Summary
The cause of pleural effusion remains uncertain in approximately 15% of patients despite exhaustive evaluation. As recently described immunoglobulin (Ig)G4‐related disease is a fibroinflammatory disorder that can affect various organs, including the lungs, we investigate whether idiopathic pleural effusion includes IgG4‐associated etiology. Between 2000 and 2012, we collected 830 pleural fluid samples and reviewed 35 patients with pleural effusions undiagnosed after pleural biopsy at Yamaguchi‐Ube Medical Center. Importantly, IgG4 immunostaining revealed infiltration of IgG4‐positive plasma cells in the pleura of 12 patients (34%, IgG4+ group). The median effusion IgG4 level was 41 mg/dl in the IgG4+ group and 27 mg/dl in the IgG4− group (P < 0·01). The light and heavy chains of effusion IgG4 antibodies of patients in the IgG4+ group were heterogeneous by two‐dimensional electrophoresis, indicating the absence of clonality of the IgG4 antibodies. Interestingly, the κ light chains were more heterogeneous than the λ light chains. The measurement of the κ and λ free light chain (FLC) levels in the pleural fluids showed significantly different κ FLC levels (median: 28·0 versus 9·1 mg/dl, P < 0·01) and κ/λ ratios (median: 2·0 versus 1·2, P < 0·001) between the IgG4+ and IgG4− groups. Furthermore, the κ/λ ratios were correlated with the IgG4+/IgG+ plasma cell ratios in the pleura of the IgG4+ group. Taken together, these results demonstrate the involvement of IgG4 in certain idiopathic pleural effusions and provide insights into the diagnosis, pathogenesis and therapeutic opportunities of IgG4‐associated pleural effusion.
Keywords: fibrinous pleuritis, free light chain, IgG4‐related disease, pleural effusion
Introduction
Pleural effusion remains common, originating from a wide range of pathologies including congestive heart failure, pneumonia and cancer 1. A diagnostic algorithm for the differentiation of a pleural effusion proposed by Light et al. has been widely accepted 2 and recommended in the British Thoracic Society pleural disease guideline 3. Nonetheless, the cause of the pleural effusion remains unclear in a substantial percentage of patients with persistently exudative effusions after the history, physical examination and biochemical and cytological tests of pleural fluid 4, 5, 6. No diagnosis has been established for up to 15% of patients, despite invasive procedures such as thoracoscopy or open pleural biopsy 4, 6, 7. Therefore, a new approach is needed to detect the cause(s) of undiagnosed pleural effusions 8, 9, 10, 11. Because immunoglobulin G4 (IgG4)‐related disease is recognized as a fibroinflammatory condition of unknown cause that can affect multiple organs including the lungs and pleura 12, 13, IgG4 might be related to certain idiopathic pleural effusions.
Hamano et al. originally reported elevated serum IgG4 concentrations in patients with autoimmune pancreatitis 14, and IgG4‐related autoimmune disease has been proposed as a new clinicopathological entity characterized by IgG4+ plasma cell infiltration 15. High serum IgG4 levels and infiltration of IgG4+ plasma cells have also been reported in other organs, including salivary and lacrimal glands 15, 16, 17, 18. Although the criteria for diagnosis of IgG4‐related disease in the lung have not been established, elevated serum IgG4 concentrations and histopathological examinations, such as marked lymphoplasmacytic infiltration including IgG4+ cells and fibrosis, have been recommended 19, 20, 21. Taniguchi et al. have reported interstitial pneumonia associated with autoimmune pancreatitis and marked infiltration of IgG4+ plasma cells in the pulmonary alveolar septum 22. Common radiological findings of IgG4‐related lung disease include hilar and mediastinal lymphadenopathy, thickening of perilymphatic interstitium with or without subpleural and/or peribronchovascular consolidation, and the pathological examination reveals lymphoplasmacytic infiltration with fibrosis, which correlates well with the radiological manifestations 23.
It has been reported that pleural effusion may occur in association with systemic IgG4‐related disease 16, 24, 25, 26, 27, 28, 29, 30. Conversely, there have been a few case reports on isolated IgG4‐related pleural effusion 26, 31, 32, but little is known about the involvement of IgG4 in the pleural effusion. In this study, we hypothesize that idiopathic pleural effusions include IgG4‐associated aetiology and demonstrate pleural infiltration of IgG4+ plasma cells in a substantial percentage of patients with idiopathic pleural effusion.
Methods
Patients
Idiopathic pleural effusion was defined as any persistent, exudative pleural effusion that remains undiagnosed after the history and physical examination, biochemical and cytological studies of pleural fluid, radiographic examinations and histopathological analysis of biopsied specimens 4, 6. Diagnosis of idiopathic pleural effusion was made after a minimum of 1‐year follow‐up (range = 1–10 years), with detailed exploration including computed tomographic (CT) scanning to exclude other causes of effusion such as malignant pleural mesothelioma and carcinomatous pleuritis, according to previous studies that mainly performed follow‐up of 1–2 years 4, 7, 33, 34, 35, 36. In this retrospective study, we accumulated 830 pleural fluid samples at Yamaguchi‐Ube Medical Center between 2000 and 2012 and reviewed 35 patients with undiagnosed pleural effusions who underwent thoracoscopy and pleural biopsy, after excluding three patients who had a malignancy during follow‐up. Biochemical data were obtained for the sera and pleural fluids when thoracentesis was conducted. Biological and bacterial analyses of sera and pleural fluids, CT scan, cytological and histological examination did not demonstrate malignancy or infectious disease in patients with pleural effusions. The patients' pleural fluids were stored at −80°C until use. This study was approved by the institutional review board of NHO Yamaguchi‐Ube Medical Center (Approval no. 26–2). Written informed consent was obtained from each patient or their family for the use of data and samples.
IgG4 immunohistochemistry
The parietal pleura were obtained from biopsy specimens. Immunostaining for IgG or IgG4 was performed by activation of the pleura with 0·1% trypsin, incubation with rabbit polyclonal anti‐human IgG antibody (Dako, Glostrup, Denmark; cat. no. A0423) or biotinylated mouse monoclonal anti‐human IgG4 antibody (clone HP‐6025; Sigma B3648; Sigma, St Louis, MO, USA) 37, 38 and HistoFine® Simple StainTM Max PO Multi (Nichirei Biosciences, Tokyo, Japan; cat. no. 724152), and development with 3,3'‐diaminobenzidine (Nichirei Bioscience; cat. no. 715301). The average number of IgG4+ plasma cells within three high‐power fields (HPFs) was calculated, and patients with the presence of > 10 IgG4+ plasma cells/HPF and an IgG4+/IgG+ cell ratio of > 40%, as described for biopsy specimens 23, 38, 39, were assigned to the IgG4+ group.
Immunoglobulin analysis of pleural fluids
IgG1, IgG2, IgG3, IgG4, IgM, IgA and IgE in pleural fluids were quantitated with Bio‐Plex Pro Assays Human Isotyping 7‐Plex (Bio‐Rad, Hercules, CA, USA), according to the manufacturer's instructions. Bio‐Plex Suspension Array System was operated with Bio‐Plex Manager (version 6·0).
Purification of IgG4 antibodies from pleural fluids
IgG4 was purified from pleural fluids by diethylaminoethyl (DEAE)‐cellulose ion exchange chromatography and subsequently by affinity chromatography on anti‐IgG4 antibody‐coupled Sepharose‐4. Pleural fluid was dialyzed against 0·01 M phosphate buffer (pH 7·0). DEAE‐cellulose (DE52; Whatman Biosystems, Chalfont St Giles, UK) in a column (ϕ1 × 30 cm) was equilibrated with 0·01 M phosphate buffer (pH 7·0). The dialyzed pleural fluid (20 ml) was passed onto the DEAE column and the fall‐through fractions containing IgG were collected. IgG4 in the IgG of the fall‐through fractions was purified with anti‐IgG4‐coupled Sepharose‐4 that had been prepared by coupling monoclonal anti‐IgG4 antibody (clone HP‐6025; Sigma) to CNBr‐activated Sepharose‐4 (GE) according to the manufacturer's instructions. The bound IgG4 was eluted with 0·1 M glycine‐HCl (pH 2·7) and neutralized immediately with 1 M Tris.
Two‐dimensional electrophoresis (2‐DE) of effusion IgG4 antibodies
2‐DE of purified IgG4 was performed as described previously 40, 41. Briefly, isoelectric focusing (IEF) gel solution contained 8·5 M urea, acrylamide/Bis (5% T, 3% C), 10% glycerol, 1·3 mM lysine and a mixture of Pharmalyte pH 3–10 (1·25%) and pH 5–8 (1·25%) (GE), which was degassed and polymerized by adding ammonium persulphate and TEMED to concentrations of 0·05 and 0·1%, respectively. Purified IgG4 was reduced in the presence of 5% β‐mercaptoethanol at room temperature for 1 h, and urea was added to a concentration of 8·5 M immediately before loading onto the capillary IEF gel (ϕ1 mm × 5 cm). IEF was run in Mini‐PROTEAN 2‐D Electrophoresis Cell (Bio‐Rad) at 200 V for 15 min, 400 V for 15 min and 750 V for 2·5 h. After IEF, capillary gel was equilibrated with sodium dodecyl sulphate (SDS) sample buffer containing 5% β‐mercaptoethanol for 30 min. After washing the capillary gel with SDS running buffer, SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) was performed at 25 mA constant per gel. After transferring the proteins onto PVDF membranes, γ4, κ and λ chains on the blots were probed with biotinylated anti‐IgG4 (Sigma; cat. no. B3648)/horseradish peroxidase‐conjugated Extavidin (Sigma; cat. no. E2886), peroxidase‐conjugated anti‐κ and anti‐λ light chain antibodies (Bio‐Rad; cat. nos STAR127P and STAR129P), respectively.
Free light chain (FLC) analysis of pleural fluids
The levels of κ and λ FLCs in pleural fluids were measured by latex‐based immunoassay using Freelite kappa kit and Freelite lambda kit (The Binding Site, Birmingham, UK). The measurement with Freelite was performed by The BN II System (Siemens, Munich, Germany) at a qualified clinical laboratory of SRL Inc. (Tokyo, Japan). The diagnostic ranges for serum κ FLC, λ FLC and κ/λ FLC ratio are 3·3–19·4 mg/l, 5·7–26·3 mg/l and 0·26–1·65, respectively 42.
Statistical analysis
The Mann–Whitney U‐test was used to assess differences in the laboratory data, pleural fluid immunoglobulin levels and FLC levels between the IgG4+ and IgG4− groups. The immunoglobulin data are expressed as median and interquartile range (IQR) unless stated otherwise. A correlation coefficient was obtained using Pearson's equation. P < 0·05 was considered statistically significant. All statistical analyses were conducted using IBM spss statistics (version 22·0; IBM, Armonk, NY, USA).
Results
Characteristics of patients' pleural fluids and pleura
Clinical and demographic information of 35 patients with idiopathic pleural effusion was obtained from the medical records (Tables 1 and 2). Biopsies from the parietal pleura of these patients demonstrated diffuse sclerosing inflammation, but no malignant cells were identified. Fibrosis was pronounced on the side of the pleural cavity, while storiform fibrosis was not seen. Diffuse lymphoplasmacytic infiltration was observed in 16 of 35 patients (Fig. 1a,b). No obliterative phlebitis was observed. IgG4 immunostaining was performed to examine whether IgG4+ plasma cells were present in the pleura of the 35 patients (Fig. 1c–h). IgG4+ plasma cells were variably detected, and the cut‐off for IgG4 positivity was set to 10 IgG4+ plasma cell counts per HPF, as proposed for biopsy specimens 23, 39. Of 35 patients, 12 patients showing > 10 IgG4+ plasma cells/HPF were assigned to the IgG4+ group (median = 31; range = 20–70; Tables 1 and 2) and 23 patients with ≤ 10 IgG4+ plasma cells/HPF to the IgG4− group (median = 0·3; range = 0–5). All patients in the IgG4+ group showed IgG4+/IgG+ cell ratios greater than 40% (Table 2). The patients in the IgG4+ group were older men with a median age of 76 years, and biochemical analysis showed lower median effusion LDH and CRP levels for this group (Table 1).
Table 1.
Clinical characteristics of patients with pleural effusions of unknown cause
|
IgG4− group (n = 23) |
IgG4+ group (n = 12) |
P | |
|---|---|---|---|
| Age (years) | 70 (63–78) | 76 (73–80) | 0·092 |
| Sex (male/female) | 22/1 | 12/0 | 0·851 |
| Serum | |||
| Total protein (g/dl) | 7·1 (6·4–7·3) | 7·2 (6·7–7·6) | 0·420 |
| Albumin/globulin | 1·00 (0·80–1·30) | 1·05 (0·85–1·20) | 0·932 |
| LDH (IU/l) | 186 (164–235) | 181 (164–227) | 0·797 |
| CRP (mg/dl) | 1·48 (0·75–2·14) | 0·83 (0·27–1·83) | 0·161 |
| Pleural fluid | |||
| Total protein (g/dl) | 4·3 (3·9–5·0) | 4·5 (3·6–5·2) | 1·000 |
| LDH (IU/l) | 356 (197–577) | 221 (145–340) | 0·049 |
| CRP (mg/dl) | 0·89 (0·51–1·24) | 0·34 (0·17–1·31) | 0·085 |
| ADA (U/l) | 21·7 (16·4–23·5) | 21·5 (15·9–29·5) | 0·719 |
| LDH in pleural fluid/serum | 1·60 (1·29–3·00) | 1·19 (0·77–1·95) | 0·079 |
Data are presented as median (interquartile range). LDH = lactate dehydrogenase; CRP = C‐reactive protein; ADA = adenosine deaminase. The normal ranges for total protein, albumin/globulin ratio, LDH and CRP in serum are 6·0–8·3 g/dl, 1·0–2·0, 120–240 IU/l and < 0·3 mg/dl, respectively. The normal range for pleural fluid ADA is < 30 U/l [2].
Table 2.
Clinical features of patients in the immunoglobulin (Ig)G4+ group.
| CT scan findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Age/sex | Effusion IgG4, mg/dl | IgG4+ PC counts/HPF * | IgG4+/IgG+ PC ratio (%) | Respiratory symptom | Pleura | Pulmonary lesion | Mediastinal lymphadenopathy | Extrapulmonary lesion | Follow‐up period (years) |
| 1 | 75M | 1133·1 | 48 | 85 | Dyspnoea on effort | (–) | (–) | (–) | (–) | 6 |
| 2 | 81M | 20·3 | 70 | 93 | Abnormality of X‐ray | Pleural plaque | (–) | (–) | (–) | 2 |
| 3 | 68M | 239·6 | 45 | 75 | Dyspnoea on effort | Pleural plaque and thickening | Ground glass attenuation | (–) | (–) | 5 |
| 4 | 76M | 44·1 | 31 | 48 | Abnormality of X‐ray | Pleural thickening | Fibrosis | (–) | (–) | 3 |
| 5 | 73M | 60·1 | 24 | 65 | Cough | Pleural thickening | Consolidation | (–) | (–) | 9 |
| 6 | 73M | 37·6 | 20 | 75 | Abnormality of X‐ray | Pleural plaque | Round atelectasis | (–) | (–) | 10 |
| 7 | 73M | 36·7 | 22 | 70 | Abnormality of X‐ray | (–) | Pneumoconiosis nodule | (–) | (–) | 9 |
| 8 | 84M | 36·3 | 26 | 81 | Abnormality of X‐ray | Pleural plaque and thickening | (–) | (–) | (–) | 3 |
| 9 | 77M | 36·5 | 31 | 69 | Abnormality of X‐ray | (–) | Consolidation | (–) | (–) | 3 |
| 10 | 78M | 36·3 | 29 | 54 | Abnormality of X‐ray | Pleural plaque | (–) | (–) | (–) | 5 |
| 11 | 67M | 50·7 | 35 | 79 | Dyspnoea on effort | Pleural plaque | (–) | (–) | (–) | 6 |
| 12 | 81M | 50·6 | 57 | 51 | Abnormality of X‐ray | (–) | (–) | (–) | (–) | 1 |
*PC = plasma cell; HPF = high‐power field (×400); CT = computed tomography.
Figure 1.
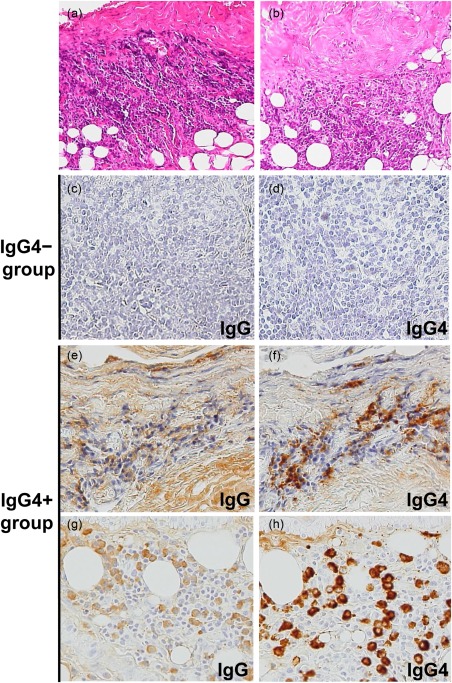
Histopathological features of the parietal pleura of patients with idiopathic pleural effusion. (a,b) Fibrous thickening of the pleura and prominent lymphoplasmacytic infiltrate in the subpleural fibrous and adipose tissue (haematoxylin and eosin staining, magnification ×100). (c–h) Immunostaining for immunoglobulin (Ig)G or IgG4, magnification ×200. (a,e,f) Case 1, effusion IgG4: 1133·1 mg/dl. (b,g,h). Case 2, effusion IgG4: 20·3 mg/dl (Table 2).
Immunoglobulin analysis of pleural fluids
Effusion IgG4 levels were significantly higher in the IgG4+ group than in the IgG4− group (median = 41 versus 27 mg/dl, P < 0·01, Fig. 2d). The proportion of IgG4 to the total IgG was also higher in the IgG4+ group than in the IgG4− group (median = 3·2 versus 1·9%, P < 0·01, Fig. 2h), which confirms higher IgG4 production in the pleura of the former group. The pleural fluid IgA levels were also elevated in the IgG4+ group (median = 403 versus 193 mg/dl, Fig. 2f), in contrast to those of IgG1, IgG2, IgG3, IgM and IgE (Fig. 2a–c,e,g).
Figure 2.
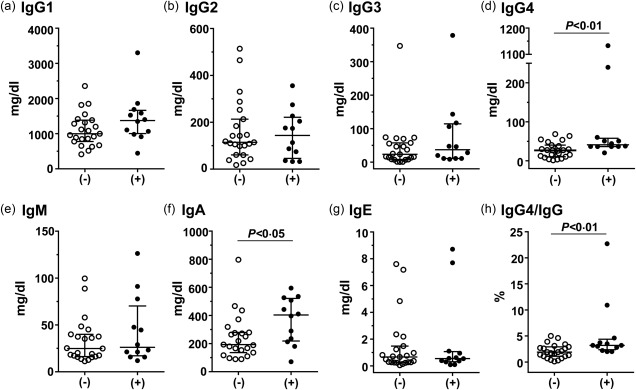
Comparison of pleural fluid levels of immunoglobulins between the immunoglobulin (Ig)G4− and IgG4+ groups. (−), IgG4− group; (+), IgG4+ group. Median and interquartile ranges are shown.
Clonality of the IgG4 antibodies of patients in the IgG4+ group
To exclude the possibility of malignant lymphoma and multiple myeloma, the clonality of the effusion IgG4 antibodies of patients in the IgG4+ group was examined by 2‐DE (Fig. 3). The control human IgG4, κ myeloma protein, was separated into four γ4 H chain spots with different isoelectric points and one κ L chain spot, but no λ L chain spot (Fig. 3a–c). In contrast, the H and L chains of the IgG4 antibodies of the patients were more heterogeneous in terms of isoelectric point and molecular weight (Fig. 3d–i). In particular, both κ and λ L chains were detected, indicating that their IgG4 antibodies are polyclonal. The numbers of the spots of the κ and λ chains were 10 and 5–6, respectively, which suggests that the κ chain predominates in the effusion IgG4 antibodies of the patients.
Figure 3.
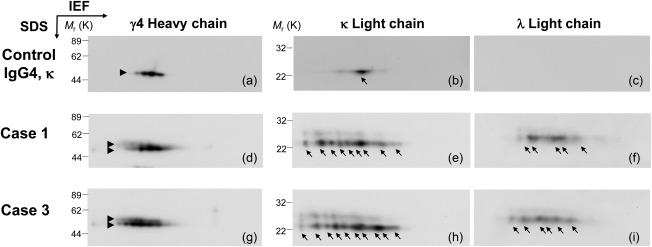
Analysis of the clonality of the effusion immunoglobulin (Ig)G4 antibodies of patients in the IgG4+ group by two‐dimensional electrophoresis (2‐DE). Control IgG4 κ myeloma protein from Sigma (cat no. I4639) (a –c). Effusion IgG4 antibodies of representative cases with abnormal IgG4 levels (d–i). The H and L chains were probed with anti‐IgG4‐Fc (left), anti‐κ chain (middle) and anti‐λ chain (right) antibodies.
Free light chains (FLC) analysis of pleural fluids
As the κ chain was associated predominantly with IgG4 H chain in the patients (Fig. 3), it was presumed that the κ‐type was also predominant in the FLCs of the pleural fluids of the IgG4+ group. FLCs are produced in excess of the H chains during immunoglobulin synthesis and secreted into the circulation. The FLC assay was developed originally to support the diagnosis of L chain multiple myeloma, and has been used to assess the excess of one L chain isotype over another by using κ/λ ratio as a surrogate for clonal expansion 43, 44, 45. Interestingly, the κ FLC levels were higher in the patients of the IgG4+ group than in the IgG4− group (median = 30·1 versus 9·1 mg/dl, P < 0·01) (Fig. 4a), whereas the median λ FLC levels were not significantly different (Fig. 4b). Importantly, the median κ/λ FLC ratio was above the normal range and significantly higher in the IgG4+ group than in the IgG4− group (2·0 versus 1·2, P < 0·001) (Fig. 4c). In a comparison of patients between the IgG4− and IgG4+ groups, the receiver operating characteristic (ROC) curve for the κ/λ FLC ratio had a sensitivity of 0·87, a specificity of 0·83 at a cut‐off value of 1·42 with an area under the curve (AUC) of 0·88 (Fig. 5). In addition, the κ/λ FLC ratios were found to be correlated with the IgG4+ plasma cell counts/HPF and the IgG4+/IgG+ cell ratios in the pleura of the IgG4+ group (Fig. 6). These results are consistent with the predominance of the κ chain in the 2‐DE patterns of the effusion IgG4 antibodies (Fig. 3).
Figure 4.
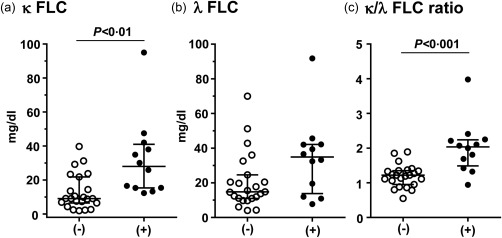
Comparison of pleural fluid levels of the κ and λ free L chains (FLC) (a,b) and κ/λ ratio (c). Median and interquartile ranges are shown. (−), Immunoglobulin (Ig)G4− group; (+), IgG4+ group.
Figure 5.
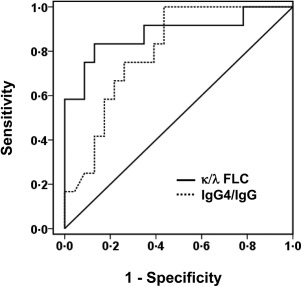
Receiver operating characteristic (ROC) analysis on diagnostic utility of immunoglobulin (Ig)G4 and κ/λ ratio for distinguishing patients between the IgG4− and IgG+ groups. Cut‐off value for κ/λ ratio, 1·42; sensitivity, 0·87; specificity, 0·83. Area under the curve (AUC), 0·88; 95% confidence interval (CI) for the AUC, 0·74 – 1·00. Cut‐off value for IgG4/IgG ratio, 2·75%; sensitivity, 0·75; specificity, 0·74; AUC, 0·80; 95% CI for the AUC, 0·66–0·94.
Figure 6.
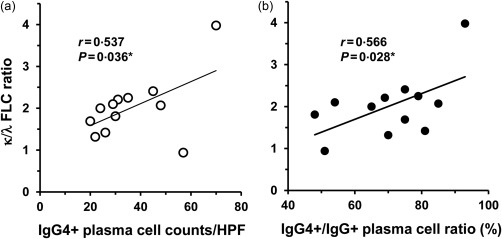
Correlation of the effusion κ/λ free L chains (FLC) ratio with immunoglobulin (Ig)G4+ plasma cell counts (a) and IgG4+/IgG+ plasma cell ratio (b) in the pleura of patients in the IgG4+ group. *One‐tailed P‐value.
Discussion
The aetiology of exudative pleural effusion sometimes remains unknown, despite thoracoscopy and histological examination of pleural biopsy specimens. In this study, we have shown that 34% of patients with idiopathic pleural effusion are associated with IgG4. To our knowledge, this study is the first to investigate the incidence of IgG4‐associated pleural effusion in patients with idiopathic pleural effusion. A pleural marker that might be related to this pleural effusion is also discussed.
Pleural effusions are manifested in some systemic IgG4‐related disease. In previous case reports on IgG4‐related disease, pleural effusion occurred as one of the symptoms of the systemic disease involving pancreas, salivary glands, etc. Nodular lesions and bronchovascular involvement are the most common pulmonary manifestations, and various combinations of pulmonary abnormalities are often found in the same patients 23, 46, 47. In contrast, the patients in this study did not show multi‐organ system involvement other than pleuritis. Considering the elevated effusion IgG4 levels, tissue IgG4+ plasma cell numbers and IgG4+/IgG+ plasma cell ratios (Table 2), however, the IgG4‐associated aetiology is evident for the patients in the IgG4+ group of this study. The patients in the IgG4+ group were elderly men and less inflammatory compared with those in the IgG4– group (Table 1), which is in agreement with characteristics of IgG4‐related disease that affects predominantly older men and progresses slowly with relatively weak inflammation signs 48. IgG4 antibodies are considered generally to be anti‐inflammatory 49, 50, but their pathogenic effects have also been reported 51, 52. At present, it is unclear whether the increased production of IgG4 antibody is a causative factor of the pleural effusion or a bystander phenomenon associated with chronic inflammatory reactions.
In differential diagnosis, sarcoidosis and multi‐centric Castleman's disease were ruled out by clinical and radiological findings, including lack of mediastinal/hilar or extrapulmonary lymphadenopathy in the chest X‐ray and CT scan examinations (Table 2). Although lymphoma/myeloma needs to be suspected when the IgG4 levels are markedly high, in this study the IgG4 antibodies were polyclonal and no patient developed malignancy during at least 1‐year follow‐up (median 5 years, Table 2). Interestingly, the effusion IgG4 levels did not correlate with the extent of pleural infiltration of IgG4+ plasma cells (cases 1 and 2, Fig. 1, Table 2). Although the effusion IgG4 level of case 2 in the IgG4+ group was as low as 20·3 mg/dl, IgG4 immunostaining exhibited dense infiltration of IgG4+ plasma cells in the pleura (Fig. 1h). The discrepancy between serum IgG4 concentrations and immunohistochemical findings has also been noted in IgG4‐related disease 53. Therefore, a biomarker other than IgG4 is needed to support the diagnosis of this pleural effusion. The measurements of FLC in the pleural fluid may be considered.
Elevated FLC levels (Fig. 4a,b) are likely to reflect the activation of polyclonal B cells that infiltrate in the pleura. Higher κ FLC levels and κ/λ ratios in the IgG4+ group than in the IgG4− group may be useful to discriminate the IgG4‐associated pleural effusion (Fig. 4). Over‐production of serum FLC and high serum κ/λ ratios have been shown recently to correlate with the disease activity of systemic lupus erythematosus 54, rheumatoid arthritis, primary Sjőgren's syndrome 55 and IgG4‐related disease 56. However, the reason for the high κ/λ ratios in these diseases is not known. One possibility to explain the high κ/λ ratios in the IgG4+ group is dominant selection of Vκ genes by possible antigen(s) that elicit pleuritis. It has been reported that the κ/λ ratios of granulocyte–macrophage colony‐stimulating factor (GM‐CSF) autoantibodies in the sera of patients with autoimmune pulmonary alveolar proteinosis are correlated with disease severity 57. The study suggests the occurrence of selective expansion of λ‐type anti‐GM‐CSF antibody‐positive B cell clones in the peripheral lymphatic tissues. However, neither autoantigens nor disease‐specific IgG4 autoantibodies have been identified in IgG4‐related disease 12. A second possibility is a preferential association of the κ L chains with the γ4 and α H chains because the levels of IgG4 and IgA were elevated in the pleural fluids (Fig. 2). The κ L chains have been shown to be associated preferentially with IgG4 and IgA H chains from the analyses of subclass distribution in 659 IgG myeloma sera 58 and 176 IgG and 62 IgA myeloma proteins 59. These studies show the mean κ/λ ratios for the IgG4 myeloma proteins as 3·0 (n = 24 58) and 2·7 (n = 11 59) and that for IgA as 2·1 (n = 42 59). The correlations of the effusion κ/λ FLC ratios with the IgG4+ cell counts and IgG4+/IgG+ cell ratios in the pleura are in agreement with this notion (Fig. 6). It has been reported that FLC can confer mast cell‐dependent hypersensitivity in mice and that increased κ FLC monomer and dimer levels and high κ/λ ratios are often found in the cerebrospinal fluid of patients with multiple sclerosis 60, 61. The pleural effusions in the IgG4+ group may be attributable to the accumulation of κ FLCs.
IgG4‐related pleural lesions are reported to be steroid‐responsive 26, 32, 62. Considering B cell activation as a possible mechanism for the pleural effusion in our study, the same therapeutic strategy with immunosuppressive agents may be applicable to our cases. One patient in the IgG4+ group who had suffered recurrent pleural effusions (Case 1, Table 2) received corticosteroids, which ameliorated the pleural effusion. Conversely, pleural effusions resolved spontaneously in a subset of patients, and so watchful waiting may be appropriate in some patients with mild pleural effusion or asymptomatic pleuritis, as described for IgG4‐related disease in other organs 63. However, criteria to justify treatment of IgG4‐related pleural lesions need to be established by future prospective studies.
This study has several limitations. This is a retrospective study with a small number of patients. One patient was followed‐up for 1 year, although more than 1‐year follow‐up is recommended for detection of occult pleural malignancy 7, 33, 36. It was not possible in all patients to assess the development of an extra‐pleural IgG4‐related lesion during follow‐up. As serum samples were not available, the serum levels of IgG4, κ and λ FLCs could not be analysed. Immunoglobulins including IgG4 were quantitated by a capture sandwich immunoassay, which is different from nephelometry used in the literature, and so the IgG4 concentrations in this study cannot be compared directly with those in the previous reports on IgG4‐related disease.
Disclosure
The authors declare no conflicts of interest.
Acknowledgements
We thank Masami Murakami and Masazumi Tanaka for technical assistance. This study was supported in part by Japan Society for the Promotion of Science under JSPS KAKENHI Grant no. 23590578 (Y. M.).
References
- 1. Porcel JM, Azzopardi M, Koegelenberg CF, Maldonado F, Rahman NM, Lee YC. The diagnosis of pleural effusions. Expert Rev Respir Med 2015; 9:801–15. [DOI] [PubMed] [Google Scholar]
- 2. Light RW. Pleural diseases, 6th edn Philadelphia, PA: Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 3. Hooper C, Lee YC, Maskell N, BTS Pleural Guideline Group . Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65: ii4–17. [DOI] [PubMed] [Google Scholar]
- 4. Ferrer JS, Munoz XG, Orriols RM, Light RW, Morell FB. Evolution of idiopathic pleural effusion: a prospective, long‐term follow‐up study. Chest 1996; 109:1508–13. [DOI] [PubMed] [Google Scholar]
- 5. Light RW. The undiagnosed pleural effusion. Clin Chest Med 2006; 27:309–19. [DOI] [PubMed] [Google Scholar]
- 6. Aleman C, Sanchez L, Alegre J et al Differentiating between malignant and idiopathic pleural effusions: the value of diagnostic procedures. Q J Med 2007; 100:351–9. [DOI] [PubMed] [Google Scholar]
- 7. Davies HE, Nicholson JE, Rahman NM, Wilkinson EM, Davies RJ, Lee YC. Outcome of patients with nonspecific pleuritis/fibrosis on thoracoscopic pleural biopsies. Eur J Cardiothorac Surg 2010; 38:472–7. [DOI] [PubMed] [Google Scholar]
- 8. Aoe K, Hiraki A, Murakami T et al Diagnostic significance of interferon‐gamma in tuberculous pleural effusions. Chest 2003; 123:740–4. [DOI] [PubMed] [Google Scholar]
- 9. Aoe K, Hiraki A, Murakami T et al Relative abundance and patterns of correlation among six cytokines in pleural fluid measured by cytometric bead array. Int J Mol Med 2003; 12:193–8. [PubMed] [Google Scholar]
- 10. Aoe K, Hiraki A, Maeda T et al Soluble receptor‐binding cancer antigen expressed on SiSo cells in pleural fluid: a potential diagnostic marker for malignant pleural effusion. Chest 2004; 126:1195–7. [DOI] [PubMed] [Google Scholar]
- 11. Porcel JM. Pleural fluid biomarkers: beyond the Light criteria. Clin Chest Med 2013; 34:27–37. [DOI] [PubMed] [Google Scholar]
- 12. Stone JH, Zen Y, Deshpande V. IgG4‐related disease. N Engl J Med 2012; 366:539–51. [DOI] [PubMed] [Google Scholar]
- 13. Della‐Torre E, Lanzillotta M, Doglioni C. Immunology of IgG4‐related disease. Clin Exp Immunol 2015; 181:191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamano H, Kawa S, Horiuchi A et al High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001; 344:732–8. [DOI] [PubMed] [Google Scholar]
- 15. Kamisawa T, Funata N, Hayashi Y et al A new clinicopathological entity of IgG4‐related autoimmune disease. J Gastroenterol 2003; 38:982–4. [DOI] [PubMed] [Google Scholar]
- 16. Zen Y, Inoue D, Kitao A et al IgG4‐related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol 2009; 33:1886–93. [DOI] [PubMed] [Google Scholar]
- 17. Stone JH, Khosroshahi A, Hilgenberg A, Spooner A, Isselbacher EM, Stone JR. IgG4‐related systemic disease and lymphoplasmacytic aortitis. Arthritis Rheum 2009; 60:3139–45. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto M, Ohara M, Suzuki C et al Elevated IgG4 concentrations in serum of patients with Mikulicz's disease. Scand J Rheumatol 2004; 33:432–3. [DOI] [PubMed] [Google Scholar]
- 19. Masaki Y, Dong L, Kurose N et al Proposal for a new clinical entity, IgG4‐positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4‐related disorders. Ann Rheum Dis 2009; 68:1310–5. [DOI] [PubMed] [Google Scholar]
- 20. Umehara H, Okazaki K, Masaki Y et al A novel clinical entity, IgG4‐related disease (IgG4RD): general concept and details. Mod Rheumatol 2012; 22:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsui S, Yamamoto H, Minamoto S, Waseda Y, Mishima M, Kubo K. Proposed diagnostic criteria for IgG4‐related respiratory disease. Respir Investig 2016; 54:130–2. [DOI] [PubMed] [Google Scholar]
- 22. Taniguchi T, Ko M, Seko S et al Interstitial pneumonia associated with autoimmune pancreatitis. Gut 2004; 53:770; author reply 770–1. [PMC free article] [PubMed] [Google Scholar]
- 23. Matsui S, Hebisawa A, Sakai F et al Immunoglobulin G4‐related lung disease: clinicoradiological and pathological features. Respirology 2013; 18:480–7. [DOI] [PubMed] [Google Scholar]
- 24. Miyake K, Moriyama M, Aizawa K et al Peripheral CD4+ T cells showing a Th2 phenotype in a patient with Mikulicz's disease associated with lymphadenopathy and pleural effusion. Mod Rheumatol 2008; 18:86–90. [DOI] [PubMed] [Google Scholar]
- 25. Rossi G, Marchioni A, Guicciardi N, Cadioli A, Cavazza A. Recurrent pleural and pericardium effusions in a white woman with IgG4‐related syndrome. Am J Surg Pathol 2009; 33:802–3. [DOI] [PubMed] [Google Scholar]
- 26. Yamamoto H, Suzuki T, Yasuo M et al IgG4‐related pleural disease diagnosed by a re‐evaluation of chronic bilateral pleuritis in a patient who experienced occasional acute left bacterial pleuritis. Intern Med 2011; 50:893–7. [DOI] [PubMed] [Google Scholar]
- 27. Sekiguchi H, Horie R, Utz JP, Ryu JH. IgG4‐related systemic disease presenting with lung entrapment and constrictive pericarditis. Chest 2012; 142:781–3. [DOI] [PubMed] [Google Scholar]
- 28. Ishida M, Hodohara K, Furuya A et al Concomitant occurrence of IgG4‐related pleuritis and periaortitis: a case report with review of the literature. Int J Clin Exp Pathol 2014; 7:808–14. [PMC free article] [PubMed] [Google Scholar]
- 29. Choi JH, Sim JK, Oh JY et al A case of IgG4‐related disease presenting as massive pleural effusion and thrombophlebitis. Tuberc Respir Dis (Seoul) 2014; 76:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waheed W, Nickerson J, Ambaye AB, Babi MA, Tandan R. IgG4‐related neuromyopathy associated with recurrent pleural effusion. J Clin Neuromuscul Dis 2015; 16:210–9. [DOI] [PubMed] [Google Scholar]
- 31. Kojima M, Nakazato Y, Kaneko Y, Sugihara S, Masawa N, Nakamura N. Cytological findings of IgG4‐related pleural effusion: a case report. Cytopathology 2013; 24:338–40. [DOI] [PubMed] [Google Scholar]
- 32. Corcoran JP, Culver EL, Psallidas I et al A 63‐year‐old man with a recurrent right‐sided pleural effusion. Thorax 2015; 70:504–7. [DOI] [PubMed] [Google Scholar]
- 33. El Solh AA, Abdo T, Pineda L, Ramadan F, Berbary E. A longitudinal study of idiopathic exudative lymphocytic pleural effusion in older people. J Am Geriatr Soc 2005; 53:1957–60. [DOI] [PubMed] [Google Scholar]
- 34. Bintcliffe OJ, Hooper CE, Rider IJ et al Unilateral pleural effusions with more than one apparent etiology. A prospective observational study. Ann Am Thorac Soc 2016; 13:1050–6. [DOI] [PubMed] [Google Scholar]
- 35. DePew ZS, Verma A, Wigle D, Mullon JJ, Nichols FC, Maldonado F. Nonspecific pleuritis: optimal duration of follow‐up. Ann Thorac Surg 2014; 97:1867–71. [DOI] [PubMed] [Google Scholar]
- 36. Janssen JP, Ramlal S, Mravunac M. The long‐term follow up of exudative pleural effusion after nondiagnostic thoracoscopy. J Bronchol 2004; 11:169–74. [Google Scholar]
- 37. Pifferi M, Di Cicco M, Bush A, Caramella D, Chilosi M, Boner AL. Uncommon pulmonary presentation of IgG4‐related disease in a 15‐year‐old boy. Chest 2013; 144:669–71. [DOI] [PubMed] [Google Scholar]
- 38. Deshpande V, Zen Y, Chan JK et al Consensus statement on the pathology of IgG4‐related disease. Mod Pathol 2012; 25:1181–92. [DOI] [PubMed] [Google Scholar]
- 39. Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J Gastroenterol 2007; 42:39–41. [DOI] [PubMed] [Google Scholar]
- 40. Mimura Y, Kabat EA, Tanaka T, Fujimoto M, Takeo K, Nakamura K. Microheterogeneity of mouse antidextran monoclonal antibodies. Electrophoresis 1995; 16:116–23. [DOI] [PubMed] [Google Scholar]
- 41. Mimura Y, Nakamura K, Tanaka T, Fujimoto M. Evidence of intra‐ and extracellular modifications of monoclonal IgG polypeptide chains generating charge heterogeneity. Electrophoresis 1998; 19:767–75. [DOI] [PubMed] [Google Scholar]
- 42. Katzmann JA, Clark RJ, Abraham RS et al Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002; 48:1437–44. [PubMed] [Google Scholar]
- 43. Bradwell AR, Carr‐Smith HD, Mead GP et al Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 2001; 47:673–80. [PubMed] [Google Scholar]
- 44. Drayson M, Tang LX, Drew R, Mead GP, Carr‐Smith H, Bradwell AR. Serum free light‐chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001; 97:2900–2. [DOI] [PubMed] [Google Scholar]
- 45. Bradwell AR, Carr‐Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003; 361:489–91. [DOI] [PubMed] [Google Scholar]
- 46. Fujinaga Y, Kadoya M, Kawa S et al Characteristic findings in images of extra‐pancreatic lesions associated with autoimmune pancreatitis. Eur J Radiol 2010; 76:228–38. [DOI] [PubMed] [Google Scholar]
- 47. Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol 2006; 41:1197–205. [DOI] [PubMed] [Google Scholar]
- 48. Umehara H, Nakajima A, Nakamura T et al IgG4‐related disease and its pathogenesis‐cross‐talk between innate and acquired immunity. Int Immunol 2014; 26:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy 2009; 39:469–77. [DOI] [PubMed] [Google Scholar]
- 50. van der Neut Kolfschoten M, Schuurman J, Losen M et al Anti‐inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007; 317:1554–7. [DOI] [PubMed] [Google Scholar]
- 51. Cornell LD, Chicano SL, Deshpande V et al Pseudotumors due to IgG4 immune‐complex tubulointerstitial nephritis associated with autoimmune pancreatocentric disease. Am J Surg Pathol 2007; 31:1586–97. [DOI] [PubMed] [Google Scholar]
- 52. Deshpande V, Chicano S, Finkelberg D et al Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol 2006; 30:1537–45. [DOI] [PubMed] [Google Scholar]
- 53. Sah RP, Chari ST. Serologic issues in IgG4‐related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol 2011; 23:108–13. [DOI] [PubMed] [Google Scholar]
- 54. Aggarwal R, Sequeira W, Kokebie R et al Serum free light chains as biomarkers for systemic lupus erythematosus disease activity. Arthritis Care Res (Hoboken) 2011; 63:891–8. [DOI] [PubMed] [Google Scholar]
- 55. Gottenberg JE, Aucouturier F, Goetz J et al Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjogren's syndrome. Ann Rheum Dis 2007; 66:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grados A, Ebbo M, Boucraut J et al Serum immunoglobulin free light chain assessment in IgG4‐related disease. Int J Rheumatol 2013; 2013:426759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nei T, Urano S, Itoh Y et al Light chain (kappa/lambda) ratio of GM‐CSF autoantibodies is associated with disease severity in autoimmune pulmonary alveolar proteinosis. Clin Immunol 2013; 149:357–64. [DOI] [PubMed] [Google Scholar]
- 58. Skvaril F, Morell A, Barandun S. The IgG subclass distribution in 659 myeloma sera. Vox Sang 1972; 23:546–51. [DOI] [PubMed] [Google Scholar]
- 59. Aucouturier P, Preud'Homme JL. Subclass distribution of human myeloma proteins as determined with monoclonal antibodies. Immunol Lett 1987; 16:55–7. [DOI] [PubMed] [Google Scholar]
- 60. Redegeld FA, van der Heijden MW, Kool M et al Immunoglobulin‐free light chains elicit immediate hypersensitivity‐like responses. Nat Med 2002; 8:694–701. [DOI] [PubMed] [Google Scholar]
- 61. Kaplan B, Livneh A, Sela BA. Immunoglobulin free light chain dimers in human diseases. ScientificWorldJournal 2011; 11:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gajewska ME, Rychwicka‐Kielek BA, Sorensen K, Kubik M, Hilberg O, Bendstrup E. Immunoglobulin G4‐related pleuritis ‐ a case report. Respir Med Case Rep 2016; 19:18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Khosroshahi A, Wallace ZS, Crowe JL, et al International consensus guidance statement on the management and treatment of IgG4‐related disease. Arthritis Rheumatol 2015; 67:1688–99. [DOI] [PubMed] [Google Scholar]


