Summary
Datura metel L. is a medicinal herb that contains withasteroids and has a wide range of biological activities. We isolated seven withasteroids from the flowers of D. metel L and examined their ability to inhibit immune responses in vitro and in vivo. Among the withasteroids, withasteroid B2 exhibited the strongest inhibitory effect on immune responses comparing B2 with other isolated compounds from D. metel L., including suppressing the differentiation of CD4+ T cells by inhibiting the expression and production of T cell lineage‐specific master regulators and cytokines and directly suppressing the cytokine‐induced Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathways. In the interleukin (IL)‐23‐induced mouse ear model of skin disease, B2 repressed disease development by inhibiting the expression of proinflammatory mediators in murine ear skin. Moreover, B2 affected the maturation of dendritic cells (DCs) in vitro which, in turn, induced T cell differentiation with an increased regulatory T cell (Treg) phenotype and decreased T helper type 17 (Th17) phenotype. This study provides new evidence that B2 might ameliorate chronic inflammatory skin diseases by suppressing pathogenic CD4+ T cell differentiation and the IL‐17+retinoic‐acid‐receptor‐related orphan receptor gamma t (RORγt)+/IL‐10+forkhead box protein 3 (FoxP3)+ ratio. These findings suggest that B2 might mediate the therapeutic effects observed in psoriasis patients following treatment with D. metel L.
Keywords: autoimmune disease, IL‐17+RORγt+/IL‐10+FoxP3+ ratio, JAK/STAT pathways
Introduction
Psoriasis is a complex, chronic, immune‐mediated inflammatory skin disorder that affects 2–3% of the population. Psoriasis is characterized by thickening, scaling or erythema in the skin, and results from the dysregulation of epidermal keratinocytes and inflammatory infiltration of leucocytes that release growth factors, cytokines and chemokines 1. Although the pathogenic mechanism of psoriasis remains unclear, it is believed to be a chronic inflammatory autoimmune disease that is mediated by T cells, particularly T helper type 1 (Th1) and Th17 cells. Accumulating evidence suggests that many innate and adaptive immune cells, including Th1 cells, and Th17‐derived cytokines, such as tumour necrosis factor (TNF‐α), interleukin (IL)‐6, IL‐1β, IL‐17A, IL‐22 and IL‐23, are involved in and interact as a network in the pathogenesis of psoriasis 2, 3, 4, 5, 6, 7. The proinflammatory cytokines are found in psoriatic lesions and peripheral blood 8. Moreover, some studies have shown that impaired regulatory T cell (Treg) functions in psoriasis patients contribute to the development of allergic inflammatory skin diseases, such as psoriasis 9, 10. Th17 cells are involved critically in the pathology of psoriasis. Consequently, retinoic‐acid‐receptor‐related orphan receptor gamma t (RORγt)+IL‐17+Th17 responses must be regulated tightly in vivo to mediate effective host defences against pathogens without causing excessive host tissue damage. CD4+CD25highforkhead box protein 3 (FoxP3)+ regulatory T cells play an important role in maintaining peripheral tolerance to self‐antigens and in counteracting the inflammatory activity of effector T helper cell subsets, and psoriasis has also been associated with an impaired suppressive capacity of Tregs 11. Although Th17 and Treg cells represent two CD4+ T cell subsets with opposing principal functions, these cell types are functionally connected 12. An imbalance of Th17 and Treg cells is involved in many autoimmune, inflammatory and allergic reactions, such as psoriasis. The recovery of these cells is thought to play an important role in the prevention and treatment of psoriasis.
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is the major signalling pathway involved in the differentiation and function of CD4+ T cells and is triggered by cytokines 13, 14, 15, 16. The JAK/STAT pathway plays a critical role in the activation of transcription factors that induce lineage‐specific master transcription factors, including T‐box transcription factor TBX21 (T‐bet), GATA‐binding protein 3 (GATA‐3), RORγt and FoxP3, during CD4+ T cell differentiation 15, 16, 17. STAT‐1 and STAT‐4 are critical for Th1 cell differentiation, whereas STAT‐5 and STAT‐6, STAT‐3 and STAT‐5 are critical for Th2, Th17 and Treg cell differentiation, respectively. These STAT proteins also collaborate with the master transcription factors to induce cytokine production by CD4+ T cells 13, 15, 16. Recently, the JAK protein has emerged as a possible new therapeutic target for the treatment of autoimmune inflammatory diseases, including psoriasis 18, 19. Several JAK inhibitors are being tested in clinical and preclinical trials with promising results. Additionally, total STAT‐3 expression has also been shown to be up‐regulated in psoriatic lesions, and a transgenic mouse model that constitutively over‐expresses activated STAT‐3 in the basal stem‐cell layer of the epidermis is able to reproduce many of the characteristic features of psoriasis 20, 21.
Withasteroids are a group of structurally diverse steroidal compounds with a C28 steroidal lactone skeleton, in which a characteristic feature is the presence of an α,β‐unsaturated δ lactone ring in the side chain; withasteroids are observed primarily in several genera of Solanaceae. In recent years, withanolides have received considerable attention because of their versatile biological activities, such as anti‐tumour 22, cytotoxic 23, 24, anti‐inflammatory 25, 26, immunosuppressive 23 and chemoprevention activities 27. Datura metel L. (baimantuoluo in Chinese), shown in Fig. 1a, is an annual herb that belongs to the Solanaceae family. The flower of D. metel L. is a traditional Chinese medicine and is listed officially in the Chinese Pharmacopoeia 28.
Figure 1.
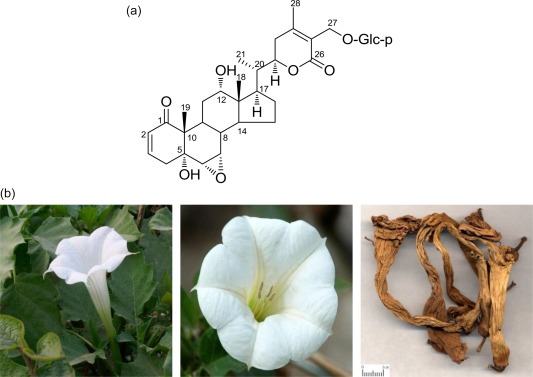
(a) Structure of B2; (b) fresh flower and dried medicinal herbs of Datura metel. [Colour figure can be viewed at wileyonlinelibrary.com]
D. metel L. has been used in Chinese folk medicine for centuries for the treatment of asthma, cough, convulsions, pain, rheumatism, convulsion and insanity 29. Professor Gang Liu, Doctor of Heilongjiang University of Traditional Chinese Medicine, discovered the perfect anti‐psoriasis effect of D. metel accidentally in clinical practice.
Chemical investigations have shown that withanolides are the main constituents of the effective part of D. metel flowers for psoriasis treatment, and a series of withanolides has been isolated 30, 31, 32, 33, 34, 35, 36. However, the active constituents and pharmacological effects of D. metel flowers for the treatment of psoriasis have not been elucidated fully. As part of a continuing project to study the active constituents of Flos Daturae for psoriasis 31, 33, 36, in this study we evaluated the ability of a series of withanolides isolated from D. metel L. to inhibit immune responses. Among these withanolides, B2 showed strong inhibitory effects on CD4+ T cell differentiation and IL‐23‐induced cutaneous inflammation in murine models and mediated these effects by inhibiting JAK/STAT signalling pathways. Moreover, we also investigated whether B2 affects the IL‐17+RORγt+/FoxP3+IL‐10+ ratio, which indicates that B2 could affect the Th17/Treg balance to cure psoriasis.
Materials and methods
Experimental animals
The experimental protocols for the animal studies were approved by the Catholic Research Institute of the Medical Science Committee on Institutional Animal Care and Use. BALB/c and C57BL/6 mice were acclimatized under specific pathogen‐free conditions for 1 week and maintained in a room at a constant temperature (23 ± 2°C) on a 12‐h light/dark cycle with free access to a laboratory chow diet and water. For the IL‐23‐induced psoriasis‐like skin inflammation model, recombinant mouse IL‐23 (eBioscience, San Diego, CA, USA) was injected intradermally into C57BL/6 mouse ears every other day for 14 days, as described previously 37. The ear thickness was measured 24 h after the final injection of IL‐23, and the ears were collected and stored at −80°C for further experiments. All animal experiments were conducted in biosafety level 3 (BSL‐3) facilities in compliance with the Ethics Committee regulations of the University of California, Los Angeles, Institutional Animal Care and Use Committee and were performed in accordance with National Institutes of Health (NIH) policies regarding the care and use of laboratory animals.
Induction of disease
Skin lesions were induced in mice through an intradermal injections into the left ear of 0·5 mg of mouse IL‐23 (eBioscience) in 20 ml of phosphate‐buffered saline (PBS) on days 0, 2, 4, 6, 8, 10, 12 and 14. The IL‐23‐injected mice were treated orally with B2 on days 3, 6, 9, 12 and 15. One group of IL‐23‐injected mice was treated with a control oligonucleotide at a dose of 15 mg/kg, and another group of mice was treated with PBS using the same dosing schedule as was used for the antagonist. The IL‐23‐induced increase in ear thickness was monitored using a Peacock dial thickness gauge, and ear samples were collected on day 18. Lesions were induced on the dorsal skin through daily intradermal injections of IL‐23 (1 mg) from days 0 to 3. The IL‐23‐treated mice were treated orally at a distal site with B2, control or PBS on days 3, 4 and 5. All the mice were killed on day 6, and samples were collected for evaluation.
Isolation and purification of B2
D. metel L. was purchased from Harbin, China in June 2013, and a voucher specimen is deposited at the National Products Research Institute, College of Pharmacy, Heilongjiang University of Traditional Chinese Medicine. B2 (> 95% purity) was extracted from the dried flowers of D. metel L. Briefly, the dried flowers of D. metel L. were extracted repeatedly with 75% ethyl alcohol (EtOH) (100 l × 3 h × 3) at room temperature. The crude extract was evaporated under a vacuum (45°C) to yield a residue (520 g), which was partitioned into water (H2O), petroleum ether and ethyl acetate (EtOAc). The EtOAc portion of the extract (400 g) was chromatographed on silica gel with dichloromethane (CH2Cl2/MeOH) (30 : 1–0 : 1, v/v) to afford fractions A–E. Fraction B (77·3 g) was rechromatographed on a silica gel column (CH2Cl2/MeOH 25 : 1–0 : 1, v/v) to yield five subfractions. B2 was obtained from fraction B after purification by CC using CH2Cl2/MeOH 20 : 1–0 : 1, v/v. Subfraction B was purified further on an octadecylsilyl (ODS) column by eluting with MeOH/H2O (1 : 9–1 : 0, v/v) and by semipreparative high‐performance liquid chromatography (HPLC) (40% MeOH/H2O, flow rate = 3 ml/min) to yield B2 (142 mg) and B4 (25 mg). Subfraction G was further purified on an ODS column by eluting with MeOH/H2O (1 : 9–1 : 0, v/v) and by semi‐preparative HPLC (55% MeOH/H2O, flow rate = 3 ml/min) to yield GB (50 mg), G1 (200 mg), G6 (75 mg), G3 (47 mg) and G5 (60 mg). Among these compounds, B2, named baimantuoluoside B, was obtained as a white amorphous powder. In the electrospray ionization mass spectrometry (ESI‐MS) analysis, a quasi‐molecular ion peak was observed at m/z 649 [(M + H)+]. The molecular formula of B2 was determined as C34H48O12 by HR‐ESI‐MS, indicating 11 degrees of unsaturation. The C(12) resonance at δC 73·8 of B2 indicated that C(12) was substituted by an α‐orientated OH group, which was confirmed by a singlet at δH 3·97 in the [1H]‐NMR spectrum. This multiplicity was characteristic of a 12α‐hydroxy‐withanolide. Therefore, B2 was elucidated as (5α, 5α, α, 22R)‐5,12‐dihydroxy‐1,26‐dioxo‐6,7; 22,26‐diepoxyerosta‐2,24‐dien‐27‐yl β‐D‐glucopyranoside.
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated from anti‐coagulant‐treated whole blood through density gradient centrifugation (Ficoll‐Hypaque; TBD Science, Tianjin, China). CD4+ T cells and CD14+ monocytes were purified using microbead isolation kits followed by magnetic‐activated cell sorting (MACS), according to the manufacturer's instructions (Miltenyi Biotec, Palo Alto, CA, USA). The purity of the isolated cells, which was determined by flow cytometry (FCM), was greater than 90%. The isolated cells (PBMCs, CD4+ T cells and monocytes) were resuspended at a concentration of 1 × 106 cells/ml in RPMI 1640 medium (Gibco‐Invitrogen, Carlsbad, CA, USA) containing L‐glutamine (2 mM), penicillin/streptomycin (100 U/ml) and 10% fetal calf serum (Gibco‐Invitrogen). Immature dendritic cells (iDCs) were generated from monocytes in the presence of recombinant human granulocyte–macrophage colony‐stimulating factor (GM‐CSF); 100 ng/ml) and IL‐4 (50 ng/ml; PeproTech, London, UK) for 6 days. On day 3, half the culture medium, including GM‐CSF and IL‐4, was refreshed. For DC maturation, 100 ng/ml lipopolysaccharide (LPS) (Sigma‐Aldrich, St Louis, MO, USA) with B2 (5 μM) or the vehicle dimethyl sulphoxide (DMSO) was added to the cells at day 6, and the cells were then cultured for 24 h. CD4+ T cells were cultured with B2 (5 μM) or the vehicle DMSO in the presence of anti‐CD3 [muromonab‐CD3 (OKT3), 0·5 μg/ml] and anti‐CD28 antibodies (15E8, 0·1 μg/ml; Miltenyi Biotec, Auburn, CA, USA) for 3 days. B2‐ or DMSO‐treated DCs were washed three times and then co‐cultured with CD4+ T cells at a ratio of 1 : 5 for 5 days.
Isolation and in‐vitro differentiation of mouse CD4+ T cells
Naive CD4+ T cells were purified from the spleens and lymph nodes of C57BL/6 mice by negative selection using a MACS column (Miltenyi Biotech). The cells were activated with plate‐bound anti‐CD3 and soluble anti‐CD28 (2 μg/ml) antibodies in RPMI‐1640 medium containing 10% fetal bovine serum (FBS), 2 mM glutamine and a 1% penicillin/streptomycin solution for 4 days. The cells were polarized under Th1‐polarizing conditions (10 ng/ml IL‐12 and 10 μg/ml anti‐IL‐4 antibody), Th2‐polarizing conditions (20 ng/ml IL‐4 and 10 μg/ml anti‐IFN‐γ antibody), Th17‐polarizing conditions (20 ng/ml IL‐6, 5 ng/ml transforming growth factor (TGF)‐β, 10 μg/ml anti‐IFN‐γ antibody and 10 μg/ml anti‐IL‐4 antibody) or Treg‐polarizing conditions (5 ng/ml TGF‐β and 10 ng/ml IL‐2). All cytokines and antibodies used for CD4+ T cell differentiation were purchased from BD Biosciences (San Jose, CA, USA).
Co‐culture of mature DCs and allogeneic CD4+ T cells
To analyse the ability of DCs matured in the presence of B2 to stimulate allogeneic CD4+ T cells, the DCs were matured in the presence and absence of B2 and subsequently cultured in the presence of allogeneic CD4+ T cells for 6 days, as described previously 38. In short, the DCs were harvested and washed to remove any B2 and then transferred into 96‐well round‐bottomed tissue culture plates. Freshly isolated CD4+ T cells were added to the wells, and the cells were co‐cultured at a DC : T cell ratio of 1 : 8 (2·5 × 104 DCs/well : 2 × 105 CD4+ T cells/well) in RPMI medium supplemented with FCS and antibiotics. For comparison, CD4+ T cells and DCs were also cultured alone. The effect of the co‐culture on T cell and DC activation was analysed by measuring the expression of surface and intracellular molecules by flow cytometry.
MTT assay
Cytotoxicity was assessed using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT) assay, as described previously 39. Briefly, splenocytes (4 × 105 cells/well) were cultured in triplicate in a 96‐well flat‐bottomed plate (Costar, Corning Incorporated, Corning, NY, USA) for 48 h. MTT (5 mg/ml) was added 4 h before the end of the culture, and then solvent [10% sodium dodecyl sulphate (SDS) and 50% N,N‐dimethyl formamide, pH 7·2) was added to dissolve the precipitate. After incubating the solution for 7 h, the optical density (OD)570 was measured using a microplate reader (Model 550; Bio‐Rad).
Proliferation assays
The proliferation of splenocytes in response to convanavalin A (ConA) was determined by [3H]‐thymidine uptake, as described previously 39. Briefly, a BALB/c splenocyte suspension (4 × 105 cells/well) was cultured with ConA (5 μg/ml) and irradiated C57BL/6 splenocytes (4 × 105 cells/well) in a 96‐well flat‐bottomed plate (Costar). The cultures were incubated for 48 h to allow ConA to induce proliferation. The cultures were then treated with 0·5 μCi [3H]‐thymidine for 8 h (ConA‐induced proliferation) before termination of the cultures. The cultured cells were harvested onto glass fibre filters. The incorporated radioactivity was measured using a Beta Scintillation Counter (MicroBeta TriLux, Wellesley, MA, USA).
Mixed lymphocyte reaction (MLR)
To investigate the effect of a series of compounds isolated from the flower of Datura L. on the immune response, we performed an MLR. Mouse T cells were isolated from the spleens and lymph nodes of C57BL/6 and BALB/C mice using a MACS column (Miltenyi Biotech). The isolated T cells (1 × 105 cells) were co‐cultured with irradiated allogeneic T cells (1 × 105 cells) in a round‐bottomed 96‐well plate for 5 days. Cell proliferation was analysed by the incorporation of [3H]‐thymidine (1 μCi/well) after 16 h of incubation.
RNA isolation and quantitative real‐time polymerase chain reaction (qRT–PCR)
The total RNA from the cells or tissues was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and cDNA was synthesized using the QuantiTect reverse transcription kit (Qiagen). qRT–PCR was performed using the KAPA SYBR Fast qPCR kit (KAPA Biosystems, Woburn, MA, USA), and the results were normalized to the expression of the housekeeping gene glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH). The PCR conditions were one cycle at 95°C for 5 min, followed by 30–40 cycles at 96°C for 20 s, 60°C for 20 s and 72°C for 20 s, and finally one cycle at 72°C for 5 min. The primers used in this experiment were purchased from Qiagen.
Measurement of cytokine production
The cytokine concentrations in the supernatants were determined by enzyme‐linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's protocol. The conditioned media were collected from mouse CD4+ T cells after differentiation for 4 days or after co‐culture with splenocytes for 24 h.
SDS‐PAGE and Western blot analysis
The samples were separated by SDS‐PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Pall Corp., Port Washington, NY, USA). The membranes were blocked for 1 h in blocking buffer (5% skimmed milk in 25 mM Tris– hydrochloric acid (HCl) (pH 8.0), 150 mM sodium chloride (NaCl) and 0·1% Tween 20) and incubated with specific primary antibodies (Genetex Inc., Irvine, CA, USA) for the target molecules overnight at 4°C. The blots were washed and incubated with horseradish peroxidase‐conjugated secondary antibodies (Genetex Inc.) for 2 h at room temperature, and the signals were detected using X‐ray film following a reaction with an electrochemiluminescence (ECL) detection kit (iNtRON Biotechnology, Korea).
Histological analysis
The histological assessments were performed as described previously 40. Formalin‐fixed, paraffin‐embedded ear tissue was sectioned at a thickness of 5 mm and the sections were stained with haematoxylin and eosin (H&E). The following parameters were assessed: (1) the level of leucocyte infiltration and vascular congestion; (2) the erosion and anabrosis of epidermal cells; and (3) the effects on the other side of the ears. The histological scores were assessed from 1 to 4. The final data are the average scores from each animal in the same group, and a higher score indicates more serious inflammation.
Measurement of surface and intracellular molecules by flow cytometry
The purity of CD14+ monocyte and CD4+ T cell isolation was confirmed by staining the cells with fluorochrome‐labelled antibodies against CD14 and CD4, respectively. For analysing the effects of co‐culturing DCs and allogeneic CD4+ T cells, the DCs were stained with fluorochrome‐labelled antibodies against CD1c, CD40, CD86, CCR7, programmed death ligand 1 (PD‐L1), human leucocyte antigen D‐related (HLA‐DR) and IL‐10 and the CD4+ T cell were stained with fluorochrome‐labelled antibodies against CD4, CD25, CD40L, CD54, CD69 and cytotoxic T lymphocyte antigen 4 (CTLA‐4), FoxP3 and IL‐10 and RORrt and IL‐17 by cells stained with fluorochrome‐labelled isotype‐matched antibodies were used as controls. Antibodies were obtained from AbD Serotec (Kidlington, UK), BD Bioscience (San Jose, CA, USA), eBioscience, Miltenyi Biotec and R&D Systems (Minneapolis, MN, USA). Ten thousand cells were acquired using fluorescence activated cell sorter (FACS)Calibur (BD Bioscience) and analysed using CellQuset (BD Bioscience). Dot‐plots of forward‐ and side‐scatter were formed, gates drawn around the DCs and the T cells and the cells analysed further using histograms. Results are expressed as the percentage of positive cells compared with cells stained with isotype control and expression levels.
Statistical analysis
The means and standard error of the means were calculated, and the differences between groups were evaluated through one‐way analysis of variance (anova) followed by Tukey's post‐hoc test when the data were distributed normally. If the data were not distributed normally, one‐way anova of ranks followed by Dunn's post‐hoc test was used to determine whether the group medians differed, but the means ± standard errors of the mean (s.e.m.) are listed in the corresponding data tables for clarity. Statistical analyses were performed using SigmaStat version 3.1. A P‐value less than 0·05 was considered statistically significant.
Results
Cytotoxicity of the drugs toward murine splenocytes
We isolated a series of seven withanolides from D. metel flowers, as described in the Materials and methods section. First, we examined the cytotoxic effect of the compounds to identify the safe dose range of the drugs. As shown in Fig. 2a, during a 48‐h culture the drugs presented a typical S‐shaped concentration–toxicity relationship for murine splenocytes. During a 48‐h culture at concentrations up to 1000 µM, the 50% cytotoxic concentration (CC50) value of GB was 59·03 ± 8·4 µmol; that of B4 was 88·82 ± 9·8 µmol; that of G1 was 4·48 ± 0·32 µmol; that of B2 (Fig 1b, 41) was 26·13 ± 4·65 µmol; that of G6 was 85·2 ± 6·8 µmol; that of G3 was 6·39 ± 0·52 µmol; and that of G5 was 7·01 ± 0·33 µmol (Fig. 2a). Because GB, B4, B2 and G6 have relatively high CC50 values, they have a favourably wide safety range. Therefore, we used concentrations of the compounds up to 100 µM in the in‐vitro experiments to exclude cytotoxic effects.
Figure 2.
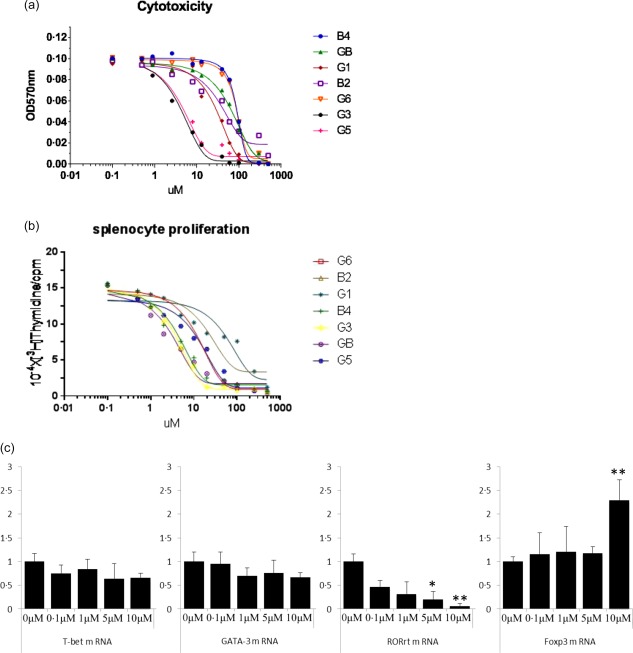
(a) Cytotoxicity profile of the drugs toward splenocytes. For cell viability measurements, BALB/c splenocytes were cultured with increasing concentrations of drugs for 48 h. The results represent the means ± standard error of the means (s.e.m.). (b) Effects of the drugs on the concanavalin A (ConA)‐induced proliferation profile of splenocytes. For the proliferation assay, BALB/c splenocytes were stimulated with ConA for 48 h at increasing concentrations of drugs in a 96‐well plate in triplicate. The results represent the means ± s.e.m. (c) B2 inhibits CD4+ T cell differentiation. Naive CD4+ T cells isolated from mice were cultured under the appropriate polarizing conditions in the presence of B2 for 4 days. Total RNA was isolated from the cells, and quantitative real‐time polymerase chain reaction (qRT–PCR) was performed to measure the mRNA levels of T cell lineage‐specific master transcription factors. The results represent the means of three independent experiments. *P < 0·001 and **P < 0·05 versus the differentiated group. [Colour figure can be viewed at wileyonlinelibrary.com]
Drugs inhibit mitogen‐induced murine splenocyte proliferation
A concentration‐dependent suppression of murine splenocyte proliferation in response to ConA was observed when the drugs were added to cell cultures. As shown in Fig. 2b, ConA‐induced proliferation was inhibited significantly by the drug treatments. The concentration‐dependencies were fitted to typical S‐shaped curves. In response to ConA, the half‐maximal inhibitory concentration (IC50) value of G6 inhibition of lymphocyte proliferation was 20·50 ± 4·06 µmol/L; the IC50 of B2 was 46·21 ± 7·65 µmol/l; the IC50 of G1 was 71·98 ± 12·1 µmol/l; the IC50 of B4 was 9·007 ± 0·86 µmol/l; the IC50 of G3 was 6·788 ± 0·21 µmol/l; the IC50 of GB was 9·381 ± 0·67 µmol/l; and the IC50 of G5 was 26·83 ± 7·3 µmol/l (Fig. 2b). Thus, B2, G1, G6 and G5 had low IC50 values, making these our test compounds.
The drugs suppress CD4+ T cell differentiation
We performed an MLR using T cells isolated from the spleens and lymph nodes of C57 black 6 (C57BL/6) and BALB/C mice, respectively, to examine the ability of the withanolide compounds isolated from the D. metel flowers to regulate immune responses. Among the seven withanolide compounds, B2 exhibited the strongest inhibitory effect on immune responses (Table 1). Thus, B2 was selected as the test compound.
Table 1.
Effects of a series of withanolide compounds isolated from Datura metel L. flowers on immune responses in mouse T cells using one‐way mixed lymphocyte reactions (MLRs)
| Compounds | Concentration (µM) | IC50 (µM) | |||
|---|---|---|---|---|---|
| 50 | 25 | 12.5 | 6.25 | ||
| B2 | 99·3 ± 3·0 | 93·1 ± 2·0 | 60·1 ± 3·4 | 32·1 ± 3·2 | 9·25 ± 1·2 |
| B4 | 97·4 ± 3·2 | 90·9 ± 3·0 | 51·9 ± 2·0 | 26·2 ± 2·0 | 10·55 ± 2·5 |
| G1 | 95·7 ± 3·4 | 70·3 ± 2·2 | 46·4 ± 5·0 | 7·1 ± 2·7 | 15·7 ± 2·3 |
| GB | 99·1 ± 4·4 | 63·3 ± 6·0 | 18·1 ± 2·1 | — | 19·41 ± 2·7 |
| G3 | 98·9 ± 3·1 | 78·7 ± 2·9 | 34·6 ± 5·7 | 7·5 ± 3·4 | 14·83 ± 1·9 |
| G4 | 67·6 ± 3·3 | 46·5 ± 6·0 | 26·1 ± 3·3 | 12·5 ± 2·5 | 27·6 ± 2·7 |
| G5 | 98·2 ± 3·5 | 76·3 ± 4·0 | 20·4 ± 4·4 | 6·8 ± 3·5 | 16·49 ± 2·1 |
We then examined the expression of the master transcription factors in mouse CD4+ T cells cultured under the appropriate polarizing conditions to determine whether B2 could affect CD4+ T cell differentiation. The mRNA levels of RORγt, which is the master transcription factor for Th17 cells, was suppressed markedly by treatment with B2, whereas the levels of T‐bet and GATA‐3, the master transcription factors for Th1 and Th2 cells, respectively, were decreased marginally. In contrast, B2 increased the mRNA levels of FoxP3, the master transcription factor for Treg cells, significantly (Fig. 2c).
B2 regulates the expression and production of CD4+ T cell lineage‐specific signature cytokines
We next examined the effects of B2 on the expression and production of selected signature cytokines in CD4+ T cell subsets (IFN‐γ, IL‐4, IL‐17A, IL‐10 and TGF‐β). These cytokines play a critical role in CD4+ T cell differentiation and are involved in the regulation of master transcription factors 16, 42. The mRNA levels of Th17 cytokines, such as IL‐17A, were decreased substantially; in contrast, the levels of the Treg cytokine IL‐10 were increased significantly following treatment with B2 at concentrations greater than 5 µM (Fig. 3a). Consistent with the mRNA levels, at concentrations greater than 5 µM, B2 inhibited the production of selected Th17 signature cytokines to a greater degree than it inhibited selected Th1 and Th2 signature cytokines when the CD4+ T cells were polarized under appropriate conditions (Fig. 3b).
Figure 3.
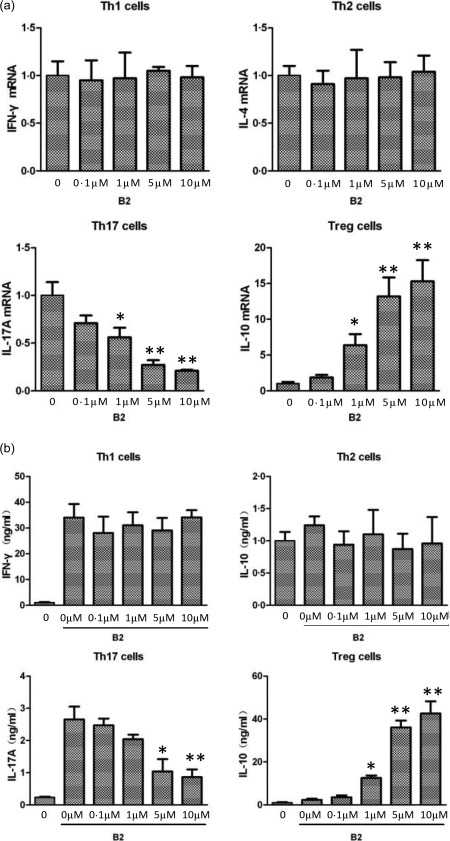
B2 inhibits the expression and production CD4+ T cell‐specific signature cytokines. (a) Total RNA was prepared from T cells differentiated using the conditions described in Fig. 2c, and quantitative real‐time polymerase chain reaction (qRT–PCR) was performed. (b) Cultured media were collected from T cells that had been differentiated using the conditions described in Fig. 2c. The cytokine concentrations were analysed by enzyme‐linked immunosorbent assay (ELISA). *P < 0·001 and **P < 0·05 versus the differentiated groups.
Unlike most proinflammatory cytokines, IL‐10 is an anti‐inflammatory cytokine that plays an important role in maintaining homeostasis and preventing inflammatory responses 43, 44. We observed that the mRNA levels and production of IL‐10 in Treg cells increased following treatment with B2 in a concentration‐dependent manner (Fig. 3a,b). These results suggest that B2 has anti‐inflammatory activity by regulating the expression of proinflammatory and anti‐inflammatory cytokines during CD4+ T cell differentiation.
B2 disrupts cytokine‐induced JAK/STAT signalling in mouse CD4+ T cells
The differentiation of CD4+ T cells is regulated by specific cytokines, and these cytokine‐induced signals are associated with the JAK/STAT pathway. Therefore, we investigated whether B2 could affect JAK/STAT signalling in mouse CD4+ T cells. B2 potently suppressed the IFN‐γ‐mediated phosphorylation of STAT‐1 and STAT‐3 in mouse CD4+ T cells, which is mediated through a JAK‐1‐ and JAK‐2‐dependent pathway (Fig. 4a). Furthermore, the JAK‐1‐ and tyrosine kinase 2 (TYK‐2)‐dependent phosphorylation of STAT‐1 and STAT‐4 was suppressed by B2 in IL‐12‐stimulated mouse CD4+ T cells (Fig. 4b). B2 also suppressed IL‐4‐induced JAK‐1‐ and JAK‐3‐dependent STAT‐6 phosphorylation (Fig. 4c). The suppressive effects of B2 on several types of cytokine‐induced STAT phosphorylation in mouse CD4+ T cells indicate collectively that B2 can potently suppress JAK/STAT‐dependent CD4+ T cell differentiation.
Figure 4.
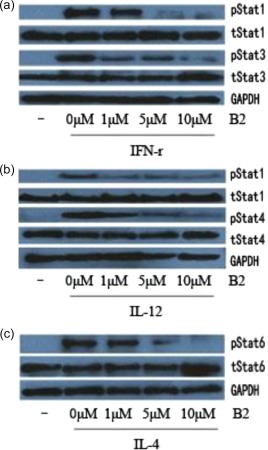
B2 inhibits cytokine‐induced Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling in mouse CD4+ T cells. Mouse CD4+ T cells were preincubated with B2 for 1 h and stimulated with interferon (IFN)‐γ (50 ng/ml) for 15 min (a), interleukin (IL)‐12 (100 ng/ml) for 30 min (b), IL‐4 (20 ng/ml (c) or IL‐2 (100 ng/ml, (d) for 15 min. Protein extracts were prepared, and Western blot analyses were performed using antibodies specific for the indicated molecules. [Colour figure can be viewed at wileyonlinelibrary.com]
B2 suppresses cutaneous inflammation in a mouse model
B2 exhibited a broad spectrum of inhibitory effects that included suppression of the JAK/STAT pathway, suggesting that B2 may be effective for the treatment of inflammatory and immune disorders. Inflammation was induced in mouse ears via intradermal injection of IL‐23 to examine the in‐vivo effects of B2 in a well‐designed animal model. IL‐23 is a proinflammatory cytokine that plays an important role in inflammatory autoimmune diseases, such as psoriasis and rheumatoid arthritis, by inducing Th17 cell‐derived cytokine‐mediated activation of the STAT‐3 signalling pathway 42, 45, 46, 47.
The intradermal injection of IL‐23 into the ears of mice clearly increased ear swelling compared with the PBS‐injected control group, which is the first hallmark of local skin inflammation (Fig. 5a). In addition, a of H&E‐stained sections revealed epidermal hyperplasia, hyperkeratosis and increased dermal inflammatory cell infiltration compared with the PBS‐injected mice. In contrast to PBS‐injected mice, the administration of B2 resulted in a significant decrease in IL‐23‐induced epidermal hyperplasia (indicated by acanthosis) and dermal inflammatory infiltrates in mice (Fig. 5b–d). Topical application of B2 on the ear skin of the mice decreased ear swelling and all pathological signs of inflammation effectively (Fig. 5a,b). These results indicate that B2 might be able to modulate cutaneous inflammation in diseases, such as psoriasis and contact dermatitis.
Figure 5.
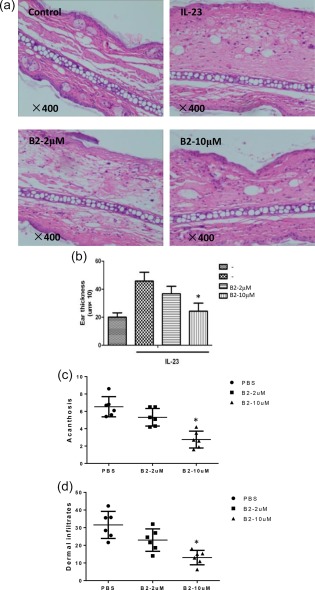
B2 ameliorates cutaneous inflammation in a mouse model. (a,b) Mouse ears were treated topically with B2 and subsequently injected intradermally with phosphate‐buffered saline (PBS) as a vehicle or mouse interleukin (IL)‐23 every other day for 14 days (n = 6 mice/group). Ear swelling was evaluated 24 h after the final injection. Histology of mouse ear sections was assessed by haematoxylin and eosin (H&E) staining. Acanthosis (c) and dermal cellular infiltrates (d) of skin injected with PBS or B2 collected from mice injected with IL‐23. *P < 0·05 and **P < 0·001 versus either IL‐23‐induced group. Scale bar = 100 mm. [Colour figure can be viewed at wileyonlinelibrary.com]
B2 suppresses the expression of proinflammatory mediators in mouse ears induced by cutaneous inflammation
During inflammatory responses, the expression levels of proinflammatory mediators are increased, and prolonged expression and production of these inflammatory mediators induce a number of inflammatory diseases. Because B2 suppressed the IL‐23‐induced progression of cutaneous inflammation in mouse ears, we performed a qRT–PCR analysis using mouse ears to assess the mRNA levels of proinflammatory mediators. Similar to the histological changes, the quantitative qRT–PCR analysis showed that the mRNA levels of T cell‐specific cytokines were elevated in the IL‐23‐injected mouse ears, whereas B2 decreased these mRNA levels and the mRNA levels of IFN‐γ, IL‐17A and IL‐22 effectively, which are Th1 and Th17 cell lineage‐specific signature cytokines, to a greater degree than it decreased the Th2 cell signature cytokine IL‐4 (Fig. 6a). This result is consistent with the results of the CD4+ T cell differentiation assay. Furthermore, treatment with B2 decreased effectively the mRNA levels of inflammatory cytokines and chemokines that are known to play a major role in psoriasis, such as chemokine (C‐X‐C motif) ligand 1 (CXCL1), activation‐regulated chemokine ligand (CCL)17, CCL20, CCL27 and CC chemokine receptor (CCR) 6, and a number of inflammatory mediators, such IL‐1α, IL‐1β, IL‐6, TNF‐α and cyclooxygenase (COX)‐2 (Fig. 6b). In contrast, the mRNA levels of an anti‐inflammatory cytokine, IL‐10, in mouse ears were increased significantly following treatment with B2 (Fig. 6b). These results suggest clearly that B2 modulates cutaneous inflammation in diseases, such as psoriasis and contact dermatitis, by regulating CD4+ T cell differentiation and the complex network of cytokine‐mediated signalling cascades.
Figure 6.
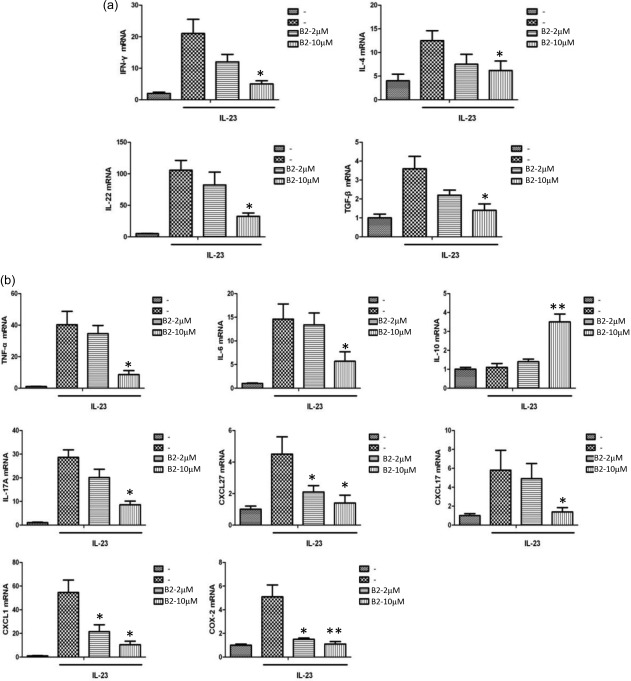
B2 inhibits the expression of pro‐inflammatory mediators in inflamed mouse ears. Total RNA was isolated from the ears of the mice described in Fig. 5, and quantitative real‐time polymerase chain reaction (qRT–PCR) was performed for proinflammatory mediators. *P < 0·05 and **P < 0·001 versus either interleukin (IL)‐23‐induced group.
Ability of B2 to reduce the RORγt+IL‐17+/FoxP3+IL‐10+ ratio in CD4+ T cells
The results described above show that B2 inhibited Th17 cell function and increased Treg cell function. Thus, we assessed the comprehensive effect of B2 on the Th17/Treg axis. Therefore, we analysed the ratio of RORγt+IL‐17+ to FoxP3+IL‐10+ allogeneic CD4+ T cells in allogeneic CD4+ T cells co‐cultured with DCs isolated from mouse peripheral blood CD14+ monocytes that were matured in the absence or presence of 10 μM B2. The proportion of allogeneic CD4+ T cells expressing RORγt and IL‐17 was reduced when the cells were co‐cultured with B2 compared with that of those cultured without B2 (Fig. 7a), indicating that B2 hampers the differentiation of Th17 cells. In addition, a higher proportion of allogeneic CD4+ T cells expressing FoxP3 and IL‐10 was found in the co‐culture system with B2 compared with that observed in the co‐culture without B2 (Fig. 7a), suggesting that B2 enhances Treg differentiation. This difference in expression resulted in a decreased ratio of allogeneic CD4+ T cells expressing RORγt and IL‐17 to those expressing FoxP3 and IL‐10 when the cells were co‐cultured with B2 compared with the ratio obtained in the co‐culture without B2 (Fig. 7b).
Figure 7.
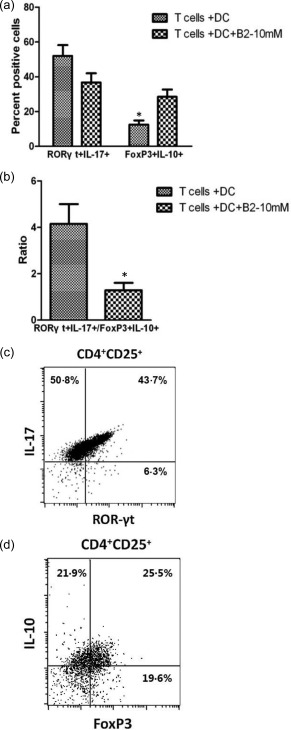
Expression of transcription factors and cytokines by allogeneic CD4+ T cells co‐cultured with dendritic cells (DCs) matured in the presence or absence of 10 μM B2. (a) Following co‐culture, the percentage of allogeneic CD4+ T cells expressing retinoic‐acid‐receptor‐related orphan receptor gamma t (RORγt)+interleukin (IL)‐17+ or forkhead box protein 3 (FoxP3)+IL‐10+ was measured by flow cytometry. (b) The ratio of RORγt+IL‐17+/FoxP3+IL‐10+ allogeneic CD4+ T cells was calculated. The results represent the means ± standard errors of the mean from six experiments. Statistically significant differences between T cells + DCs and T cells + DCs + B2 were calculated by one‐way analysis of variance (anova), and significant P‐values are indicated. (c) A representative dot‐plot of CD4+ T cells stained with antibodies against RORγt and IL‐17. (d) A representative dot‐plot of CD4+CD25+ T cells stained with antibodies against FoxP3 and IL‐10.
Discussion
Psoriasis, a chronic skin disease, is known to be the most prevalent autoimmune disease in humans. The precise aetiology of psoriasis remains poorly understood, but is thought to result from immune dysfunction 46. In Chinese medicine, various plants, including D. metel L. (Yangjinhua), have been used for the treatment of psoriasis. Among the constituents of D. metel L., withasteroids, including withasteroid B (B2), have been reported to be the active compounds for the treatment of psoriasis. However, the effects of this plant on the immune response, particularly with regard to CD4+ T cell differentiation and signal transduction, have not been elucidated. In this study, we showed that B2 regulated CD4+ T cell differentiation and the immune response, thereby ameliorating IL‐23‐induced, psoriasis‐like, dermatitis‐like inflammatory skin disease. Moreover, B2 affected the maturation of DCs in vitro and this effect, in turn, induced T cell differentiation towards an increased Treg phenotype and a decreased Th17 phenotype. These findings suggest that B2 might be involved in the therapeutic effects observed in psoriasis patients following treatment with D. metel L.
T cell receptor (TCR) activation triggered by antigen‐presenting cells causes CD4+ T cells to differentiate into distinct Th cell lineages, and this process is influenced by cytokines, master transcription factors and STAT proteins 16, 47. Our study showed that B2 affected the expression and production of Th cell lineage‐specific master transcription factors and cytokines. Of the master transcription factors, T‐bet (the Th1 master regulator) and RORγt (the Th17 master regulator) were more susceptible to B2 than the Th2 master transcription factor GATA‐3, and these inhibitory effects were correlated with the mRNA levels and production of Th cell lineage‐specific cytokines. In addition, B2 increased the mRNA levels of FoxP3 significantly (the Treg master transcription factor), as well as the cell lineage‐specific cytokines. In general, Th1 cells are known to be involved in organ‐specific autoimmunity, Th17 cells are involved in systemic autoimmune diseases and cancers and Treg cells are responsible for immunosuppressive activities by producing their specific cytokines 12, 48. The inhibitory effect of B2 on CD4+ T cell differentiation suggests that this compound may ameliorate various immune‐related diseases that are caused by an aberrantly regulated immune response, particularly diseases in which the pathology is mediated by Th1 and Th17 cells rather than Th2 cells, such as psoriasis.
Psoriasis is a T cell‐mediated skin disorder that is accepted to be mediated mainly by Th17 cells 47, 49, but has also been associated with dysfunctional Tregs 11. We studied the effects of co‐culturing allogeneic CD4+ T cells with DCs matured in the presence or absence of B2 to further elucidate its immunomodulatory effects. The results revealed a reduced IL‐17+RORγt+/IL‐10+FoxP3+ ratio for the CD4+ T cells that were co‐cultured with DC‐B2 compared with those that were co‐cultured with DC‐C alone, indicating a decrease in Th17 cells and an increase in Tregs. As two important immune cell lineages, Tregs are regarded as immunoregulators that function to prevent an excessive immune response, whereas Th17 cells are normally involved in defence against extracellular bacterial and fungal infections. A precise balance between Treg and Th17 cells would not only be a critical prerequisite for healthy homeostasis, but also would direct the outcome of the immune responses 12.
The development of psoriasis is known to be associated with STAT‐3 activation, which is necessary for Th17 cell development and is activated by cytokines 48, 50. In addition, inhibition of STAT‐3 activation by treatment with a STAT‐3 inhibitor in a mouse model and in psoriasis patients provided effective therapeutic activities in psoriatic lesions 51. In addition to inhibiting the expression of Th17 and Treg cell lineage‐specific master transcription factors and cytokines, B2 also effectively inhibited cytokine‐induced activation of the STAT proteins STAT‐1, STAT‐3, STAT‐4, STAT‐5 and STAT‐6. The JAK/STAT signalling pathway transduces cytokine‐mediated signals, and STAT proteins are important transcription factors in CD4+ T cell differentiation 16, 42. Activation of the JAK/STAT signalling cascade in abnormal conditions is associated with a wide range of inflammatory diseases and cancers. Therefore, regulation of the aberrantly activated JAK/STAT signalling cascade is a valuable therapeutic target for the treatment of human diseases, including psoriasis. Thus, the inhibitory activity of B2 on CD4+ T cell differentiation was mediated by its combined regulatory effects on multiple signalling cascades at multiple levels, including the expression of master transcription factors, the production and expression of T cell lineage‐specific cytokines, and the cytokine‐induced activation of STAT proteins. Our results indicate that STAT‐3 is one of the important factors in the development of psoriasis and that B2 might be used as a possible therapeutic small molecule for the treatment of autoimmune diseases, including psoriasis.
We further investigated whether B2 could inhibit IL‐23‐induced psoriasis‐like skin inflammation in mice to determine the effect of B2 in vivo. In this model, IL‐23 induces skin inflammation by triggering the infiltration of mononuclear cells into the epidermis and dermis, followed by Th17 cell differentiation and STAT‐3 signalling pathway activation 37, 52, 53, 54. We observed that B2 ameliorated the cutaneous inflammatory responses in this model by inhibiting the expression of various proinflammatory mediators. These results suggest that B2 may ameliorate inflammation in chronic skin inflammatory diseases by suppressing pathogenic CD4+ T cell differentiation and the overall immune response.
In summary, we observed that B2, which was isolated from D. metel L. flowers, strongly inhibited CD4+ T cell differentiation and immune responses by regulating JAK/STAT signaling and the development of psoriasis‐like skin diseases in an animal model. Moreover, B2 decreased the IL‐17+RORγt+/IL‐10+FoxP3+ ratio in co‐cultures of CD4+ T cells with DC‐B2. These results provide new evidence that B2 might ameliorate CD4+ T cell‐mediated inflammatory skin diseases, implying that it is a promising candidate treatment for immune cell‐mediated skin inflammatory diseases.
Some limitations of this study should be addressed. First, our study used animal models of IL‐23‐induced psoriasis, and although this model shares many characteristics with human psoriasis, the causes of psoriasis are still unclear. Thus, research studies using mice with imiquimod‐induced dermatitis, transgenic mice, or a severe combined immunodeficiency disease (SCID) mouse psoriasis skin xenograft model, which develops a psoriasis‐like skin disease, are necessary to confirm the immune efficacy of B2. Secondly, we hypothesized that the use of B2 alone might not completely inhibit the symptoms. Thus, the treatment of psoriasis should comprise a combination therapy with other withasteroids isolated from D. metel L. Further studies are needed to evaluate the efficacy of combination therapies.
Author contributions
Y. S. isolated the mouse CD4+ T cells, differentiated them in vitro, performed the MTT and proliferation assays, mixed lymphocyte reactions and statistical analysis. Q. W. co‐cultured the mature DCs and allogeneic CD4+ T cells and performed the histological analysis. B. Y. isolated all withasteroid compounds from D. metel L. on 1 July 2014. G. C. and H. K. designed the entire study. This research was supported by the National Natural Science Foundation of China (Grant no. 81603416), and by China Postdoctoral Science Foundation (Grant no. 2016M600267), and by Young talent training program of Heilongjiang Provincial Education Department (Grant No. is UNPYSCT‐2016207), and by the Seventh Heilongjiang Postdoctoral Special Fund (postdoctoral young talent program), and by a Project of the Department of Education of Heilongjiang Province (Grant nos 12541755 and 12541752).
Disclosure
The authors declare that there are no disclosures.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant no. 81603416), and by China Postdoctoral Science Foundation (Grant no. 2106M600267), and by the Seventh Heilongjiang Postdoctoral Special Fund (postdoctoral young talent program), and by a Project of the Department of Education of Heilongjiang Province (Grant nos 12541755 and 12541752).
Contributor Information
G. Cheng, Email: gcheng@mednet.ucla.edu
H. Kuang, Email: hxkuang56@163.com
References
- 1. Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol 2012; 9:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elloso MM, Gomez‐Angelats M, Fourie AM. Targeting the Th17 pathway in psoriasis. J Leukoc Biol 2012; 92:1187–97. [DOI] [PubMed] [Google Scholar]
- 3. Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol 2004; 135:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin DA, Towne JE, Kricorian G et al The emerging role of IL‐17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013; 133:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Eng J Med 2009; 361:496–509. [DOI] [PubMed] [Google Scholar]
- 6. Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev 2014; 13:490–5. [DOI] [PubMed] [Google Scholar]
- 7. Raychaudhuri SP. Role of IL‐17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol 2013; 44:183–93. [DOI] [PubMed] [Google Scholar]
- 8. Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010; 130:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL‐17A‐producing cells and are found in lesional skin. J Invest Dermatol 2011; 131:1853–60. [DOI] [PubMed] [Google Scholar]
- 10. Zielinski CE, Zuberbier T, Maurer M. Immunoregulation in cutaneous allergy: prevention and control. Curr Opin Allergy Clin Immunol 2012; 12:498–503. [DOI] [PubMed] [Google Scholar]
- 11. Sugiyama H, Gyulai R, Toichi E et al Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol 2005; 174:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lochner M, Wang Z, Sparwasser T. The special relationship in the development and function of T helper 17 and regulatory T cells. Prog Mol Biol Transl Sci 2015; 136:99–129. [DOI] [PubMed] [Google Scholar]
- 13. Egwuagu CE. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine 2009; 47:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy KM, Reiner SL. Decision making in the immune system: the lineage decisions of helper T cells . Nat Rev Immunol 2002; 2:933–44. [DOI] [PubMed] [Google Scholar]
- 15. Zhu J, Paul WE. Peripheral CD4+ T‐cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev 2010; 238:247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010; 28:445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shuai K, Liu B. Regulation of JAK–STAT signalling in the immune system. Nat Rev Immunol 2003; 3:900–11. [DOI] [PubMed] [Google Scholar]
- 18. Ghoreschi K, Laurence A, O'Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be?. Nat Immunol 2009; 10:356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O'Shea JJ. Therapeutic targeting of Janus kinases. Immunol Rev 2008; 223:132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sano S, Chan KS, Carbajal S et al Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med 2005; 11:43–9. [DOI] [PubMed] [Google Scholar]
- 21. Wolk K, Haugen HS, Xu W et al IL‐22 and IL‐20 are key mediators of the epidermal alterations in psoriasis while IL‐17 and IFN‐γ are not. J Mol Med 2009; 87:523–36. [DOI] [PubMed] [Google Scholar]
- 22. Jayaprakasam B, Zhang Y, Seeram NP, Nair MG. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci 2003; 74:125–32. [DOI] [PubMed] [Google Scholar]
- 23. Habtemariam S. Cytotoxicity and immunosuppressive activity of withanolides from Discopodium penninervium . Planta Med 1997; 63:15–7. [DOI] [PubMed] [Google Scholar]
- 24. Minguzzi S, Barata LE, Shin YG et al Cytotoxic withanolides from Acnistus arborescens . Phytochemistry 2002; 59:635–41. [DOI] [PubMed] [Google Scholar]
- 25. Budhiraja RD, Sudhir S, Garg KN. Antiinflammatory activity of 3 β‐Hydroxy‐2,3‐dihydro‐withanolide F. Planta Med 1984; 50:134–6. [DOI] [PubMed] [Google Scholar]
- 26. Jayaprakasam B, Nair MG. Cyclooxygenase‐2 enzyme inhibitory withanolides from Withania somnifera leaves. Tetrahedron 2003; 59:841–9. [Google Scholar]
- 27. Su B‐N, Park EJ, Nikolic D et al Activity‐guided isolation of novel norwithanolides from deprea s ubtriflora with potential cancer chemopreventive activity. J Org Chem 2003; 68:2350–61. [DOI] [PubMed] [Google Scholar]
- 28. National Committee of Chinese Pharmacopoeia . Pharmacopoeia of the People's Republic of China. Beijing: Chemical Industry Press, 2005. [Google Scholar]
- 29. Jiangsu New Medical College . The dictionary of medicinal plant. Shanghai: Shanghai Science and Technology Press, 2006. [Google Scholar]
- 30. Kuang HX, Yang BY, Xia YG, Wang QH. Two new withanolide lactones from Flos Daturae . Molecules 2011; 16:5833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuang H, Yang BTL, Xia Y, Dou D. Baimantuoluosides A–C, three new withanolide glucosides from the flower of Datura metel L. Helv Chim Acta 2009; 92:1315–23. [Google Scholar]
- 32. Yang B‐Y, Xia Y‐G, Liu Y et al New antiproliferative and immunosuppressive withanolides from the seeds of Datura metel . Phytochem Lett 2014; 8:92–6. [Google Scholar]
- 33. Yang BY, Xia YG, Wang QH, Dou DQ, Kuang HX. Baimantuoluosides D–G, four new withanolide glucosides from the flower of Datura metel L. Arch Pharm Res 2010; 33:1143–8. [DOI] [PubMed] [Google Scholar]
- 34. Yang B‐Y, Xia Y‐G, Wang Y‐Y, Wang Q‐H, Kuang H‐X. Two novel norwithasteroids with unusual six‐ and seven‐membered ether rings in side chain from Flos Daturae . Evid Based Compl Alternat Med 2013; 2013:352019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang B, Wang Q, Xia Y, Feng W, Kuang H. Withanolide compounds from the flower of Datura metel L. Helv Chim Acta 2007; 90:1522–8. [Google Scholar]
- 36. Yang B, Wang Q, Xia Y, Feng W, Kuang H. Baimantuoluolines D–F, three new withanolides from the flower of Datura Metel L. Helv Chim Acta 2008; 91:964–71. [Google Scholar]
- 37. Lee YS, Cheon IS, Kim BH, Kwon MJ, Lee HW, Kim TY. Loss of extracellular superoxide dismutase induces severe IL‐23‐mediated skin inflammation in mice. J Invest Dermatol 2013; 133:732–41. [DOI] [PubMed] [Google Scholar]
- 38. Freysdottir J, Omarsdottir S, Ingólfsdóttir K, Vikingsson A, Olafsdottir ES. In vitro and in vivo immunomodulating effects of traditionally prepared extract and purified compounds from Cetraria islandica . Int Immunopharmacol 2008; 8:423–30. [DOI] [PubMed] [Google Scholar]
- 39. Wu QL, Fu YF, Zhou WL et al Inhibition of S‐adenosyl‐L‐homocysteine hydrolase induces immunosuppression. J Pharmacol Exp Ther 2005; 313:705–11. [DOI] [PubMed] [Google Scholar]
- 40. Luo Q, Gu Y, Zheng W et al Erlotinib inhibits T‐cell‐mediated immune response via down‐regulation of the c‐Raf/ERK cascade and Akt signaling pathway. Toxicol Appl Pharmacol 2011; 251:130–6. [DOI] [PubMed] [Google Scholar]
- 41. Yang BY, Xia YG, Chen D, Kuang HX. Chemical constituents from the flower of Datura metel . Chinese J Natural Med 2010; 8:429–32. [Google Scholar]
- 42. Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008; 112:1557–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999; 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rutz S, Ouyang W. Regulation of interleukin‐10 and interleukin‐22 expression in T helper cells. Curr Opin Immunol 2011; 23:605–12. [DOI] [PubMed] [Google Scholar]
- 45. Grasis JA, Tsoukas CD. Itk: the rheostat of the T cell response. J Signal Transduct 2011; 2011:297868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun 2007; 8:1–12. [DOI] [PubMed] [Google Scholar]
- 47. van Beelen AJ, Teunissen MB, Kapsenberg ML, de Jong EC. Interleukin‐17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol 2007; 7:374–81. [DOI] [PubMed] [Google Scholar]
- 48. Tokura Y, Mori T, Hino R. Psoriasis and other Th17‐mediated skin diseases. J UOEH 2010; 32:317–28. [DOI] [PubMed] [Google Scholar]
- 49. Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev 2008; 223:87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakajima K, Kanda T, Takaishi M et al Distinct roles of IL‐23 and IL‐17 in the development of psoriasis‐like lesions in a mouse model. J Immunol 2011; 186:4481–9. [DOI] [PubMed] [Google Scholar]
- 51. Miyoshi K, Takaishi M, Nakajima K et al Stat3 as a therapeutic target for the treatment of psoriasis: a clinical feasibility study with STA‐21, a Stat3 inhibitor. J Invest Dermatol 2011; 131:108–17. [DOI] [PubMed] [Google Scholar]
- 52. Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL‐23 and Th17 cytokines. Curr Rheumatol Rep 2007; 9:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 2012. 181:8–18. [DOI] [PubMed] [Google Scholar]
- 54. Toussirot E. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets 2012; 11:159–68. [DOI] [PubMed] [Google Scholar]


