Abstract
Background
Previous studies disclosed the pivotal role of methyltransferase complex G9a/Glp in the pathogenesis of neuropathic hypersensitivity induced by peripheral nerve injury. We observed that higher dose of G9a inhibitor improved nociceptive behavior, but the lower dose worsened pain. The aim of this study is to extensively observe the differential effect of various dosages of G9a/Glp inhibitors on nerve injury-induced allodynia.
Materials and methods
After approval by the institutional ethical committee on pain research in conscious animals, C57BL/6 mice were used for measuring nociceptive behavior evoked with von Frey filaments after spared nerve injury. G9a/Glp inhibitor BIX01294 or UNC0638 was injected through the pre-buried intrathecal catheter. The dose–response curves of behavioral changes were depicted when inhibitors were administered once in bolus at the 14th day post spared nerve injury. Withdrawal behaviors were compared during the 49 days’ observation window after spared nerve injury with various dosages of inhibitors injected intrathecally for 14 days.
Results
Dose–behavior curves of a single bolus of both BIX01294 and UNC0638 displayed a “V”-shaped responses of allodynia withdrawal from lower through higher dose when measured at the 14th day post spared nerve injury. A threshold dose of 10.0 µg for BIX01294 and 80.0 µg for UNC0638 significantly worsened allodynia. However, daily bolus intrathecal injection for 14 days of both inhibitors lower or higher than these threshold doses prominently improved nociceptive behavior, producing contrasting results. On the same animal, threshold dose followed by a lower or higher dose with a 14 days’ interval also showed contrast effect on nociceptive behavior, and a lower or higher dose to threshold dose sequence of inhibitor administration was vice versa.
Conclusions
Methyltransferase complex G9a/Glp has a threshold role in mediating peripheral nerve injury-induced hypersensitivity at its low level versus high level through inhibiting and facilitating the nociceptive behavior, respectively.
Keywords: G9a/Glp complex, pain-related behavior, dose–response curve, methyltransferase, inhibitor
Introduction
Chronic neuropathic pain is a worldwide public health problem and a kind of devastating suffering for patients. However, current therapy for neuropathic pain is still unsatisfactory. Emerging evidence indicates that epigenetic regulation plays an important role in the development or maintenance of persistent pain and possibly the transition of acute pain to chronic pain, thus shedding light in a new therapeutic direction for neuropathic pain.1,2
As two related mammalian lysine methyltransferases, G9a and G9a-like protein (Glp) often exist as a G9a/Glp heteromeric complex and independently induce H3K9me2 or DNA methylation to induce target gene transcription inhibition.3 Diverse role of G9a/Glp-related epigenetic modulation has been explored in various pain conditions. Following spared nerve ligation (SNL), G9a plays a dominant role in transcriptional repression of potassium channel genes in the dorsal root ganglion (DRG) including Kcna4, Kcnd2, Kcnq2, Kcnma1, and selective G9a inhibition or knockout completely restored these potassium channel expression and attenuated pain hypersensitivity.4 Similarly, SNL increased G9a and H3K9me2 expression in the DRG neurons, and blocking these increases rescued Kcna2 expression in these neurons and ameliorated neuropathic pain.5 However, in mouse hind paw, complete Freunds adjuvant injection-induced inflammatory pain, upregulated methyl CpG binding protein 2 was observed to inhibit G9a expression in the central nucleus of the amygdala, and Cre-induced G9a knockdown may further deteriorate pain.6 These studies further suggest G9a/Glp-related epigenetic regulation may play a complex role underlying pain modulation.
Our previous study showed that G9a expression was upregulated in ventral tegmental area (VTA) following spared nerve injury (SNI), which further decreased tyrosine hydroxylase expression via methylating its gene Th, and the resulted disinhibition of dopaminergic descending inhibitory transmission finally contributing to pain development. Intraventricular injection of a dose of 300 µg G9a inhibitor BIX01294 significantly improved pain symptoms.7 Incidentally, we observed that a relatively small dose of BIX01294 might further worsen allodynia. This differential effect of BIX01294 and the studies of others enabled us to extensively explore different levels of G9a/Glp on changes in nociceptive behavior following peripheral nerve injury.
In the present study, various dosages of G9a/Glp inhibitors BIX01294 and UNC0638 were intrathecally injected following SNI, and corresponding nociceptive behaviors were observed. Our study showed a threshold dose of 10.0 µg for BIX01294 or 80.0 µg for UNC0638 decreased pain threshold, while lower or higher than these threshold doses produced contrasting effect on pain behavior. Thus, for the first time, we uncovered methyltransferase complex G9a/Glp that might have a dual role in mediating peripheral nerve injury-induced hypersensitivity at its low level versus high level through inhibiting and facilitating the nociceptive behavior, respectively.
Materials and methods
Subjects and peri-surgical care
After approval by the institutional ethical committee, a total of 410 male C57BL/6 mice (from Nanjing Medical University, Nanjing, China) weighing 20–25 g, 7–9 weeks of age, were used for behavioral tests in accordance to the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. The peri-surgical treatment was reported previously.7 Briefly, animals were housed in a cage on a 12:12 h light/dark cycle with 23 ± 1℃. After randomization, each mouse was placed in a test box with three mirrored sides for a 10-min habituation period, and then the test sessions took place. No food or water was available to the mice during the experiment. After the experiment, a lethal dose of pentobarbital was injected to euthanize each mouse.
Study protocol
First, the mechanical withdrawal behavior was observed for a period of 98 days post SNI evoked with von Frey filaments. Next, various dosages of G9a inhibitor BIX01294 (0.625, 1.25, 2.5, 5.0, 10.0, 20.0, 40.0, and 80.0 µg) or Glp inhibitor UNC0638 (5.0, 10.0, 20.0, 40.0, 80.0, 160.0, 320.0, and 640.0 µg) were, respectively, intrathecally injected through the pre-buried catheter once in bolus at the 14th day post SNI, and nociceptive behavior was detected at the same day. Besides, the effect of a three days’ intrathecal injection of BIX/UNC or their solvent dimethyl sulfoxide (DMSO) on nociceptive behavior in sham mice was observed. Further, various dosages of both BIX01294 and UNC0638 were intrathecally injected for a 14 days’ duration, and withdrawal behaviors were compared for 49 days post SNI. Finally, nociceptive behavior after BIX01294/UNC0638 treatment on the same animal was observed with a crossover intervention that was a threshold dose followed by a lower or higher dose with a 14 days’ interval, as well as an inverse sequence.
G9a and Glp share as high as 80% sequence identity in their respective SET domains, which are also BIX01294 and UNC0638 binding sites.8 BIX01294 and UNC0638 inhibit both G9a and Glp potently and selectively over a wide range of epigenetic as well as nonepigenetic targets, while UNC0638 is considered less toxic to cells compared to BIX01294.8,9 In our study, BIX01294 and UNC0638 were injected intrathecally using a microsyringe in a volume of 5 µL over 30 s, and then 10 µL saline was used to flush the catheter dead space. For the DMSO-treated sham mice, a total of 15 µL of DMSO only was given.
Intrathecal catheterization
Mice underwent intrathecal catheterization (ALZET Osmotic Pumps, Cupertino, CA) one week before nerve injury as described previously.10 In brief, cephalic–cervical area was shaved and sterilized, a midline incision was performed, and then the paravertebral muscles were dissected from the spinous processes. With the help of a surgical microscope, a hole (1 × 1 mm) was drilled through cisternal membrane until the dura was exposed. The catheter was inserted for 2.5 mm, fixed with tissue glue, and further secured on the fascia of paravertebral muscle. Finally, sodium penicillin 10,000 IU was given intramuscularly against infection. The mice would be excluded (∼10%) if neurological deficits were exhibited after catheterization, and in our study, a total of 300 mice underwent intrathecal catheterization, and 25 of them (8%) were excluded from final statistical analysis because of neurological deficits. The intrathecal catheter was not removed until the day of sacrifice.
Spared nerve injury
The animal model of chronic neuropathic pain was established by SNI as described elsewhere.11 In brief, animals were anesthetized with isoflurane, and the tibial and common peroneal branches of the sciatic nerve were ligated and sectioned distally, but the sural nerve was left intact. For sham-operated ones, the sciatic nerve was merely exposed but not ligated and dissected.
Pain threshold measurement
Pain threshold was assessed using the von Frey filaments (Stoelting Co., Wood Dale, IL) as described previously.12 In brief, the filaments were applied to the central surface of the hind paw plantar for a maximal 10 s to detect the stimulus threshold via triggering a withdrawal reaction. The stimulation was initiated from the weakest filament (0.407 g). The stimulus increment was based on the response of the mouse to the current filament: if the paw withdrew, the same filament was again used 60 s later; but if not, the next stronger filament was presented. If with the same filament, the mouse paw withdrew in two consecutive tests, no further testes were need. Withdrawal responses were used to determine the absolute threshold, that is, the 50% withdrawal threshold, by adjusting to a Gaussian integral psychometric function using a maximum likelihood method.
Statistical analysis
Data are presented as the mean ± SEM and analyzed using GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA). When there were two testing groups, Student’s t test was used to analyze the intergroup difference. One-way analysis of variance followed by the Bonferroni post hoc tests for multiple comparisons were used for all other data when necessary. All reported p values are two sided, and a p value of less than 0.05 was considered to be statistically significant.
Results
Characteristic behavioral changes post SNI
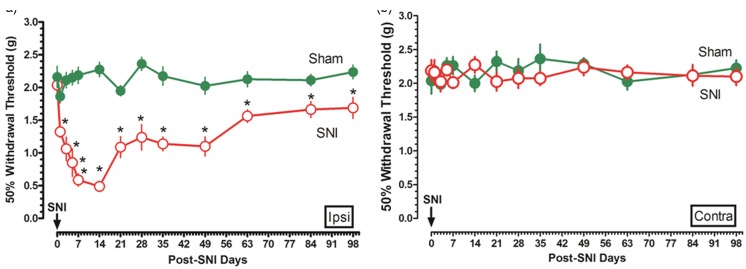
Ipsilateral and contralateral mechanical withdrawal threshold was measured for a period of 98 days post SNI. The ipsilateral mechanical withdrawal threshold trajectory showed that the nerve injury induced an immediate, remarkably, and consistent reduction in pain threshold, peaked at the 14th day after nerve injury and progressively recovered thereafter, which was still significantly lower than the sham-operated ones at the end of 98 days’ observational period (Figure 1(a)). However, no withdrawal threshold differences were found in the contralateral injured nerve (Figure 1(b)).
Figure 1.
Characteristic behavioral changes post SNI. During the 98 days’ postinjury observational period, ipsilateral and contralateral withdrawal threshold in sham and SNI mice was measured ((a) and (b), n = 15 in each group, respectively). Data are shown as mean ± SEM; *p < 0.05 versus sham. SNI: spared nerve injury.
Dose–behavior curves of various dosages of G9a/Glp inhibitor
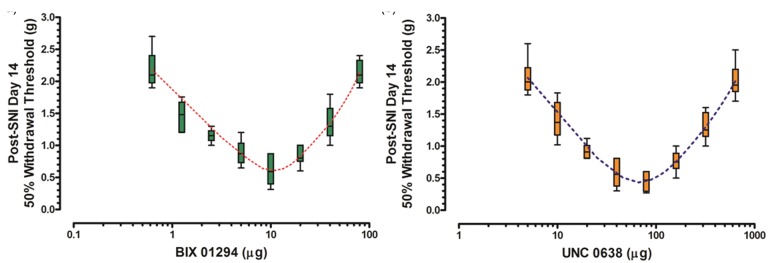
One of the following dosages of G9a/Glp inhibitor BIX01294: 0.625, 1.25, 2.5, 5.0, 10.0, 20.0, 40.0, and 80.0 µg was intrathecally injected, respectively, through the pre-buried catheter once in bolus at the 14th day post SNI, and the nociceptive behaviors were detected at the same day. With the dose of BIX01294 increased from 0.0625 to 10 µg, the mechanical withdrawal threshold progressively decreased; however, with the dose further increased from 10.0 to 80.0 µg, pain threshold gradually increased, showing a prominent “V” shape on the dose–behavior curve (Figure 2(a)).
Figure 2.
Dose–behavior curves of various dosages of G9a/Glp inhibitor. Dose–behavior curves of various dosages of G9a/Glp inhibitors BIX01294 and inhibitor UNC0638 were depicted ((a) and (b), n = 5 for each dose group). Data are shown as mean ± SEM; the dotted lines denote the theoretical dose–behavior curves. SNI: spared nerve injury.
Likewise, various dosages of G9a/Glp inhibitor UNC0638: 5.0, 10.0, 20.0, 40.0, 80.0, 160.0, 320.0, and 640.0 µg were intrathecally injected, and mechanical withdrawal threshold was recorded as well. Similar “V”-shaped dose–behavior curve was observed for UNC0638 with the lowest mechanical threshold at a dose of 80.0 µg (Figure 2(b)).
Behavior changes of BIX01294/UNC0638 in sham-operated mice
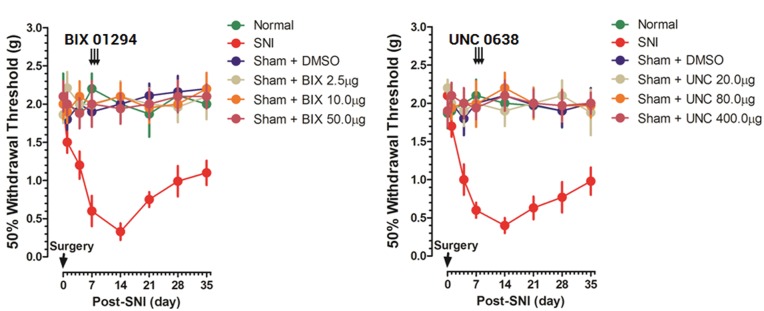
To explore the effect of G9a/Glp inhibitors on basal mechanical sensitivity, different doses of BIX01294 (2.5, 10.0, or 50.0 µg), UNC0638 (20.0, 80.0, or 400.0 µg), or equal volume of solvent DMSO were intrathecally injected daily in bolus for three days in sham mice from the seventh day postsurgery, and nociceptive behaviors were compared with those in normal and SNI mice for a 35 days’ period. The behavioral trajectory showed neither BIX (Figure 3(a)) nor UNC (Figure 3(b)) had effect on mechanical withdrawal threshold in sham mice, reflecting their specific for SNI pathologic process.
Figure 3.
Behavior changes of BIX01294/UNC0638 in sham-operated mice. Different doses of BIX01294 (2.5, 10.0, and 50.0 µg), UNC0638 (20.0, 80.0, and 400.0 µg), or equal volume of DMSO was intrathecally injected daily in bolus for three days in sham mice. Pain-related behavior trajectory in normal, SNI, and BIX/UNC/DMSO-treated mice was presented ((a) and (b), n = 5–7 for each dose group). Data are shown as mean ± SEM. DMSO: dimethyl sulfoxide; SNI: spared nerve injury.
Behavior changes of different dosages of inhibitor-treated animals
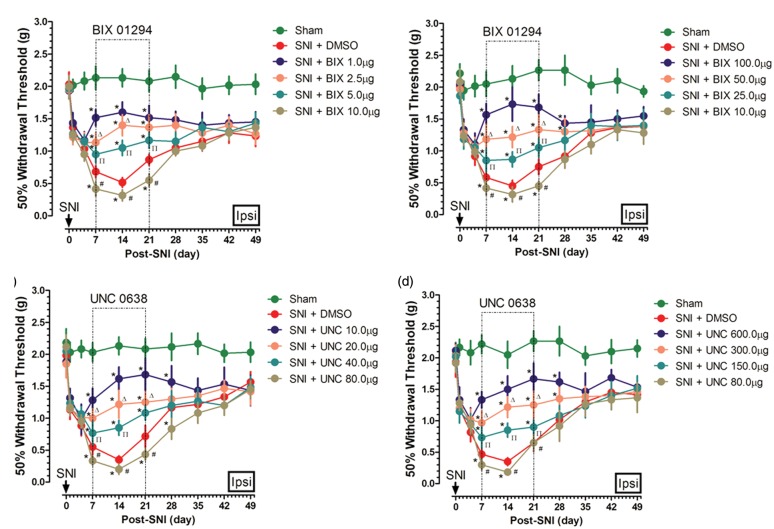
According to the dose–behavior curves of BIX01294/UNC0638, low and high doses of BIX01294/UNC0638 might have different effects on nociceptive behaviors. To further understand such differential pain regulation, each of the following dosages of BIX01294: 1.0, 2.5, 5.0, 10.0, 25.0, 50.0, and 100.0 µg, as well as UNC0638: 10.0, 20.0, 40.0, 80.0, 150.0, 300.0, and 600.0 µg, respectively, was intrathecally injected daily in bolus from the seventh post SNI for 14 days, and ipsilateral nociceptive behaviors were observed for a 49 days’ period.
For BIX01294, a dose of 10.0 µg significantly worsen nerve injury-induced allodynia during the 14 days’ treatment period; however, both lower (1.0, 2.5, and 5.0 µg) and higher doses (25.0, 50.0, and 100.0 µg) improved nociceptive behaviors, showing a contrary effect. Further, the pain improvement effect of BIX01294 was dose-dependent and greater with a far lower (1.0 > 2.5 > 5.0 µg) or far higher dose (100.0 > 50.0 > 25.0 µg; Figure 4(a) and (b)). Likewise, UNC0638 has a similar role in affecting nerve injury-induced allodynia with a threshold dose at 80.0 µg to worsen pain. Dose-dependent pain improvement effect of UNC0638 was also observed with a lower (10.0 > 20.0 > 40.0 µg) or higher (600.0 > 300.0 > 150.0 µg) dose, showing a contrasting effect (Figure 4(c) and (d)). These results were in agreement with the findings of Figure 2.
Figure 4.
Behavior changes of different dosages of inhibitor-treated animals. Various dosages of BIX01294 or equal volume of DMSO were intrathecally injected daily in bolus for 14 days in SNI mice, and ipsilateral nociceptive behaviors were observed for a 49 days’ period ((a) and (b), n = 5–7 in each group). Similarly, the pain behaviors change of various doses of UNC0638 or equal volume of DMSO was also observed ((c) and (d), n = 5–7 in each group). Data are shown as mean ± SEM; *p < 0.05 versus sham, #p < 0.05 versus SNI + DMSO, Δp < 0.05 versus SNI + BIX 1.0 µg for (a), SNI + BIX 100.0 µg for (b), SNI + UNC 10.0 µg for (c), and SNI + UNC 600.0 µg for (d), Πp < 0.05 versus SNI + BIX 2.5 µg/UNC for (a), SNI + BIX 50.0 µg for (b), SNI + UNC 20.0 µg for (c), and SNI + UNC 300.0 µg for (d). DMSO: dimethyl sulfoxide; SNI: spared nerve injury.
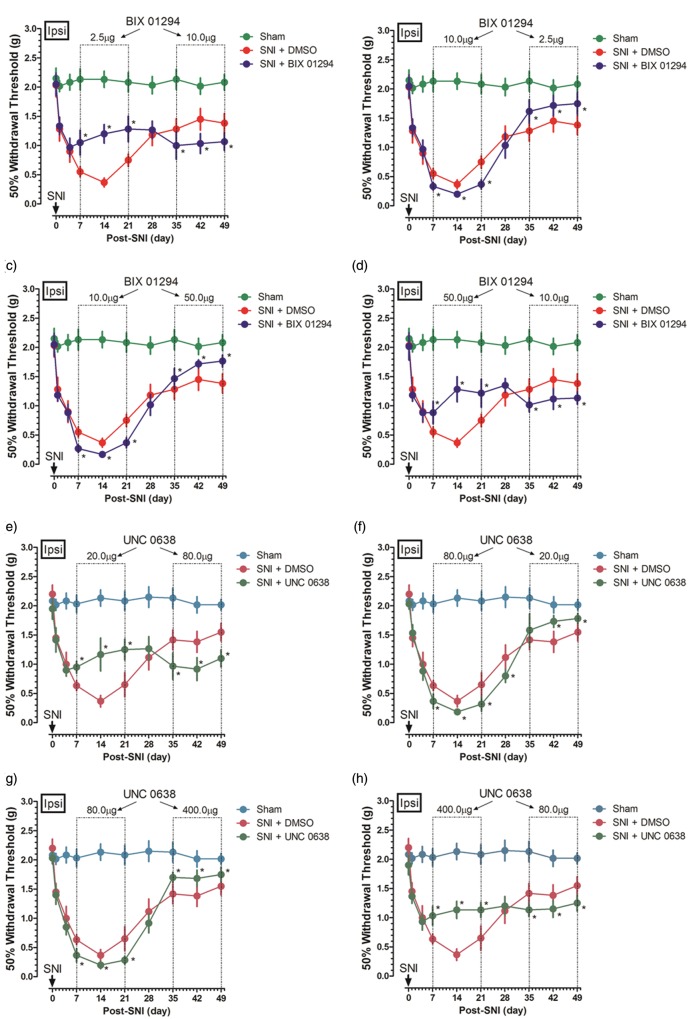
Effect of different dosages of inhibitors on nociceptive behavior in the same mice
To further clarify G9a/Glp inhibitors’ potential role in pain change, a crossover intervention was applied to the same mice. A 14-day intrathecal injection of BIX01294 2.5 µg or 50.0 µg increased withdrawal threshold; however, after a 14 days’ washout, BIX01294 10.0 µg always worsened nociceptive behaviors (Figure 5(a) and (d)), the effect which also applied after an inverse treatment sequence (Figure 5(b) and (c)). Similar role was observed with UNC0638; a 14-day intrathecal injection of UNC0638 20.0 µg or 400.0 µg increased while 80.0 µg decreased withdrawal threshold with a 14 days’ interval in the same mice, independent of treatment sequence (Figure 5(e) to (h)).
Figure 5.
Effect of different dosages of inhibitors on nociceptive behavior in the same mice. A crossover intervention was designed to clarify the G9a/Glp inhibitors’ potential role in pain change in the same mice. The nociceptive behavior was depicted with a lower or a higher dose of BIX01294 2.5 µg and 50.0 µg, respectively, followed with a threshold dose 10 µg intrathecal injection, as well as an inversely treatment sequence ((a)–(d), n = 5 in each group). Similarly, the nociceptive behavior was depicted with a lower or a higher dose of UNC0638 20.0 µg and 400.0 µg, respectively, followed with a threshold dose of 80.0 µg intrathecal injection, as well as an inversely sequence ((e)–(h), n = 5–7 in each group). Data are shown as mean ± SEM; *p < 0.05 versus SNI. DMSO: dimethyl sulfoxide; SNI: spared nerve injury.
Discussion
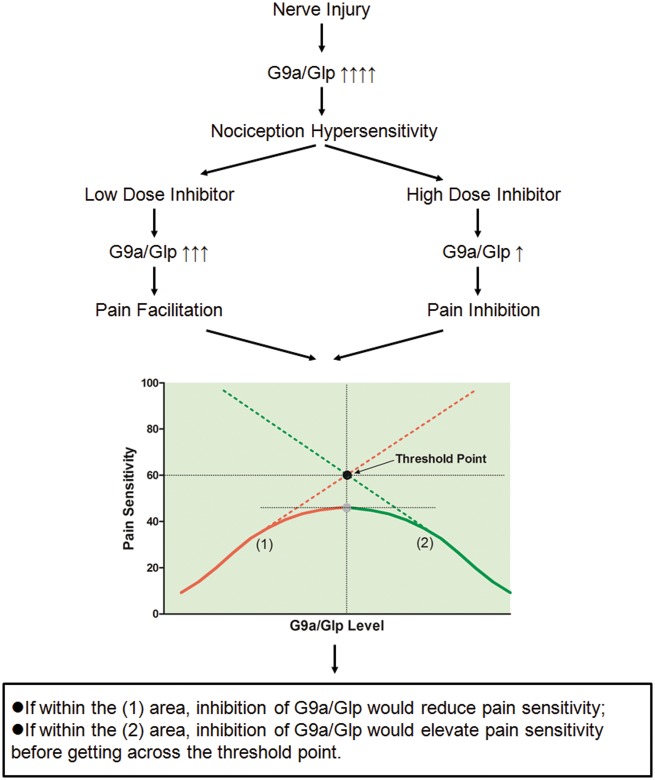
The salient finding of our study was that different dosages of G9a/Glp inhibitors BIX01294/UNC0638 might have differential effect on nociceptive behaviors after peripheral nerve injury. For the first time, we uncovered that methyltransferase complex G9a/Glp might have a threshold role in mediating peripheral nerve injury-induced hypersensitivity at its low level versus high level via inhibiting and facilitating the nociceptive behavior, respectively. The theoretical model of such “bell-shaped” relationship between G9a/Glp and peripheral nerve injury-induced hypersensitivity was shown in Figure 6.
Figure 6.
Theoretical model of the dual role of G9a/Glp in peripheral nerve injury-induced hypersensitivity. According to the abovementioned behavioral results of BIX01294/UNC0638, it disclosed that methyltransferase complex G9a/Glp might have a threshold role in mediating peripheral nerve injury-induced hypersensitivity at its low level versus high level via inhibiting and facilitating the pain-related behavior, respectively. The suggested theoretical model of the threshold role of G9a/Glp is shown in Figure 5. G9a/Glp expression consistently increased after nerve injury demonstrated by ours and the other studies, significantly contributing to pain hypersensitivity.4,7,17 A low dose of BIX01294/UNC0638 might not fully inhibit G9a/Glp activity, whose level was still within the (2) area, under which range inhibition of G9a/Glp to a level just as higher as the threshold point would elevate pain sensitivity. However, a relative high-dose BIX01294/UNC0638 might inhibit G9a/Glp activity to a lower level within the (1) area; under which range, inhibition of G9a/Glp would reduce pain sensitivity. Overall, the level of G9a/Glp has a “bell-shaped” relation with peripheral nerve injury-induced hypersensitivity. Glp: G9a-like protein.
The response of the peripheral nervous system to injury may go together with alteration in epigenetic modulation. On the contrary, we suggested G9a/Glp-related epigenetic regulation acted dually underlying the development of neuropathic pain and associated symptoms.
Different types of pain condition may have different levels of G9a/Glp; therefore, it needs to be generalized for the declaration. In Dr. Laumet’s study,4 G9a expression consistently increased after SNL, and a dose of intrathecal 10 µg BIX01294 largely normalized genome-wide gene expression in the injured DRG and gradually improved pain hypersensitivity to the baseline. This pain inhibitory effect of 10 µg BIX01294 was inconsistent with ours possibly because of the following two reasons. On one hand, the animal models were different. Both SNL and SNI are widely used neuropathic pain animal models, but SNL is more likely to induce more severe pain with quicker onset and longer duration than that of the SNI. Such difference in injury severity may underlie the potential G9a/Glp expression differential. On the other hand, G9a activity was time dependently upregulated during the 21 days’ observation period post SNL in Laumet’s study; however, BIX01294 was only given during the first eight days, at which time duration it was hard to determine to what extent the inhibition of G9a expression. Such 10 µg BIX01294 might inhibit G9a level to the (2) area at this intervene point. In another report of Liang et al.,5 SNL increased G9a and H3K9me2 expression in the DRG neurons, and blocking these increases rescued Kcna2 expression in these neurons and ameliorated neuropathic pain, other than distinct nerve injury, the methodology of G9a knockout which theoretically significantly decreased G9a level might explain the observed pronociceptive other than dual effect of G9a.
Even in our study, it was difficult to determine the real level of G9a/Glp due to its nuclear location as shown in Figure 5. To achieve such obvious dual regulatory effect, the level of G9a/Glp should keep away from the threshold point, either far higher or far lower. In fact, BIX01294/UNC0638 inhibits only enzymatic function of G9a/Glp, so the abovementioned G9a/Glp level more likely reflects its enzymatic activity rather than the protein or mRNA level.13
Other than different types of pain condition, the final transcription inhibitory targets of G9a/Glp also contributed to its dual role in pain sensitivity. If within the (1) area in Figure 5, G9a/Glp may be a trigger of inhibitory neural molecules, for example, dopamine. Nerve injury-induced pain hypersensitivity was considered as the results of the enhanced ascending facilitation and the diminished descending inhibition.14 Dopaminergic descending inhibitory pathway has been shown to modulate nociceptive transmission and enhance antinociception.15 Our previous study had suggested that G9a/Glp complex downregulated tyrosine hydroxylase expression in the VTA, and the resulted disinhibition of dopaminergic descending inhibitory pathway partially contributed to peripheral nerve injury-induced neuropathic pain.7 However, if within the (2) area in Figure 5, G9a/Glp may be a trigger of excitatory neural molecules, for example, glutamate. During neuropathic pain, elevated glutamatergic neurotransmission in the CNS is observed, which is accompanied by lower effectiveness of opioid antinociceptive drugs.16 The reduction in the inhibitory effect of the opioid on synaptic glutamate release from primary afferent nerves could be normalized by G9a inhibition, which further suggested a potential role of G9a stimulating effect for glutamate release following nerve injury.17
Level of G9a/Glp at different nerve locations may have various effects on pain sensitivity under different pain conditions. Supraspinal regions such as the amygdala, the anterior cingulate cortex, the prefrontal cortex, the insular cortex, the nucleus accumbens, and the VTA, as well as the spinal regions display robust interconnections and form a network capable of exerting top-down regulation of nociceptive input, coordinately regulating chronic pain development.18 The final influence for pain may be facilitatory or inhibitory depending on the pattern of activity within specific nerve location.
We need to acknowledge that there are still several limitations to this study. First of all, the dual effect of G9a/Glp was indirectly demonstrated by their inhibitor BIX01294/UNC0638 and not tested by other methodologies such as knockout or knockdown of G9a/Glp. Further, the underlying mechanisms of this dual effect of G9a/Glp on pain hypersensitivity following nerve injury are not explored in our study. G9a/Glp-related H3K9me2 expression or DNA methylation status following BIX01294/UNC0638 was not detected in our study. Last, BIX01294/UNC0638 was only administrated intrathecally, and neither transcriptional inhibitory targets of G9a/Glp nor region specific G9a/Glp expression was examined. So, more detailed data are needed to explore the underlying epigenetic mechanisms.
Conclusions
In conclusion, our results for the first time uncover methyltransferase G9a/Glp might have a threshold role in mediating peripheral nerve injury-induced hypersensitivity at its low level versus high level via inhibiting and facilitating the nociceptive behavior, respectively. This “bell-shaped” relationship between G9a/Glp and pain sensitivity (Figure 6) expands our understanding of the epigenetic plastic regulation underlying neuropathic pain and suggests much more precise therapies for neuropathic pain treatment.
Acknowledgment
This work was partially presented to the 2014 Annual Meeting of The American Society of Anesthesiologists (New Orleans, LA) as a poster (A1081).
Author Contributions
Xian Wang, Xiaofeng Shen, and Shaolei Ma contributed equally to this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Natural Scientific Foundation of China (NSFC, 81271242, 81371248, and 81600960) and the Jiangsu Provincial Medical Youth Talent (QNRC2016103).
References
- 1.Descalzi G, Ikegami D, Ushijima T, et al. Epigenetic mechanisms of chronic pain. Trends Neurosci 2015; 38: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai G, Ren K, Dubner R. Epigenetic regulation of persistent pain. Transl Res 2015; 165: 177–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tachibana M, Matsumura Y, Fukuda M, et al. G9a/GLP complexes independently mediate H3K9 and DNA methylation to silence transcription. EMBO J 2008; 27: 2681–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laumet G, Garriga J, Chen SR, et al. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015; 18: 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang L, Gu X, Zhao JY, et al. G9a participates in nerve injury-induced Kcna2 downregulation in primary sensory neurons. Sci Rep 2016; 6: 37704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Tao W, Hou YY, et al. MeCP2 repression of G9a in regulation of pain and morphine reward. J Neurosci 2014; 34: 9076–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Shen X, Bao S, et al. Dopaminergic inhibition by G9a/Glp complex on tyrosine hydroxylase in nerve injury-induced hypersensitivity. Mol Pain 2016; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Y, Zhang X, Horton JR, et al. Structural basis for G9a-like protein lysine methyltransferase inhibition by BIX-01294. Nat Struct Mol Biol 2009; 16: 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vedadi M, Barsyte-Lovejoy D, Liu F, et al. A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells. Nat Chem Biol 2011; 7: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu S, Wu H, Wang X, et al. Tumor suppressor menin mediates peripheral nerve injury-induced neuropathic pain through potentiating synaptic plasticity. Neuroscience 2012; 223: 473–485. [DOI] [PubMed] [Google Scholar]
- 11.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Feng SW, Fuzhou W, et al. Modeled behavior of neuropathic pain with social defect in rats: a preliminary methodology evaluation. Med Sci Monit Basic Res 2014; 20: 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epsztejn-Litman S, Feldman N, Abu-Remaileh M, et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nat Struct Mol Biol 2008; 15: 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracey I, Dickenson A. SnapShot: pain perception. Cell 2012; 148: 1308–1308.e2. [DOI] [PubMed] [Google Scholar]
- 15.Potvin S, Grignon S, Marchand S. Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse 2009; 63: 390–402. [DOI] [PubMed] [Google Scholar]
- 16.Osikowicz M, Mika J, Przewlocka B. The glutamatergic system as a target for neuropathic pain relief. Exp Physiol 2013; 98: 372–384. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Chen SR, Laumet G, et al. Nerve injury diminishes opioid analgesia through lysine methyltransferase-mediated transcriptional repression of mu-opioid receptors in primary sensory neurons. J Biol Chem 2016; 291: 8475–8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest 2010; 120: 3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]