Abstract
Background
Multiple sclerosis is a polysymptomatic disease. Little is known about relative contributions of the different multiple sclerosis symptoms to self-perception of health.
Objectives
To investigate the relationship between symptom severity in 11 domains affected by multiple sclerosis and self-rated health.
Methods
Multiple sclerosis patients in two multiple sclerosis centers assessed self-rated health with a validated instrument and symptom burden with symptoMScreen, a validated battery of Likert scales for 11 domains commonly affected by multiple sclerosis. Pearson correlations and multivariate linear regressions were used to investigate the relationship between symptoMScreen scores and self-rated health.
Results
Among 1865 multiple sclerosis outpatients (68% women, 78% with relapsing–remitting multiple sclerosis, mean age 46.38 ± 12.47 years, disease duration 13.43 ± 10.04 years), average self-rated health score was 2.30 (‘moderate to good’). Symptom burden (composite symptoMScreen score) highly correlated with self-rated health (r = 0.68, P < 0.0001) as did each of the symptoMScreen domain subscores. In regression analysis, pain (t = 7.00), ambulation (t = 6.91), and fatigue (t = 5.85) contributed the highest amount of variance in self-rated health (P < 0.001).
Conclusions
Pain contributed the most to multiple sclerosis outpatients’ perception of health, followed by gait dysfunction and fatigue. These findings suggest that ‘invisible disability’ may be more important to patients’ sense of wellbeing than physical disability, and challenge the notion that physical disability should be the primary outcome measure in multiple sclerosis.
Keywords: Multiple sclerosis, self-rated health, disability, self-report, symptom
Background
The goal of treatment in multiple sclerosis (MS) is typically described as prevention of long-term physical disability.1 From a more patient-centric perspective, however, the goal may be better defined as ‘preserving the sense of health’, broadly understood as ‘a state of complete physical, mental and social wellbeing’.2 Maintaining the sense of health and wellbeing is not only important as the goal into itself, but also because negative perception of health correlates with risk of mortality.3 A large community health survey revealed that patients with MS report worse self-reported health than the general public.4 As we seek to improve self-rated health (SRH) in MS, it is important to understand better which MS symptoms contribute the most to adverse perception of health in MS.
The question of which symptoms have the strongest impact on SRH in MS has been investigated in several studies which yielding discrepant findings.5–9 Some studies have focused on the impact of physical disability and fatigue on SRH to the exclusion of other symptoms.10 Others have only accounted for impact of cognitive and psychological aspects, but did not account for such commonly affected domains, such as vision or sensory function.9,11 Many studies used disability instruments that were not validated for MS populations,5,6,8 or enrolled very disabled,9 or relatively few patients (n = 50–217), which limited the generalizability of results.10,12,13 These limitations may explain why the investigations yielded inconsistent results.
To circumvent these limitations, we utilized an instrument validated for MS that solicits information on symptom severity in 11 domains commonly affected by MS, and administered this instrument to a large and unselected sample of MS outpatients.
Methods
Population
We included all consecutive patients with neurologist-diagnosed MS (2010 McDonald’s criteria),14 who completed symptoMScreen and SRH questionnaires during their routine care visit at NYU Multiple Sclerosis Comprehensive Care Center in New York, NY, USA, and RWJ Barnabas Multiple Sclerosis Comprehensive Care Center in Livingston, NJ, USA, between February 2015 and February 2016. In order to meet the institutional review board’s exempt-review status, we excluded patients younger than 18 years old, and those who could not follow written instructions in English.
Instruments
Symptom severity was assessed with SymptoMScreen (SyMS) (Supplementary Appendix A), an in-house-developed and validated battery of seven-point Likert scales for the following 11 distinct domains commonly affected by MS: mobility, dexterity, vision, fatigue, cognition, bladder function, sensory, spasticity, pain, depression, and anxiety. Higher scores indicate more severe symptom endorsement, while lower scores indicate no limitations due to symptoms. SyMS composite and individual domain scores have previously been shown to have strong psychometric properties – highly consistent reliability, strong criterion validity, and strong convergent and divergent validity.15
General health was assessed with a validated five-point question adopted by the World Health Organization (WHO) for the study of aging and general health: ‘In general, how would you rate your health today (please circle one)? Very good (1) – Good (2) – Moderate (3) – Bad (4) – Very bad (5)’ (http://www.who.int/healthinfo/systems/WHO-INDEPTH_SAGE_A.pdf?ua=1).
The patient determined disease steps (PDDS) is a self-reported, eight-point scale that measures global neurological impairment in MS (published online at http://www.narcoms.org/sites/default/files/PDDS_Letter_without_PS_2016.pdf). PDDS correlates strongly with Expanded Disability Status Scale (EDSS), the ‘gold standard’ of disability assessment in MS.16,17
SyMS, the WHO general health questionnaire, and PDDS are all instruments that are collected at our clinics as part of routine clinical care. We chose these relatively concise questionnaires in order to limit the time burden on our patients, and to avoid incomplete forms these questionnaires provide the most complete and unbiased representation regarding our clinical sample. Moreover, the WHO general health questionnaire has been widely used worldwide and is not subject to cultural or language biases.
Procedures
We included all patients who satisfied our inclusion criteria and completed SyMS, PDDS, and SRH questionnaire during a routine clinic visit. If a patient completed several surveys during a one-year study period, the initial survey was used unless it was not the most complete survey; otherwise the most complete survey was used. Treating neurologists confirmed MS diagnosis, and assigned disease subtype. The study was approved by the institutional review boards of NYU School of Medicine and Saint Barnabas Medical Center.
Statistical analyses
All data analyses were conducted using R software.18 Initial analysis revealed that there was a complex pattern of missing data among the predictors and the SRH responses, averaging around 10% missing. Therefore, missing data were imputed using the multivariate imputation by chained equations (MICE) method in R prior to running correlations and regressions.19 This method has been shown to accurately control for the effect of missing data on statistical inference.
After controlling for missing variables, responses were analyzed for normality and skewness. Univariate associations between SRH response and SyMS scores and demographic variables were analyzed using Pearson correlations. Subsequently, linear multivariable regression was run predicting SRH from the SyMS domains controlling for demographic variables, age, gender, and MS center; P < 0.05 was considered significant.
Results
Demographics
A total of 1865 MS patients completed the SyMS, PDDS and SRH questionnaire during the one-year study period. The sample was 68% women, with a mean age of 46.38 ± 12.47 years (range 18–85), a mean disease duration of 13.43 ± 10.04 years (range 1–58); 78% of patients had the relapsing form of MS. Disability of the patients ranged from ‘none’ (PDDS = 0) to bedbound (PDDS = 8), with a mean PDDS score of 2.12 ± 2.16 (in between ‘moderate disability’ and ‘early gait disability’), and median PDDS of 1 (‘mild disability’).
The mean SRH response was 2.30 ± 0.88 (in between ‘good’ and ‘moderate’). The distribution of SRH scores was as follows: 1% of respondents indicated their health was ‘very bad’, 7% ‘bad’, 32% ‘moderate’, 41% ‘good’ and 19% ‘very good’. The responses between the suburban (Livingston, NJ, USA) and urban (NYU, Manhattan, NY, USA) MS centers were not significantly different (P > 0.05).
Pearson correlations
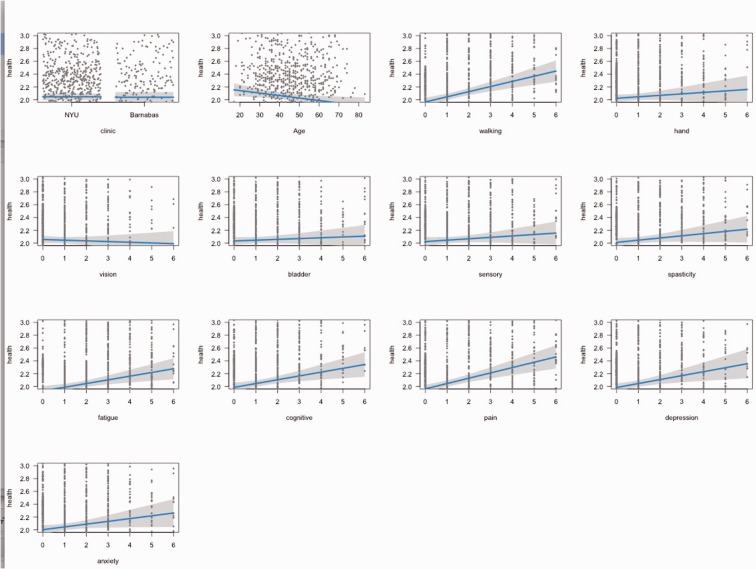
The SyMS composite score was highly correlated with general health and explained almost 50% of the variability in SRH (r = 0.68, P < 0.0001), indicating that greater overall symptom severity was associated with adverse health. Each of the SyMS domains were significantly correlated with SRH (Table 1). The magnitude of correlations varied from r = 0.57 for pain (highest) to r = 0.42 for vision (lowest). SRH was moderately correlated with PDDS (r = 0.39, P < 0.0001). SRH did not correlate with age (r = 0.09, P > 0.05) or disease duration (r = 0.03, P > 0.05). A chi-square analysis revealed no significant differences across SRH with regard to gender or MS subtype. Effect plots demonstrate the association between symptom domains on SyMS with general health, holding all of the other predictors in the model constant (Figure 1).
Table 1.
Pearson correlations to assess relationship between self-rated health and SymptoMScreen domains.
| Sympto MScreen domain | Correlation with SRH | P value |
|---|---|---|
| Walking | 0.52 | <0.0001 |
| Hand function | 0.50 | <0.0001 |
| Vision | 0.43 | <0.0001 |
| Fatigue | 0.57 | <0.0001 |
| Cognition | 0.52 | <0.0001 |
| Bladder | 0.45 | <0.0001 |
| Sensory | 0.52 | <0.0001 |
| Spasticity | 0.55 | <0.0001 |
| Pain | 0.57 | <0.0001 |
| Depression | 0.51 | <0.0001 |
| Anxiety | 0.51 | <0.0001 |
SRH: self-rated health.
Figure 1.
Visual effects plots between each predictor and general health holding the other predictors constant.
Multivariate regression analyses
Multiple linear regression analysis was used to develop a model for predicting patients’ SRH from SyMS domains, MS center location and age (Table 2). Although age was not significantly correlated with the total SRH score, it was retained in the model because it has been shown in the literature to affect persons’ rating of general health.20 Gender and disease type were not included in the model because they were not predictive of SRH in the univariate analyses. PDDS was not included in the multivariate model due to its multicollinearity with the mobility domain in SyMS, therefore PDDS makes no unique contribution to SRH beyond what is included in the SyMS. Age had a small and inverse effect on SRH (i.e. higher age was associated with slightly better SRH). The following seven SyMS domains significantly predicted partial effects in the full model (P < 0.05): gait, spasticity, fatigue, cognition, pain, depression and anxiety. Four domains – vision, sensory, bladder, and hand function – did not make a significant contribution to SRH.
Table 2.
Multiple linear regression predicting self-reported health from SymptoMScreen domains.
| Estimate | Standard error | t value | P value | |
|---|---|---|---|---|
| Intercept | 1.703 | 0.063 | 27.158 | <0.001*** |
| MS center | −0.003 | 0.032 | −0.105 | 0.916 |
| Age | −0.004 | 0.001 | −3.326 | <0.001*** |
| Walking | 0.087 | 0.013 | 6.691 | <0.001*** |
| Hand | 0.007 | 0.015 | 0.458 | 0.647 |
| Vision | −0.013 | 0.014 | −0.959 | 0.338 |
| Bladder | 0.003 | 0.012 | 0.213 | 0.831 |
| Sensory | 0.015 | 0.015 | 1.020 | 0.308 |
| Spasticity | 0.040 | 0.016 | 2.534 | 0.011* |
| Fatigue | 0.081 | 0.014 | 5.854 | <0.001*** |
| Cognitive | 0.039 | 0.015 | 2.608 | 0.009** |
| Pain | 0.094 | 0.013 | 6.997 | <0.001*** |
| Depression | 0.056 | 0.017 | 3.352 | <0.001*** |
| Anxiety | 0.040 | 0.017 | 2.366 | 0.018* |
Significant at P < 0.05.
Significant at P < 0.01.
Significant at P < 0.001.
R2 = 0.64.
The three strongest predictors of SRH in our model were pain (P < 0.001), followed by walking (P < 0.001) and fatigue (P < 0.001). Every one unit increase in self-reported pain (on a Likert scale of 0–6), was associated with an increase of 0.09 in a self-reported health Likert scale.
Conclusions
In the large and unselected MS clinic population, patients reported their general health as in between ‘good’ and ‘moderate’, or between scores of ‘2' and ‘3' on a one-to-five point scale from ‘very good' to ‘very bad'. Our clinic sample was largely comprised of relapsing MS patients with relatively lower levels of disability, with a median PDDS of 1. This is consistent with recent studies from the United States observing relatively very low average disability scores in their MS clinic populations – mean EDSS recorded in the large UCSF (San Francisco, USA)21 and Partners (Boston, USA) MS centers was 1.5.22 While clinicians and medical assistants made every effort to document responses on all patients that were seen in the clinic, it is probable that the most disabled patients (e.g. nursing home residents) are underrepresented in our sample.
Self-rated general health was highly correlated with overall symptom burden (composite SyMS score). This finding supports the validity of the SyMS composite score as a clinically relevant outcome measure. When we examined associations between each of the 11 domain subscales of SyMS with SRH in a multivariate model, seven domains (pain, gait dysfunction, fatigue, depression, cognition, anxiety, spasticity) significantly predicted SRH, while four domains did not (vision, sensory, bladder, and hand function). It is noteworthy that the domain that accounted the most to patients’ perception of health – pain – is not included in the most widely used outcome measure in MS, EDSS. Gait dysfunction was the second highest predictor of SRH, followed by fatigue.
Our work suggests that ‘invisible’ symptoms are as important to patients’ sense of wellbeing as the physical disability visible to the examiner. These findings, corroborated by some of the previous studies,8,11–13 call into question the overwhelming emphasis on ambulation and physical disability as the primary, and often the only, outcome measure in clinical trials and observational studies of MS. Importantly, not all of the ‘invisible’ symptoms were significant predictors of general health. Bladder symptoms, for example, did not contribute significantly to SRH in the multivariate model. This could be due to the relatively low levels of disability in our sample, as bladder dysfunction strongly correlates with overall disability,23 as well as relatively low ranking in importance that patients assign to bladder dysfunction compared to fatigue, for example. This consideration might also partially explain the low degree of persistence on the various classes of medications for overactive bladder.24
Use of the validated single-item instruments adopted by WHO in their global studies of SRH2 allowed us to collect a large volume of data on consecutive (unselected) patients seen in our centers. However, the use of single items to measure a complex domain has been criticized because ‘a single item is unlikely to represent well the broad scope of that domain’.25 This critique is accurate and applicable to our work. Another obvious limitation is the cross-sectional design of the study, which precludes any statements of causation or sensitivity to change.
SyMS shares the limitation of all patient-reported outcome (PRO) measures in that responses depend entirely on patients’ ability to understand and interpret the questions. Reassuringly, patient-reported and clinician-recorded disability have a shown very high degree of correlation,16,26 although the more subjective symptoms, such as fatigue, could be interpreted more variably by patients and clinicians. Recall bias is also a concern, but is partially mitigated in the case of SyMS, which asks patients to assess their present symptom burden. Despite these known limitations, PROs add an important dimension to our understanding of disease, and are increasingly used in clinical studies of MS and other diseases.
Self-assessments of wellbeing and symptom burden may be confounded by mood, pain or fatigue, as was shown in a recent study in which depression had an impact on self-report of the various symptoms of MS.27 This confounding of other symptoms with depression is an important issue that is part of the broader subject of how symptoms in one domain affect symptoms in other domains. For example, the controversy of how much and what constitutes fatigue versus depression has not yet been fully untangled. Untangling this very complex interrelationship between the different symptoms requires more than can be done with the data at hand and warrants further study.
Despite these limitations, our work provides evidence that some ‘subjective’ domains contribute more to patients’ sense of their health than do the ‘objective’ neurological disability. Insofar as preservation of the sense of health and wellbeing is an essential goal of care in a chronic neurological condition, our findings have important implications for clinical practice and clinical research in MS.
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental data
Supplementary material is available for this article online.
References
- 1.Trojano M, Pellegrini F, Fuiani A, et al. New natural history of interferon-beta-treated relapsing multiple sclerosis. Ann Neurol 2007; 61: 300–306. [DOI] [PubMed] [Google Scholar]
- 2.Constitution of the World Health Organization. In: World Health Organization. Handbook of basic documents, 5th ed. Geneva: Palais des Nations, 1952, pp. 3–20.
- 3.Assari S. Gender differences in the predictive role of self-rated health on short-term risk of mortality among older adults. SAGE Open Med 2016; 4: 1–8. [DOI] [PMC free article] [PubMed]
- 4.Jones SL, Warren S, Turpin KVL, et al. The burden of multiple sclerosis: a community health survey. Health Qual Life Outcomes 2008; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marck CH, Hadgkiss EJ, Weiland TJ, et al. Physical activity and associated levels of disability and quality of life in people with multiple sclerosis: a large international survey. BMC Neurol 2014; 14: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy N, Confavreux C, Haas J, et al. Quality of life in multiple sclerosis in France, Germany, and the United Kingdom. J Neurol, Neurosurg Psychiatry 1998; 65: 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothwell PM. Quality of life in multiple sclerosis. J Neurol, Neurosurg Psychiatry 1998; 65: 433–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes A, While A, Mathes L, et al. Health problems and health-related quality of life in people with multiple sclerosis. Clin Rehabil 2006; 20: 67–78. [DOI] [PubMed] [Google Scholar]
- 9.Lobentanz IS, Asenbaum S, Vass K, et al. Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand 2004; 110: 6–13. [DOI] [PubMed] [Google Scholar]
- 10.Tabrizi FM, Radfar M. Fatigue, sleep quality, and disability in relation to quality of life in multiple sclerosis. Int J MS Care 2015; 17: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motl RW, McAuley E. Symptom cluster and quality of life: preliminary evidence in multiple sclerosis. J Neurosci Nursing: Journal of the American Association of Neuroscience Nurses 2010; 42: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cioncoloni D, Innocenti I, Bartalini S, et al. Individual factors enhance poor health-related quality of life outcome in multiple sclerosis patients. Significance of predictive determinants. J Neurol Sci 2014; 345: 213–219. [DOI] [PubMed] [Google Scholar]
- 13.Benedict RHB, Wahlig E, Bakshic R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci 2005; 231: 29–34. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald Criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green R, Kalina J, Ford R, et al. SymptoMScreen: a tool for rapid assessment of symptom severity in MS across multiple domains. Appl Neuropsychol: Adult 2017; 24: 183–189. [DOI] [PubMed] [Google Scholar]
- 16.Learmonth YC, Motl RW, Sandroff BM, et al. Validation of Patient Determined Disease Steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013; 13: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohol M, Orav E, Weiner H. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology 1995; 45: 251–255. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. www.R-project.org/ (accessed 15 August 2017).
- 19.van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. J Stat Software 2011; 45.3: 1–67. [Google Scholar]
- 20.Roberts G, Stuifbergen AK. Health appraisal models in multiple sclerosis. Soc Sci Med 1998; 47: 243–253. [DOI] [PubMed] [Google Scholar]
- 21.Cree B, Gourraud P-A, Oksenberg J, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016; 80: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
- 23.Zecca C, Riccitelli GC, Disanto G, et al. Urinary incontinence in multiple sclerosis: prevalence, severity and impact on patients’ quality of life. Eur J Neurol 2016; 23: 1228–1234. [DOI] [PubMed] [Google Scholar]
- 24.Sussman D, Yehoshua A, Kowalski J, et al. Adherence and persistence of mirabegron and anticholinergic therapies in patients with overactive bladder: a real-world claims data analysis. Int J Clin Pract 2017; 1: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobart J. Rating scales for neurologists. J Neurol, Neurosurg Psychiatry 2003; 74(Suppl 4): iv22–iv26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey KS, Cutter G, Green R, et al. Single-question patient-reported disability strongly correlates with Expanded Disability Status Scale. Presented orally at the 31st Congress European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Barcelona, Spain, October 2015.
- 27.Beeney JE, Arnett PA. Endorsement of self-report neurovegetative items of depression is associated with multiple sclerosis disease symptoms. J Int Neuropsychol Soc 2008; 14: 1057–1062. [DOI] [PubMed] [Google Scholar]