalytic subunit of PI3K transmits some of the signalling downstream of ICOS, principally by recruitment of PI3K to tyrosine within its cytoplasmic YFMF motif.71, 72 Like ICOS‐deficient T cells, p110δ‐deficient T cells show normal priming but are unable to enter primary follicles, and so numbers of Tfh cells are substantially reduced in GC.72 Those that do form have defects in ICOS‐mediated IL‐21, IL‐4 and cMaf mRNA expression.72 The guanine nucleotide exchange factor Vav1 contributes to PI3K activation in T cells. Vav1 −/− T cells are unable to provide B‐cell help during the GC response and show defects in IL‐4 and c‐Maf mRNA expression.73, 74 However, ICOS‐dependent up‐regulation of Bcl6 expression and development of CXCR5+ Tfh‐like cells is unaffected in p110δ‐deficient mice.71, 72 Instead these events are dependent upon the p85α component of PI3K, which forms a complex with osteopontin and moves to the nucleus to protect Bcl6 from ubiquitination‐dependent proteosomal degradation.75 ICOS is superior to CD28 for regulating expression of the transcription factor Klf2.76, 77 This is probably because ICOS preferentially recruits the p110/p50α isoform of PI3K that is known to have an elevated lipid kinase activity and so a higher potential to promote phosphorylation of the transcription factor FOXO1.78 This induces the nuclear exclusion of FOXO1, rendering it functionally inactive. This is critical because FOXO1 suppresses Tfh cell differentiation through negative regulation of Bcl6 and positive regulation of Klf2 expression.76, 77 ICOS also induces Akt (which also mediates phosphorylation of FOXO1) and the E3 ubiquitinase, ITCH, which degrades FOXO1.79 This demonstrates that there is redundancy in the pathways downstream of ICOS that suppress FOXO1, to promote Tfh cell differentiation.
Figure 2.
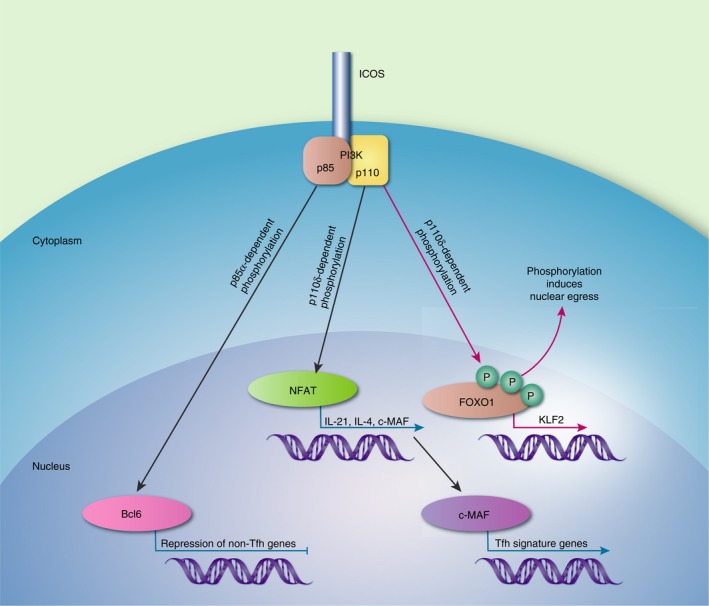
The roles of phosphatidlyinositol 3‐kinase (PI3K) subunits in inducible T‐cell co‐stimulator (ICOS) ‐mediated T follicular helper (Tfh) cell differentiation. The regulatory p85α subunit forms a complex with osteopontin (OPN). This complex migrates to the nucleus to protect B‐cell lymphoma 6 (Bcl6) from degradation. Signals through the catalytic p110δ subunit are responsible for nuclear factor of activated T cell (NFAT) activation allowing transcription of interleukin‐21 (IL‐21), IL‐4 and c‐MAF. The transcription factor c‐MAF drives the expression of Tfh cell genes. PI3K also induces phosphorylation of FOXO1, promoting its nuclear egress and so preventing it from activating the transcription factor KLF2, which inhibits Tfh cell differentiation.
The expression of ICOS is tightly controlled. This is partly achieved through post‐transcriptional mechanisms that regulate the stability of ICOS mRNA. RNA‐binding proteins Roquin 1 and Roquin 2 bind to the 3′ untranslated region of ICOS mRNA, facilitating its degradation.66, 80 Mice with a single mutation in the Rc3h1 locus (Sanroque, which codes for a mutant form of Roquin 1 which is able to bind target mRNA but unable to direct mRNA degradation) and mice deficient in both Roquin 1 and Roquin 2 have increased levels of ICOS expression in all T cells, including naive T cells.64, 81 This results in increased numbers of Tfh cells and the generation of spontaneous GC. In addition, ICOS expression is regulated by the micro‐RNA, MiR‐146a.82 Micro‐RNAs target mRNAs for degradation and/or suppress their translation.83 MiR‐146a is highly expressed in Tfh cells and in its absence there is an accumulation of Tfh cells.84 MiR‐146a represses multiple canonical Tfh cell transcripts including Icos, Slamf1, Cd84, Stat1, Cxcr4 and Notch1. Roquin 1 enhances dicer‐mediated processing of MiR‐146a, resulting in increased numbers of Tfh and GC B cells and increased expression of key Tfh mRNAs including ICOS. MiR‐17‐92 also regulates Tfh cell differentiation by restraining expression of genes important for the differentiation of other T‐cell lineages.85 It also promotes ICOS signalling by reducing the levels of the PI3K repressors PTEN and PHLPP2, thereby facilitating PI3K signalling.85
OX40
OX40 is expressed on activated T cells and disruption of OX40–OX40L interactions during the peak of the immune response perturbs Tfh cell differentiation.86, 87, 88 Transgenic mice that overexpress OX40L on DC show increased numbers of Tfh cells, whereas OX40‐deficient mice show impaired CD4 T‐cell responses.89, 90, 91, 92 CD40‐dependent maturation of DC results in the up‐regulation of OX40L on T cells, although OX40–OX40L interactions are most instructive in the T–B‐cell conjugates at later times of the immune response.93, 94 The role of OX40 in Tfh cell differentiation appears to be dependent upon the context of the immunization. Augmenting OX40 signalling during early stages of lymphocytic choriomeningitis virus infection can direct the CD4 T‐cell response away from a Tfh cell fate.95 OX40 signalling can also induce terminal differentiation of CD4 cells and enhance CD8 cell lytic capability in a tumour environment.96, 97 OX40 co‐stimulation increases T‐cell proliferation and CXCR5 expression during the later stages of activation, contributing to the aberrant Tfh cell response in systemic lupus erythematosus.89, 98, 99, 100 In vitro OX40 co‐stimulation induces expression of Tfh cell‐associated molecules and confers B‐cell helper function.100 However, this can be mimicked by simply increasing the level of TCR signalling, implying that OX40 merely amplifies the TCR signal. In agreement with this, OX40‐deficient humans have normal Tfh differentiation and antibody responses.101
Like ICOS, OX40 is a strong activator of the PI3K, AKT and NF‐κB signalling pathways.71, 94, 102, 103 It also enhances NFAT accumulation in CD4 T cells during antigenic stimulation.104, 105 OX40 is also a target of Roquin‐1 and Roquin‐2 through direct binding of their mRNA and 3′ untranslated region‐dependent post‐transcriptional repression.106 Combined ablation of Roquin1 and Roquin2 induced the expression of OX40 and the activation of the alternative NF‐κB pathway, resulting in elevated expression of Relb and IRF4.106
PD‐1
T‐cell activation results in PD‐1 expression.107, 108 It is highly expressed by Tfh cells, memory T cells and exhausted CD8 T cells and its function may be cell‐type‐dependent.30, 109, 110 Although the role of PD‐1 in terminating T‐cell responses and T‐cell exhaustion have been studied extensively, its role in regulating Tfh cells is less well explored. PD‐1 signalling is triggered by interaction with either PD‐L1 (expressed on many cells, including activated B cells and Tfh cells) and PD‐L2 (expressed on DC, macrophages and B‐1 cells).111, 112
Engagement of PD‐1 results in the formation of microclusters of PD‐1 with the TCR.113, 114 The tyrosine phosphatases, SHP1 and SHP2 are recruited to the intracellular tail of PD‐1 where they decrease the phosphorylation status of CD3ζ chain immunoreceptor tyrosine‐based activation motifs, attenuating ZAP‐70 activation and inhibiting T‐cell activation.115, 116, 117 PD‐1 engagement inhibits CD4+ T‐cell proliferation and cytokine production.118, 119, 120 These effects of PD‐1 can be overcome by strong signalling through CD28 and/or IL‐2R.118, 120, 121, 122 Recent work has highlighted the role that PD‐1 plays in suppressing CD28 signalling.123, 124 CD28 is preferred over the TCR as a target for dephosphorylation by PD‐1‐recruited SHP2.123 Consistent with this, the response to anti‐PD‐1 therapy requires CD28 signalling.123 Hence it is the balance of the signals received from stimulatory and inhibitory receptors that determines the final outcome of T‐cell fate.
Metabolic studies of CD8 T cells receiving PD‐1 signals showed that they are unable to engage in glycolysis, glutaminolysis or metabolism of branched‐chain amino acids and display an increased rate of fatty acid oxidation.125 They also have substantial spare respiratory capacity, allowing production of energy under conditions of stress.125 PD‐1 ligation can also alter the metabolic programme of pre‐activated CD4+ T cells, reprogramming their metabolism from glycolysis to fatty acid oxidation thereby preventing effector cell development. The bioenergetic properties of PD‐1 stimulated T cells display similarities to those of memory T cells, which sustain their survival due to catabolic metabolism of fatty acid oxidation. Whereas the effect of PD‐1 stimulation in Tfh cells remains unknown, it is tempting to speculate that it prevents excessive proliferation and provides them with longevity. Indeed, comparison of Tfh and Th1 cells generated during acute viral infection showed that Tfh cells are less proliferative than Th1 cells.36 This is accompanied by a reduction in glycolysis and an inability to maximally engage in aerobic glycolysis while maintaining IL‐21 secretion. In addition, Bcl6 has been shown to repress the expression of genes involved in glycolysis.126 Furthermore, single‐cell RNA‐Seq of T cells during malaria infection showed that at the bifurcation point where T cells differentiate into either Th1 or Tfh cells, Th1 cells cycled faster and expressed more genes associated with glycolysis than their Tfh counterparts.127 The high levels of PD‐1 expressed on Tfh cells may reduce glycolysis in these cells, resulting in maintenance of their phenotype with sustained cytokine production in the B‐cell follicle. Mice deficient in both PD‐L1 and PD‐L2, or PD‐1 show diminished numbers of long‐lived plasma cells and higher levels of GC B‐cell death following immunization.128 This result is linked to increased numbers of Tfh cells and decreased IL‐4 and IL‐21 mRNA levels in these Tfh cells.128 In addition, regulatory B cells express high levels of PD‐L1, which attenuates T‐cell activation and regulates Tfh cell differentiation 129.
SLAM
The role of SLAM in Tfh cell differentiation came to light with the realization that mutations in SLAM‐associated protein (SAP) result in a lymphoproliferative disease. Patients with mutations in SAP have no natural killer T cells and impaired humoral immunity characterized by reduced Tfh cell differentiation.130 SAP‐deficient mice have a T‐cell‐intrinsic defect in humoral responses, characterized by poor GC formation, low antibody titres and a scarcity of memory B cells and long‐lived plasma cells.131, 132 SAP is an adapter protein that can be recruited by SFRs. There are seven SFRs expressed on haematopoietic cells: 2B4, Ly9, CRACC, CD48, SLAM, CD84 and Ly108.133, 134 However, SFRs are able to signal through other SH2 domain‐containing molecules (such as Fyn), particularly in the context of SAP deficiency.135, 136 Intravital imaging showed that SAP‐deficient T cells are effectively activated by antigen‐bearing DC but are unable to maintain stable conjugates with antigen‐specific B cells, resulting in a poor GC response.131 Tfh cells express high amounts of the SFRs CD84 and Ly108.137, 138 Cd84 −/− mice show a reduction in GC formation and impaired humoral responses, most likely due to their inability to form stable B–T‐cell conjugates.138 Mice deficient in SLAM are defective in IL‐4 production, suggesting that formation of T–B‐cell conjugates enables Tfh cell cytokine production.138 Yet the severe defect in GC formation seen in SAP‐deficient mice has not been recapitulated in any single SLAM family receptor knockout mouse and deletion of all seven SFRs has no effect on the GC response.139 Interestingly, Chen et al. showed that in the absence of SAP, SFR signalling is inhibitory in Tfh cells and suppresses humoral immunity.139 Genetic deletion of Ly108 reverses the phenotype of SAP‐deficient mice.137 Ly108 can associate with both SAP and SHP‐1 and both molecules compete for the same immunoreceptor tyrosine‐switch motif suggesting that Ly108 can act as a rheostat for T–B‐cell interactions. In Tfh cells, antibody‐mediated cross‐linking of SFRs induces the phosphorylation of tyrosine residues on SHP1 and the biochemical suppression of SHP1 can alleviate SFR‐mediated inhibition in SAP‐deficient T cells.139 Hence, it appears that SAP works by preventing the coupling of SFRs to inhibitory signalling pathways, but that this activity is not required for T–B‐cell conjugates that form in the GC.
Conclusions
Co‐receptors provide critical and unique signals to drive effective Tfh cell differentiation. They can act as rheostats for TCR signalling, tempering or elevating the intracellular signals transmitted following antigen engagement. They also provide signals to guide the migration of emerging Tfh cells and regulate expression of transcription factors, cytokines and co‐receptors. The activation status and location of T cells within the environment of the secondary lymphoid organ determines which co‐receptors are engaged and how. In the first phase of Tfh differentiation, DC provide the antigenic stimulus. This is accompanied by CD28 co‐stimulation, which drives Tfh differentiation through proliferation and expression of other co‐receptors. This allows the emerging Tfh cell to respond to cytokines, migratory signals and further co‐stimulatory and inhibitory signals. During the second and third phases of Tfh differentiation ICOS, OX40, CD40L and SFRs provide stimulatory signals that not only amplify antigenic signals but also provide unique signals that are critical for driving Tfh differentiation. The activation‐induced expression of the inhibitory receptors CTLA‐4 and PD‐1 are critical during these stages of differentiation, enabling tight control of Tfh cell proliferation, critical for optimal immune responses. The evolution of B cells able to produce high‐affinity antibodies depends upon competition among B‐cell clones. This is partly achieved by limiting Tfh cell help. In conditions where there are excessive numbers of Tfh cells there is a lower selection pressure so low‐affinity and self‐reactive B‐cell clones are not purged from the GC. Furthermore, in conditions where Tfh cell numbers are not restricted (e.g. when the PD‐1 signalling pathway is blocked) mRNA expression of key cytokines is reduced and there are fewer resultant antibody‐secreting cells and memory B cells. Restricting Tfh cell proliferation provides a limited number of cells able to provide high‐quality help to B cells. Understanding the mechanisms used to govern this will underpin immunization strategies, selecting more stringently for the development of ‘fit’ Tfh cells and aiding development of treatments for diseases with aberrant Tfh cell function.
Disclosure
The authors state that there are no competing interests.
Acknowledgements
We thank Dr D. Hill and Dr M. Turner for critical appraisal of this manuscript. This work was supported by funding from the Biotechnology and Biological Sciences Research Council and the European Research Council (637801).
Contributor Information
Louise M. C. Webb, Email: louise.webb@babraham.ac.uk
Michelle A. Linterman, Email: Michelle.Linterman@babraham.ac.uk
References
- 1. Suan D, Sundling C, Brink R. Plasma cell and memory B cell differentiation from the germinal center. Curr Opin Immunol 2017; 45:97–102. [DOI] [PubMed] [Google Scholar]
- 2. Bannard O, Cyster JG. Germinal centers: programmed for affinity maturation and antibody diversification. Curr Opin Immunol 2017; 45:21–30. [DOI] [PubMed] [Google Scholar]
- 3. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol 2016; 34:335–68. [DOI] [PubMed] [Google Scholar]
- 5. Read KA, Powell MD, Oestreich KJ. T follicular helper cell programming by cytokine‐mediated events. Immunology 2016; 149:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL‐2‐quenching dendritic cells. Nature 2016; 533:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qi H. T follicular helper cells in space‐time. Nat Rev Immunol 2016; 16:612–25. [DOI] [PubMed] [Google Scholar]
- 8. Fazilleau N, Mark L, McHeyzer‐Williams LJ, McHeyzer‐Williams MG. Follicular helper T cells: lineage and location. Immunity 2009; 30:324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keck S, Schmaler M, Ganter S, Wyss L, Oberle S, Huseby ES et al Antigen affinity and antigen dose exert distinct influences on CD4 T‐cell differentiation. Proc Natl Acad Sci USA 2014; 111:14852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knowlden ZA, Sant AJ. CD4 T cell epitope specificity determines follicular versus non‐follicular helper differentiation in the polyclonal response to influenza infection or vaccination. Sci Rep 2016; 6:28287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM et al Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell 2013; 153:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor MJ, Husain K, Gartner ZJ, Mayor S, Vale RD. A DNA‐based T cell receptor reveals a role for receptor clustering in ligand discrimination. Cell 2017; 169:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benson RA, MacLeod MK, Hale BG, Patakas A, Garside P, Brewer JM. Antigen presentation kinetics control T cell/dendritic cell interactions and follicular helper T cell generation in vivo . Elife 2015; 4:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH et al Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci USA 2016; 113:E6639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Remarque EJ, van Beek WC, Ligthart GJ, Borst RJ, Nagelkerken L, Palache AM et al Improvement of the immunoglobulin subclass response to influenza vaccine in elderly nursing‐home residents by the use of high‐dose vaccines. Vaccine 1993; 11:649–54. [DOI] [PubMed] [Google Scholar]
- 16. Chen WH, Cross AS, Edelman R, Sztein MB, Blackwelder WC, Pasetti MF. Antibody and Th1‐type cell‐mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine 2011; 29:2865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pilkinton MA, Nicholas KJ, Warren CM, Smith RM, Yoder SM, Talbot HK et al Greater activation of peripheral T follicular helper cells following high dose influenza vaccine in older adults forecasts seroconversion. Vaccine 2017; 35:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu YL, Metz DP, Chung J, Siu G, Zhang M. B7RP‐1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol 2009; 182:1421–8. [DOI] [PubMed] [Google Scholar]
- 19. Victoratos P, Kollias G. Induction of autoantibody‐mediated spontaneous arthritis critically depends on follicular dendritic cells. Immunity 2009; 30:130–42. [DOI] [PubMed] [Google Scholar]
- 20. Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol 2005; 5:853–65. [DOI] [PubMed] [Google Scholar]
- 21. Ueno H. T follicular helper cells in human autoimmunity. Curr Opin Immunol 2016; 43:24–31. [DOI] [PubMed] [Google Scholar]
- 22. Maine CJ, Marquardt K, Cheung J, Sherman LA. PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. J Immunol 2014; 192:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al Bcl6 and Blimp‐1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009; 325:1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD et al Bcl6 mediates the development of T follicular helper cells. Science 2009; 325:1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL et al The transcriptional repressor Bcl‐6 directs T follicular helper cell lineage commitment. Immunity 2009; 31:457–68. [DOI] [PubMed] [Google Scholar]
- 26. Goenka R, Barnett LG, Silver JS, O'Neill PJ, Hunter CA, Cancro MP et al Cutting edge: dendritic cell‐restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol 2011; 187:1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barnett LG, Simkins HM, Barnett BE, Korn LL, Johnson AL, Wherry EJ et al B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol 2014; 192:3607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dahlgren MW, Gustafsson‐Hedberg T, Livingston M, Cucak H, Alsen S, Yrlid U et al T follicular helper, but not Th1, cell differentiation in the absence of conventional dendritic cells. J Immunol 2015; 194:5187–99. [DOI] [PubMed] [Google Scholar]
- 29. Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A et al Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013; 38:596–605. [DOI] [PubMed] [Google Scholar]
- 30. Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene‐1high germinal center‐associated subpopulation. J Immunol 2007; 179:5099–108. [DOI] [PubMed] [Google Scholar]
- 31. Glatman Zaretsky A, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med 2009; 206:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R et al Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 2010; 33:241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu P, Shih C, Qi H. Ephrin B1‐mediated repulsion and signaling control germinal center T cell territoriality and function. Science 2017; 365:707–17. [DOI] [PubMed] [Google Scholar]
- 34. Shulman Z, Gitlin AD, Weinstein JS, Lainez B, Esplugues E, Flavell RA et al Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 2014; 345:1058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaeth M, Eckstein M, Shaw PJ, Kozhaya L, Yang J, Berberich‐Siebelt F et al Store‐Operated Ca2+ entry in follicular T cells controls humoral immune responses and autoimmunity. Immunity 2016; 44:1350–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM et al The Interleukin‐2–mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T Helper 1 and follicular B helper T cells. Immunity 2015; 43:690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez GJ, Hu JK, Pereira RM, Crampton JS, Togher S, Bild N et al Cutting Edge: NFAT transcription factors promote the generation of follicular helper T cells in response to acute viral infection. J Immunol 2016; 196:2015–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bernard D, Hansen JD, Du Pasquier L, Lefranc MP, Benmansour A, Boudinot P. Costimulatory receptors in jawed vertebrates: conserved CD28, odd CTLA4 and multiple BTLAs. Dev Comp Immunol 2007; 31:255–71. [DOI] [PubMed] [Google Scholar]
- 39. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23:515–48. [DOI] [PubMed] [Google Scholar]
- 40. Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I et al ICOS is an inducible T‐cell co‐stimulator structurally and functionally related to CD28. Nature 1999; 397:263–6. [DOI] [PubMed] [Google Scholar]
- 41. Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co‐receptor signalling. Nat Rev Immunol 2003; 3:544–56. [DOI] [PubMed] [Google Scholar]
- 42. Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol 2005; 6:152–62. [DOI] [PubMed] [Google Scholar]
- 43. Yong PF, Salzer U, Grimbacher B. The role of costimulation in antibody deficiencies: ICOS and common variable immunodeficiency. Immunol Rev 2009; 229:101–13. [DOI] [PubMed] [Google Scholar]
- 44. Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K et al A single amino acid alteration in cytoplasmic domain determines IL‐2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS). J Exp Med 2003; 197:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watanabe R, Harada Y, Takeda K, Takahashi J, Ohnuki K, Ogawa S et al Grb2 and Gads exhibit different interactions with CD28 and play distinct roles in CD28‐mediated costimulation. J Immunol 2006; 177:1085–91. [DOI] [PubMed] [Google Scholar]
- 46. Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM et al CTLA‐4 controls follicular helper T‐cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci USA 2015; 112:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wakamatsu E, Mathis D, Benoist C. Convergent and divergent effects of costimulatory molecules in conventional and regulatory CD4+ T cells. Proc Natl Acad Sci USA 2013; 110:1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walker LS, Gulbranson‐Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M et al Compromised OX40 function in CD28‐deficient mice is linked with failure to develop CXC chemokine receptor 5‐positive CD4 cells and germinal centers. J Exp Med 1999; 190:1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Platt AM, Gibson VB, Patakas A, Benson RA, Nadler SG, Brewer JM et al Abatacept limits breach of self‐tolerance in a murine model of arthritis via effects on the generation of T follicular helper cells. J Immunol 2010; 185:1558–67. [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Ma CS, Ling Y, Bousfiha A, Camcioglu Y, Jacquot S et al Dual T cell‐ and B cell‐intrinsic deficiency in humans with biallelic RLTPR mutations. J Exp Med 2016; 213:2413–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roncagalli R, Cucchetti M, Jarmuzynski N, Gregoire C, Bergot E, Audebert S et al The scaffolding function of the RLTPR protein explains its essential role for CD28 co‐stimulation in mouse and human T cells. J Exp Med 2016; 213:2437–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walker LS, Wiggett HE, Gaspal FM, Raykundalia CR, Goodall MD, Toellner KM et al Established T cell‐driven germinal center B cell proliferation is independent of CD28 signaling but is tightly regulated through CTLA‐4. J Immunol 2003; 170:91–8. [DOI] [PubMed] [Google Scholar]
- 53. Linterman MA, Denton AE, Divekar DP, Zvetkova I, Kane L, Ferreira C et al CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. Elife 2014; 3:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA‐4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 2014; 41:1026–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM et al Trans‐endocytosis of CD80 and CD86: a molecular basis for the cell‐extrinsic function of CTLA‐4. Science 2011; 332:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen‐4 and immune checkpoint blockade. J Clin Invest 2015; 125:3377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Riley JL, Blair PJ, Musser JT, Abe R, Tezuka K, Tsuji T et al ICOS costimulation requires IL‐2 and can be prevented by CTLA‐4 engagement. J Immunol 2001; 166:4943–8. [DOI] [PubMed] [Google Scholar]
- 58. Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS). Curr Opin Immunol 2010; 22:326–32. [DOI] [PubMed] [Google Scholar]
- 59. Warnatz K, Bossaller L, Salzer U, Skrabl‐Baumgartner A, Schwinger W, van der Burg M et al Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood 2006; 107:3045–52. [DOI] [PubMed] [Google Scholar]
- 60. Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH et al ICOS co‐stimulatory receptor is essential for T‐cell activation and function. Nature 2001; 409:97–101. [DOI] [PubMed] [Google Scholar]
- 61. Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. J Immunol 2001; 166:3659–62. [DOI] [PubMed] [Google Scholar]
- 62. Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A et al ICOS is essential for effective T‐helper‐cell responses. Nature 2001; 409:105–9. [DOI] [PubMed] [Google Scholar]
- 63. Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A et al ICOS deficiency is associated with a severe reduction of CXCR5+ CD4 germinal center Th cells. J Immunol 2006; 177:4927–32. [DOI] [PubMed] [Google Scholar]
- 64. Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM et al A RING‐type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 2005; 435:452–8. [DOI] [PubMed] [Google Scholar]
- 65. Yu D, Tan AH, Hu X, Athanasopoulos V, Simpson N, Silva DG et al Roquin represses autoimmunity by limiting inducible T‐cell co‐stimulator messenger RNA. Nature 2007; 450:299–303. [DOI] [PubMed] [Google Scholar]
- 66. Heissmeyer V, Vogel KU. Molecular control of Tfh‐cell differentiation by Roquin family proteins. Immunol Rev 2013; 253:273–89. [DOI] [PubMed] [Google Scholar]
- 67. McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V et al ICOS is critical for CD40‐mediated antibody class switching. Nature 2001; 409:102–5. [DOI] [PubMed] [Google Scholar]
- 68. Xu H, Li X, Liu D, Li J, Zhang X, Chen X et al Follicular T‐helper cell recruitment governed by bystander B cells and ICOS‐driven motility. Nature 2013; 496:523–7. [DOI] [PubMed] [Google Scholar]
- 69. Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W et al T–B‐cell entanglement and ICOSL‐driven feed‐forward regulation of germinal centre reaction. Nature 2015; 517:214–8. [DOI] [PubMed] [Google Scholar]
- 70. Rolf J, Fairfax K, Turner M. Signaling pathways in T follicular helper cells. J Immunol 2010; 184:6563–8. [DOI] [PubMed] [Google Scholar]
- 71. Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW et al Inducible costimulator promotes helper T‐cell differentiation through phosphoinositide 3‐kinase. Proc Natl Acad Sci USA 2009; 106:20371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM et al Phosphoinositide 3‐kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol 2010; 185:4042–52. [DOI] [PubMed] [Google Scholar]
- 73. Gulbranson‐Judge A, Tybulewicz VL, Walters AE, Toellner KM, MacLennan IC, Turner M. Defective immunoglobulin class switching in Vav‐deficient mice is attributable to compromised T cell help. Eur J Immunol 1999; 29:477–87. [DOI] [PubMed] [Google Scholar]
- 74. Tanaka Y, So T, Lebedeva S, Croft M, Altman A. Impaired IL‐4 and c‐Maf expression and enhanced Th1‐cell development in Vav1‐deficient mice. Blood 2005; 106:1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. A p85α‐osteopontin axis couples the receptor ICOS to sustained Bcl‐6 expression by follicular helper and regulatory T cells. Nat Immunol 2015; 16:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai CY, Emslie E et al ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity 2015; 42:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ et al ICOS maintains the T follicular helper cell phenotype by down‐regulating Kruppel‐like factor 2. J Exp Med 2015; 212:217–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Calnan DR, Brunet A. The FoxO code. Oncogene 2008; 27:2276–88. [DOI] [PubMed] [Google Scholar]
- 79. Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu YC. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol 2014; 15:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Athanasopoulos V, Ramiscal RR, Vinuesa CG. ROQUIN signalling pathways in innate and adaptive immunity. Eur J Immunol 2016; 46:1082–90. [DOI] [PubMed] [Google Scholar]
- 81. Pratama A, Ramiscal RR, Silva DG, Das SK, Athanasopoulos V, Fitch J et al Roquin‐2 shares functions with its paralog Roquin‐1 in the repression of mRNAs controlling T follicular helper cells and systemic inflammation. Immunity 2013; 38:669–80. [DOI] [PubMed] [Google Scholar]
- 82. Srivastava M, Duan G, Kershaw NJ, Athanasopoulos V, Yeo JH, Ose T et al Roquin binds microRNA‐146a and Argonaute2 to regulate microRNA homeostasis. Nat Commun 2015; 6:6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jiang SH, Shen N, Vinuesa CG. Posttranscriptional T cell gene regulation to limit Tfh cells and autoimmunity. Curr Opin Immunol 2015; 37:21–7. [DOI] [PubMed] [Google Scholar]
- 84. Pratama A, Srivastava M, Williams NJ, Papa I, Lee SK, Dinh XT et al MicroRNA‐146a regulates ICOS‐ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nat Commun 2015; 6:6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baumjohann D, Kageyama R, Clingan JM, Morar MM, Patel S, de Kouchkovsky D et al The microRNA cluster miR‐17 approximately 92 promotes TFH cell differentiation and represses subset‐inappropriate gene expression. Nat Immunol 2013; 14:840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weinberg AD, Morris NP, Kovacsovics‐Bankowski M, Urba WJ, Curti BD. Science gone translational: the OX40 agonist story. Immunol Rev 2011; 244:218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tahiliani V, Hutchinson TE, Abboud G, Croft M, Salek‐Ardakani S. OX40 cooperates with ICOS to amplify follicular Th cell development and germinal center reactions during infection. J Immunol 2017; 198:218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T‐cell biology and immune disease. Immunol Rev 2009; 229:173–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brocker T, Gulbranson‐Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40‐ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur J Immunol 1999; 29:1610–6. [DOI] [PubMed] [Google Scholar]
- 90. Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B et al OX40‐deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity 1999; 11:699–708. [DOI] [PubMed] [Google Scholar]
- 91. Gaspal FM, Kim MY, McConnell FM, Raykundalia C, Bekiaris V, Lane PJ. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol 2005; 174:3891–6. [DOI] [PubMed] [Google Scholar]
- 92. Gaspal F, Withers D, Saini M, Bekiaris V, McConnell FM, White A et al Abrogation of CD30 and OX40 signals prevents autoimmune disease in FoxP3‐deficient mice. J Exp Med 2011; 208:1579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J Exp Med 2003; 197:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity 2005; 22:621–31. [DOI] [PubMed] [Google Scholar]
- 95. Boettler T, Choi YS, Salek‐Ardakani S, Cheng Y, Moeckel F, Croft M et al Exogenous OX40 stimulation during lymphocytic choriomeningitis virus infection impairs follicular Th cell differentiation and diverts CD4 T cells into the effector lineage by upregulating Blimp‐1. J Immunol 2013; 191:5026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hirschhorn‐Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM et al OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med 2009; 206:1103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hirschhorn‐Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H et al Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med 2012; 209:2113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW et al OX40 costimulation promotes persistence of cytomegalovirus‐specific CD8 T cells: a CD4‐dependent mechanism. J Immunol 2007; 179:2195–202. [DOI] [PubMed] [Google Scholar]
- 99. Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T‐cell co‐stimulatory molecule OX40. Nat Rev Immunol 2004; 4:420–31. [DOI] [PubMed] [Google Scholar]
- 100. Jacquemin C, Schmitt N, Contin‐Bordes C, Liu Y, Narayanan P, Seneschal J et al OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity 2015; 42:1159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Byun M, Ma CS, Akcay A, Pedergnana V, Palendira U, Myoung J et al Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med 2013; 210:1743–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Song J, So T, Croft M. Activation of NF‐κB1 by OX40 contributes to antigen‐driven T cell expansion and survival. J Immunol 2008; 180:7240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. So T, Choi H, Croft M. OX40 complexes with phosphoinositide 3‐kinase and protein kinase B (PKB) to augment TCR‐dependent PKB signaling. J Immunol 2011; 186:3547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci USA 2006; 103:3740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Croft M. Control of immunity by the TNFR‐related molecule OX40 (CD134). Annu Rev Immunol 2010; 28:57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vogel KU, Edelmann SL, Jeltsch KM, Bertossi A, Heger K, Heinz GA et al Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity 2013; 38:655–68. [DOI] [PubMed] [Google Scholar]
- 107. Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD‐1 expression upon T cell activation. J Immunol 2008; 181:4832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ et al Regulation of PD‐1, PD‐L1, and PD‐L2 expression during normal and autoimmune responses. Eur J Immunol 2003; 33:2706–16. [DOI] [PubMed] [Google Scholar]
- 109. Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V et al Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27:670–84. [DOI] [PubMed] [Google Scholar]
- 110. Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH et al Phenotype, function, and gene expression profiles of programmed death‐1(hi) CD8 T cells in healthy human adults. J Immunol 2011; 186:4200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Turnis ME, Andrews LP, Vignali DA. Inhibitory receptors as targets for cancer immunotherapy. Eur J Immunol 2015; 45:1892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS et al T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non‐Th1/Th2 effector cells that provide help for B cells. J Immunol 2004; 173:68–78. [DOI] [PubMed] [Google Scholar]
- 113. Yokosuka T, Takamatsu M, Kobayashi‐Imanishi W, Hashimoto‐Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012; 209:1201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J et al PD‐1 inhibits T‐cell receptor induced phosphorylation of the ZAP70/CD3ζ signalosome and downstream signaling to PKCθ . FEBS Lett 2004; 574:37–41. [DOI] [PubMed] [Google Scholar]
- 115. Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP‐1 and SHP‐2 associate with immunoreceptor tyrosine‐based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004; 173:945–54. [DOI] [PubMed] [Google Scholar]
- 116. Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y et al Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002; 169:5538–45. [DOI] [PubMed] [Google Scholar]
- 117. Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD‐1 immunoreceptor inhibits B cell receptor‐mediated signaling by recruiting src homology 2‐domain‐containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA 2001; 98:13866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I et al PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat Immunol 2001; 2:261–8. [DOI] [PubMed] [Google Scholar]
- 119. Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA et al Tissue expression of PD‐L1 mediates peripheral T cell tolerance. J Exp Med 2006; 203:883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nurieva R, Thomas S, Nguyen T, Martin‐Orozco N, Wang Y, Kaja MK et al T‐cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J 2006; 25:2623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H et al Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR et al PD‐1:PD‐L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL‐2. Eur J Immunol 2002; 32:634–43. [DOI] [PubMed] [Google Scholar]
- 123. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA et al T cell costimulatory receptor CD28 is a primary target for PD‐1‐mediated inhibition. Science 2017; 355:1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL et al Rescue of exhausted CD8 T cells by PD‐1‐targeted therapies is CD28‐dependent. Science 2017; 355:1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN et al PD‐1 alters T‐cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 2015; 6:6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V et al Bcl‐6 directly represses the gene program of the glycolysis pathway. Nat Immunol 2014; 15:957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lonnberg T, Svensson V, James KR, Fernandez‐Ruiz D, Sebina I, Montandon R et al Single‐cell RNA‐seq and computational analysis using temporal mixture modelling resolves Th1/Tfh fate bifurcation in malaria. Sci Immunol 2017; 2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Good‐Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD‐1 regulates germinal center B cell survival and the formation and affinity of long‐lived plasma cells. Nat Immunol 2010; 11:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD‐L1hi B cells are critical regulators of humoral immunity. Nat Commun 2015; 6:5997. [DOI] [PubMed] [Google Scholar]
- 130. Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D et al The X‐linked lymphoproliferative‐disease gene product SAP regulates signals induced through the co‐receptor SLAM. Nature 1998; 395:462–9. [DOI] [PubMed] [Google Scholar]
- 131. Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP‐controlled T‐B cell interactions underlie germinal centre formation. Nature 2008; 455:764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hu J, Havenar‐Daughton C, Crotty S. Modulation of SAP dependent T: B cell interactions as a strategy to improve vaccination. Curr Opin Virol 2013; 3:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol 2007; 25:337–79. [DOI] [PubMed] [Google Scholar]
- 134. Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol 2011; 29:665–705. [DOI] [PubMed] [Google Scholar]
- 135. Veillette A, Dong Z, Perez‐Quintero LA, Zhong MC, Cruz‐Munoz ME. Importance and mechanism of ‘switch’ function of SAP family adapters. Immunol Rev 2009; 232:229–39. [DOI] [PubMed] [Google Scholar]
- 136. Dong Z, Veillette A. How do SAP family deficiencies compromise immunity? Trends Immunol 2010; 31:295–302. [DOI] [PubMed] [Google Scholar]
- 137. Kageyama R, Cannons JL, Zhao F, Yusuf I, Lao C, Locci M et al The receptor Ly108 functions as a SAP adaptor‐dependent on‐off switch for T cell help to B cells and NKT cell development. Immunity 2012; 36:986–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cannons JL, Qi H, Lu KT, Dutta M, Gomez‐Rodriguez J, Cheng J et al Optimal germinal center responses require a multistage T cell: B cell adhesion process involving integrins, SLAM‐associated protein, and CD84. Immunity 2010; 32:253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chen S, Cai C, Li Z, Liu G, Wang Y, Blonska M et al Dissection of SAP‐dependent and SAP‐independent SLAM family signaling in NKT cell development and humoral immunity. J Exp Med 2017; 214:475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


