Abstract
Objective:
The objective was to report patterns of physical activity and their relationship to wound healing success in patients with diabetic foot ulcers protected with removable or irremovable offloading devices.
Methods:
Forty-nine people with diabetic foot ulcers were randomized to wear either a removable cast walker (RCW) or an irremovable instant total contact cast (iTCC). Primary outcome measures included change in wound size, physical activities including position (ie, sitting, standing, lying) and locomotion (speed, steps, etc). Outcomes parameters were assessed on weekly basis until wound healing or until 12 weeks.
Results:
A higher proportion of patients healed at 12 weeks in the iTCC group (P = .038). Significant differences in activity were observed between groups starting at week 4. RCW patients became more active than the iTCC group (75% higher duration of standing, 100% longer duration of walking, and 126% longer unbroken walking bout, P < .05). Overall, there was an inverse association between rate of weekly wound healing and number of steps taken per day (r < –.33, P < .05) for both groups. RCW patients had a significant inverse correlation between duration of daily standing and weekly rate of healing (r = –.67, P < .05). Standing duration was the only significant predictor of healing at 12 weeks.
Conclusion:
The results from this study suggest significant differences in activity patterns between removable and irremovable offloading devices. These patterns appear to start diverging at week 4, which may indicate a decline in adherence to offloading. Results suggest that while walking may delay wound healing, unprotected standing might be an even more unrealized and sinister culprit.
Keywords: offloading, diabetic foot ulcer, physical activity monitoring, wearable sensors, wound healing, adherence
Diabetes is a global epidemic and is one of the most significant public health challenges of our day. It is estimated that 642 million people worldwide will have diabetes by 2040,1 of which about 50% will develop peripheral neuropathy (PN).2 Largely because of PN and loss of protective sensation, lower extremity complications of diabetes constitute a major public burden in both the developed and developing world and affect 15-25% of those with PN.2-5 The most common complication, the diabetic foot wound, occurs most frequently when pressure and shear (cycles of stress) are multiplied by activity (episodes of initiating movement, walking, and standing).6,7 Management of physical activity and its overall pattern including postures and weight-bearing activities in patients with diabetic foot disease is poorly understood.7-10 Clinicians are cautious about advising extra activity to their patients with diabetic foot ulcers (DFU). There is concern about excessive foot loading causing a delay with healing of DFU because of repetitive moderate stress.11,12 However, the published data regarding this association are not entirely clear. Furthermore, there are few if any data evaluating the role of prolonged low-grade pressure on healing.
While walking on an unprotected wound may be plausibly detrimental to healing, the role of exercise on health benefits cannot be ignored, even in patients with DFU. Recent evidence suggests that exercise causes a trend of increased joint mobility, reduced psychological distress, and increased peripheral blood flow, which might reduce the risk of falling and contribute in accelerating wound healing.13-16 On the other hand, prolong immobilization of foot may lead to deconditioning, muscle atrophy and weakness17,18 and ultimately alter quality of life and wellbeing of patient even after successful wound healing. Thus it stands to reason to mobilize foot and encourage patients with DFU to be active and mobile. However, it is unclear whether weight-bearing activities even with protective offloading would suppress the impact of repetitive stress on plantar wound and its negative impact on success of wound healing.
Several studies have explored the physical activity levels in individuals at high risk for DFU.19-23 To our knowledge, none have explored activity pattern including postures (i.e., sitting, standing, lying, walking) and locomotion characteristics (e.g., number of taken steps, unbroken walking bouts, walking speed, postural transition, etc.) in individuals with DFU. Few studies suggested that exercise activity may have positive effect on physiological (e.g., oxygen) and psychological (e.g., stress) functioning and thus could enhance rates of wound healing.15,24,25 However, none of these studies explored the effect of physical activity pattern in patients with a DFU. Thus, there are no standardized guidelines available to dose physical activity in this population and clinicians are generally concerned about excessive loading of the foot leading to poor wound healing outcomes.8 There exists only a paucity of data (more specifically evidence from a single randomized study) investigating the levels and profiles of physical activity in this population.26 Therefore, the purpose of the present study was to report the patterns of physical activity as a function of removable and irremovable offloading modality in people with DFU.
Methods
Forty-nine eligible subjects with confirmed diabetes and PN, age 18 or older with noninfected, non ischemic, plantar neuropathic foot ulcers were entered into this prospective randomized controlled trail. Subjects with major foot amputation, active Charcot arthropathy, ankle brachial index (ABI) of 0.5 or less,27 history of alcohol or substance abuse within 6 months, or unable to keep research appointments were excluded. If subjects had noncompressible vessels (ABI > 1.2), we measured toe pressures to determine a toe brachial index (TBI). A TBI > 0.65 was required for enrollment. In addition, we excluded those patients, who could not be accommodated in a standard removable cast walker or were unable to walk a distance of minimum 20 minutes with or without an assistive device.
Subjects were recruited from 2 clinical sites including Hamad Medical Co (HMC) in Doha, Qatar and the Southern Arizona Limb Salvage Alliance (SALSA) clinic at the University of Arizona Health System, USA. The study received local institutional review board (IRB) approval from the University of Arizona and Hamad Medical Corporation. All subjects were given written informed consent before recruitment.
Using a computer generated randomization list, participants were assigned to one of the two off-loading modalities; removable cast walker (RCW, DH Offloading Walker, Ossur, Reykjavik, Iceland) and instant total contact cast (iTCC, the same RCW wrapped with a cohesive bandage, rendering it irremovable; Figure 1).28,29 Sequentially numbered, opaque envelopes that contained the study group assignment were provided to each site. At the time of randomization, an envelope was opened by the study coordinator to identify the study group assignment.
Figure 1.
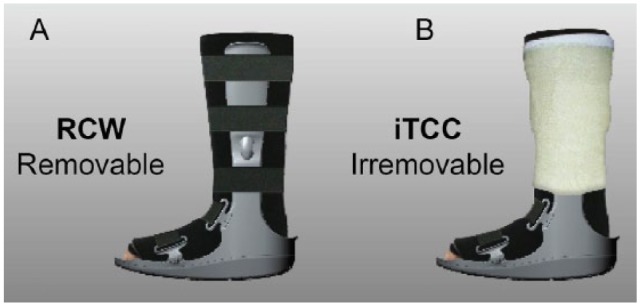
Patients were randomized to one of two offloading modalities: (A) removable cast walker and (B) instant total contact cast.
All subjects received standard of care including wound debridement and moisture retentive dressings by a wound care specialist. This methodology was described in previous studies.30,31 Subjects in RCW group were instructed to cleanse the wound daily and apply a dressing. They were instructed to inspect the wound at each dressing change and how to detect signs that the wound is worsening. They were asked to report these signs to the study coordinator immediately. Subjects in this group were also instructed to only walk with the RCW in place and to leave it on at all times. Subjects randomized to the iTCC group did not have daily dressing changes due to the irremovable nature of the device. Instead they received both written and oral instructions on device care, bathing, and signs of cast deterioration. On weekly basis, the iTCC was removed for the purpose of wound care and wound assessment similar to subjects in the RCW group and reapplied at the end of wound care and assessment.
Outcomes including change in wound size, wound closure, and daily physical activities were assessed at baseline and then on weekly basis until wound healing or at 12 weeks, whichever came first. The baseline was defined the first visit when patient visited the wound clinic for the purpose of wound care. Wound assessment included measurement of length, width, and depth of the ulcer before and after debridement. If there was more than 1 ulcer, the largest ulcer meeting all the inclusion and exclusion criteria was enrolled. Other ulcers were treated in the same manner as the study ulcer. We evaluated wounds at each clinical visit to ensure the absence of infection. At each study visit the study coordinator took photographs of the wound, which were planimetrically measured using a 3-D imaging system (Silhouette, ARANZ Systems, Christchurch, New Zealand) and assessed by a clinician unaware of specific study allocation. This provides measures of wound area, length and width. Pre and post treatment photos were taken. Areas of new epithelium or partial thickness ulceration were not included. We estimated changes in wound area compared to previous visit wound size to estimate rate of weekly wound healing. An ulcer was considered “healed” when it is fully epithelialized with no drainage.
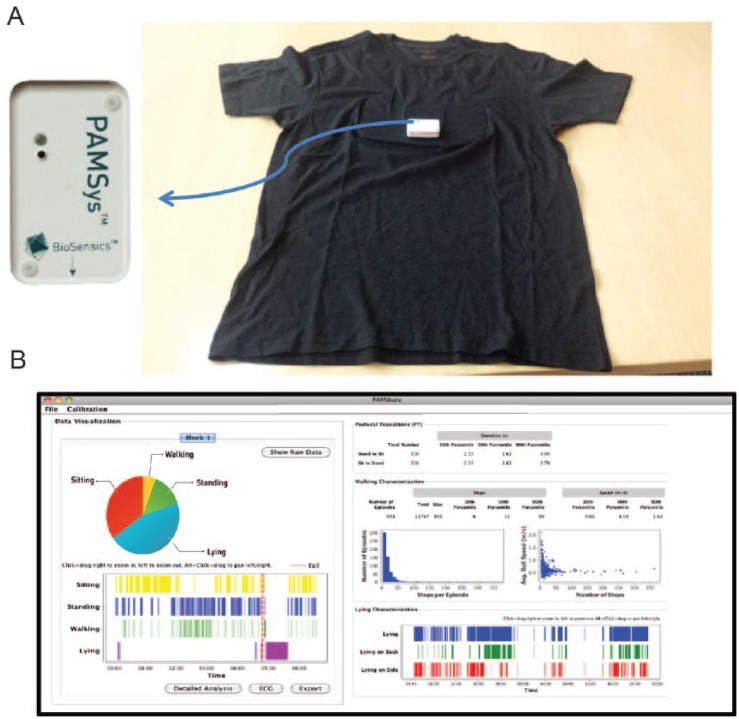
Spontaneous daily physical activity was monitored using a validated and an unobtrusive wearable sensor (PAMSys™, BioSensics LLC, MA, USA; Figure 2)32 incorporated in a comfortable shirt (PAMShirt™) worn by participants for 48 hours at baseline and once every week for 48 hours until 12 weeks. Patient adherence in wearing the PAMShirt™ was assessed based on measuring acceleration fluctuation indicator of respiration as described in earlier publication.23 Activity was quantified by percentage of each main posture (i.e., sitting, standing, lying, and walking), total number of steps, number of unbroken walking episode, gait speed, longest unbroken episode of walking, number and duration of postural transition (i.e., sit-to-stand and stand-to-sit) per day.32-34
Figure 2.
(A) Spontaneous activity was measured using a validated wearable sensor (PAMSys™, Biosensics LLC, Boston, MA, USA) integrated in a comfortable underwear shirt (PAMShirt™). (B) The device provides information about body posture (e.g., sitting, standing, lying, walking), locomotion (e.g. number of steps, number of unbroken walking bout, speed, etc.), and postural transition (e.g., sit-to-stand and stand-to-sit).
To examine whether the presence of DFU may limit spontaneous daily physical activities in DPN patients, the results of this study was retrospectively compared with our previous study23 in which similar activity monitoring protocol was used to monitor 48-hour spontaneous daily physical activities in 13 DPN patients without active ulcers (age: 59 ± 8 years, BMI: 34.6 ± 4.2 kg/m2).
Results are expressed as mean ± standard deviation (SD). ANOVA and Fisher’s exact test (or chi-square, as appropriate) was used to examine between-group differences in descriptive data. Repeated measures ANOVA test was used to examine between-group differences in weekly wound healing outcomes. When a significant difference (defined as P < .050) was found the Student–Newman–Keuls correction was used as the post hoc test to assess pairwise comparisons. Spearman correlation coefficient was used to examine correlation between activity characteristics and wound outcomes. Logistic regression model (forward conditional) was used to identify significant activities predictors to successful wound healing. The person who analyzed the data was blind to the type of intervention. The collected physical activity data were also compared retrospectively with previously collected data7 from diabetic patients without foot ulcers (n = 13, age: 59 ± 8, BMI: 34.6 ± 4.2) to examine whether presence of foot ulcers may impact spontaneous daily physical activities. Statistical analyses were performed using SPSS® version 20 (IBM, Armonk, NY, USA).
Results
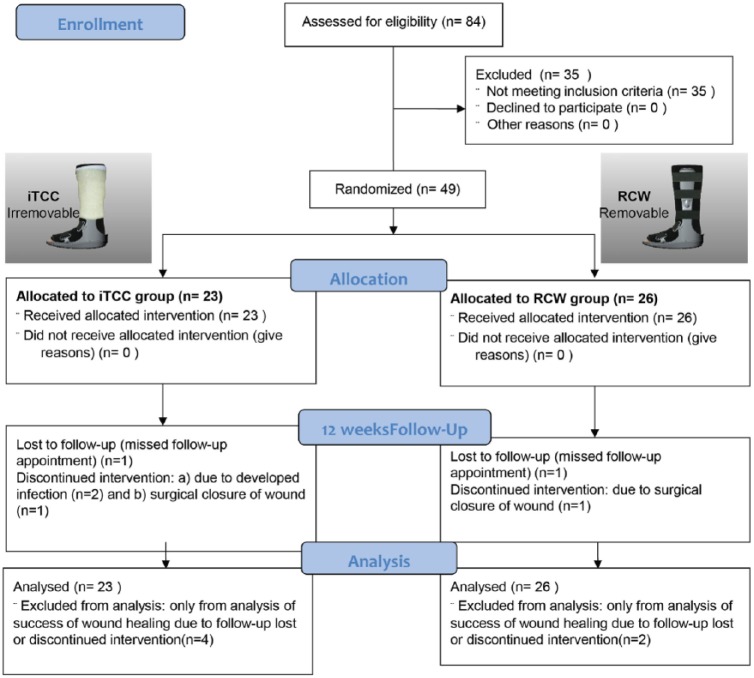
Eighty-four subjects with confirmed diabetes and peripheral neuropathy (DPN) were screened for the purpose of this study, out of which 49 subjects (age = 53.7 ± 7.7 years, BMI = 29.2 kg/m2, 93% male, VPT = 41.7 ± 22.5 volts, and HbA1C = 10.3 ± 2.4%) satisfied the inclusion and exclusion criteria and randomized to iTCC and RCW groups (Figure 3). There were no differences in the descriptive characteristics between groups (Table 1). The baseline wound area sizes were ranged from 0.16 cm2 to 36.8 cm2 for the RCW group and between 0.36 cm2 to 39.0 cm2 for the iTCC group. Six subjects were excluded from the study due to developed infection (2 subjects in iTCC group), surgical closure of wound (1 in iTCC and 1 in RCW group), and missed follow-up appointments (1 in iTCC and 1 in RCW group). All participants were ambulatory and 81% did not use any assistive device for walking.
Figure 3.
Consort flowchart. Patients were randomized to one of two offloading modalities: (A) removable cast walker and (B) instant total contact cast.
Table 1.
Population Descriptive Characteristics.
| iTCC (n = 23) | RCW (n = 26) | P value | 95% CI | |
|---|---|---|---|---|
| Age (years) | 52.1 ± 8.2 | 54.8 ± 7.3 | .268 | [–7.6, 2.2] |
| BMI (kg/m2) | 30.8 ± 6.6 | 27.8 ± 5.4 | .141 | [–1.1, 7.2] |
| Gender (% male) | 89% | 96% | .562 | — |
| HbA1C (%) (mmol/mol) | 10.3 ± 1.7% (89 mmol/mol) | 10.3 ± 2.8% (89 mmol/mol) | .988 | [–2.2, 2.3] |
| VPT (volt) | 49.9 ± 25.6 | 36.3 ± 19.9 | .107 | [–3.1, 30] |
| Ambulatory status (% of walking without assistive device) | 78% | 88% | .157 | — |
| Baseline wound area size (cm2) | 6.46 ± 8.48 | 10.13 ± 12.00 | .229 | [–9.71, 2.38] |
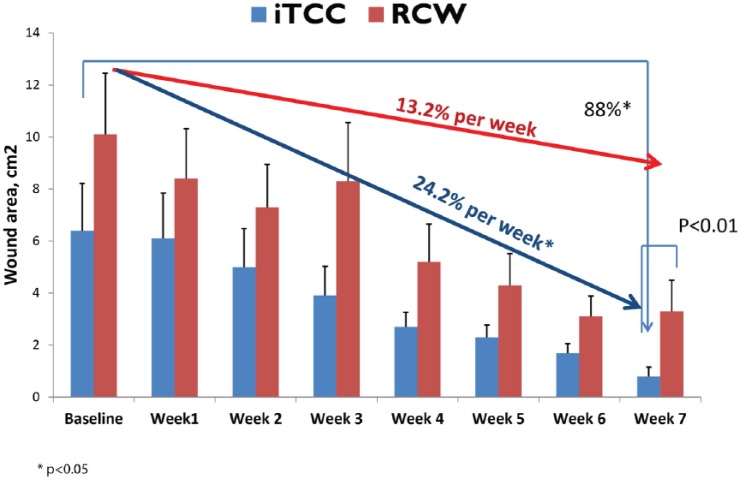
At 12 weeks, significantly more people healed in the iTCC group compared to patients offloaded with its removable counterpart (70% iTCC vs 40% RCW, P = .049). The weekly rate of wound healing was 13.2% in the RCW group versus 24.2% in iTCC (P < .001). Starting from week 7, the wound area was significantly smaller in iTCC group (0.8 ± 1.7 cm2 in iTCC v. 3.3 ± 6.1 cm2 in RCW, P = .010; Figure 4).
Figure 4.
Change in wound area over 7 weeks for iTCC and RCW groups.
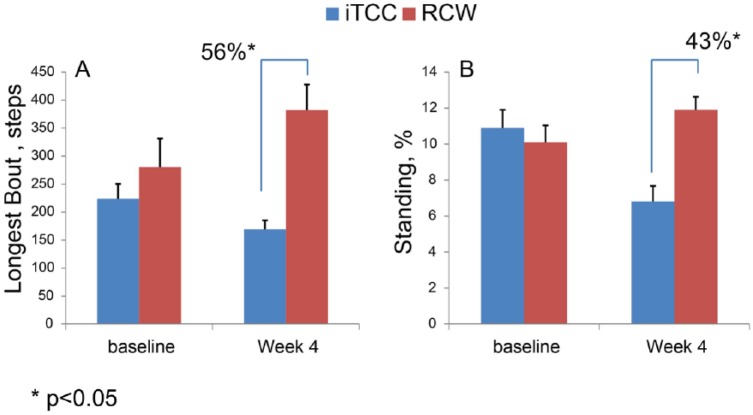
While at baseline the activity pattern was almost the same between 2 groups (Table 2), there were a number of differences in activity assessments between groups at the end of study visit and starting from week 4 (Table 3). At baseline, only the number of postural transitions were significantly lower in the iTCC group (P = .037). Interestingly, at the end of follow-up (last assessment before complete healing or at 12 weeks, whichever came first), a number of differences in activity assessments between groups were observed, which indicate that patients in the RCW group have changed their activity behavior which may explain the differences in wound healing success between groups. Starting from week 4, while all participants were still active in the study, the RCW population had a 50% longer walking period (P = .049, 95% CI = 0.01% to 4.8% of 24-hour activities), 56% longer unbroken walking episodes (P = .048, 95% CI = 0.3 steps to 425 steps; Figure 5A), and 43% longer average daily standing period (P = .028, 95% CI = 0.6% to 9.4% 24-hour activities; Figure 5B). Interestingly, patients in the iTCC group spent nearly twice as much time lying on their sides than did the group in the RCW (P = .005), which may be explained by restriction caused by iTCC offloading while sleeping.
Table 2.
Baseline Activity Characterization.
| Type of physical activity | iTCC (n = 23) | RCW (n = 26) | P value | 95% CI | ||
|---|---|---|---|---|---|---|
| Type of posture (% of total activity) | Lying (%) | Total | 40.8 ± 26.4 | 45.9 ± 18.2 | .544 | [–22, 12] |
| Lying-supine (%) | 17.0 ± 13.1 | 2.4 ± 14.6 | .772 | [–14, 10] | ||
| Lying-sides (%) | 23.9 ± 17.9 | 25.6 ± 13.7 | .527 | [–14, 7.6] | ||
| Sitting (%) | 44.0 ± 21.9 | 39.7 ± 14.7 | .536 | [–9.6, 18.2] | ||
| Standing (%) | 10.9 ± 4.8 | 10.1 ± 4.8 | .734 | [–3.9, 5.5] | ||
| Walking (%) | 4.3 ± 3.8 | 4.3 ± 2.6 | .970 | [–2.3, 2.4] | ||
| Postural transition | Number per day | 76 ± 39 | 114 ± 50 | .037 | [–75, –2.5] | |
| Duration (sec) | 4.0 ± 0.8 | 3.9 ± 0.6 | .861 | [–.5, .6] | ||
| Locomotion | # steps | 3912 ± 2525 | 5273 ± 3337 | .256 | [–3765, 1043] | |
| # episodes of walking | 167 ± 237 | 238 ± 117 | .332 | [–128, 45] | ||
| Longest walking episode (step) | 222 ± 124 | 280 ± 261 | .494 | [–231, 114] | ||
| Speed (m/s) | 0.69 ± 0.9 | 0.72 ± 0.06 | .332 | [–.08, .03] | ||
Table 3.
Follow-Up Activity Characterization at 4 Weeks.
| Type of physical activity | iTCC (n = 23) | RCW (n = 26) | P value | 95% CI | ||
|---|---|---|---|---|---|---|
| Type of posture (% of total activity) | Lying (%) | Total | 57.2 ± 21.4 | 4.0 ± 1.2 | .046 | [0.4, 34] |
| Lying-supine (%) | 21.7 ± 11.6 | 21.4 ± 13.2 | .952 | [–13.6, 14.4] | ||
| Lying-sides (%) | 35.6 ± 12.6 | 18.8 ± 7.9 | .005 | [6.0, 27.8] | ||
| Sitting (%) | 33.6 ± 18.8 | 43.3 ± 10.5 | .198 | [–25, 5.7] | ||
| Standing (%) | 6.8 ± 4.2 | 11.9 ± 3.7 | .028 | [–9.4, –0.6] | ||
| Walking (%) | 2.4 ± 2.1 | 4.8 ± 2.1 | .049 | [–4.8, –0.01] | ||
| Postural transition | Number per day | 106 ± 54 | 129 ± 44 | .373 | [–76, 30] | |
| Duration (sec) | 3.9 ± 0.7 | 4.0 ± 0.3 | .981 | [–0.55, 0.54] | ||
| Locomotion | # steps | 2994 ± 2551 | 5902 ± 3090 | .070 | [–6172, 272] | |
| # bouts | 166 ± 128 | 279 ± 98 | .065 | [–234, 8] | ||
| Longest episode (step) | 169 ± 78 | 382 ± 232 | .048 | [–425, –0.3] | ||
| Speed (m/s) | 0.72 ± 0.10 | 0.69 ± 0.04 | .434 | [–0.05, 0.11] | ||
Figure 5.
While daily physical activities were almost similar at baseline, at week 4, participants in the RCW group had (A) 56% longer continuous walking episode and (B) 43% longer standing duration (P < .05).
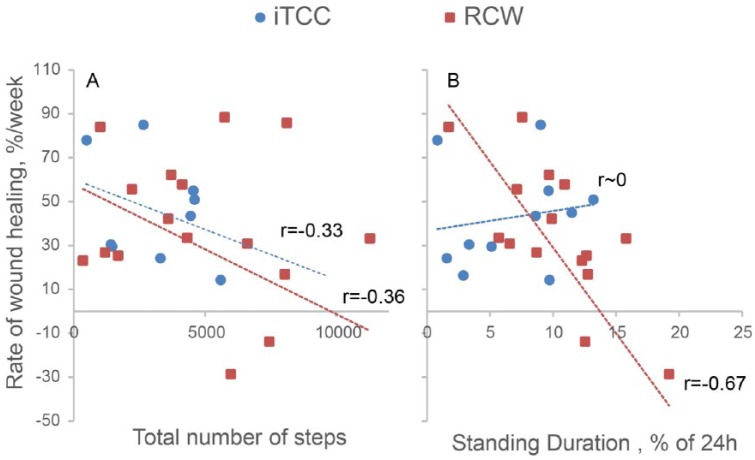
Comparison between the amount of wound size reduction at week 7 and activity parameters suggest no significant association between activities at baseline and wound healing success. Results revealed a significant negative association between the rate of weekly wound healing and average of number of taken steps per day irrespective of offloading type (Figure 6A). In addition, while no association was observed between daily percentage of standing and rate of weekly wound healing in iTCC group, a significant negative correlation (r = –.67, P < .001) was observed in the RCW (Figure 6B).
Figure 6.
Association between rate of weekly wound healing and (A) number of taken steps per day, (B) standing duration per 24 hours.
Among measurable parameters at baseline, only baseline wound size and type of offloading were associated with success of wound healing (Table 4). Although baseline physical activities were not different between healers and nonhealers, two key physical activity parameters measured during the final treatment visit (12 weeks or the week before healing, whichever came first) were different between healers and nonhealers (Table 4). Specifically, healers had an average 50% shorter duration of standing (P = .025) and a 44% shorter longest unbroken walking episode per day (P = .024). Interestingly among measurable variables, only last visit standing period per day was a significant predictor for success of wound healing (odds ratio = 0.663, P = .013).
Table 4.
Descriptive Difference Between Healers and Nonhealers.
| Healed (n = 22) | Nonhealed (n = 21) | P value | |
|---|---|---|---|
| Ambulatory status (% of nonuser of assistive devices) | 76% | 86% | .226 |
| Type of offloading (% of iTCC) | 68% | 38% | .049 |
| Age (years) | 53.4 ± 8.1 | 53.8 ± 7.8 | .884 |
| BMI (kg/m2) | 29.1 ± 6.3 | 29.6 ± 6.2 | .805 |
| VPT (volt) | 47.9 ± 28.6 | 35.8 ± 12.8 | .158 |
| HbA1C | 9.86 ± 2.66 | 11.00 ± 1.99 | .285 |
| Baseline wound area (cm2) | 5.71 ± 8.24 | 11.95 ± 12.18 | .041 |
| Baseline lying (% of 24 hours) | 36.5 ± 21.4 | 50.1 ± 20.0 | .090 |
| Baseline sitting (% of 24 hours) | 47.0 ± 18.5 | 36.7 ± 14.8 | .116 |
| Baseline standing (% of 24 hours) | 9.8 ± 5.3 | 11.2 ± 6.7 | .518 |
| Baseline walking (% of 24 hours) | 5.27 ± 3.8 | 3.50 ± 2.02 | .120 |
| Baseline number of steps per day | 5304 ± 3567 | 4312 ± 2658 | .398 |
| Baseline number of walking episodes per day | 464 ± 226 | 427 ± 218 | .654 |
| Baseline longest continuous walking per day, steps | 299 ± 297 | 225 ± 124 | .370 |
| Last visit lying (% of 24 hours) | 55.9 ± 27.2 | 43.3 ± 12.3 | .055 |
| Last visit sitting (% of 24 hours) | 36.5 ± 40.7 | 40.7 ± 11.3 | .627 |
| Last visit standing (% of 24 hours) | 5.7 ± 4.0 | 11.4 ± 3.9 | .025 |
| Last visit walking (% of 24 hours) | 1.93 ± 1.41 | 4.6 ± 2.4 | .057 |
| Last visit number of steps per day | 2595 ± 2056 | 5586 ± 3186 | .104 |
| Last visit number of walking episodes per day | 278 ± 209 | 538 ± 220 | .058 |
| Last visit longest continuous walking per day, steps | 190 ± 72 | 340 ± 234 | .235 |
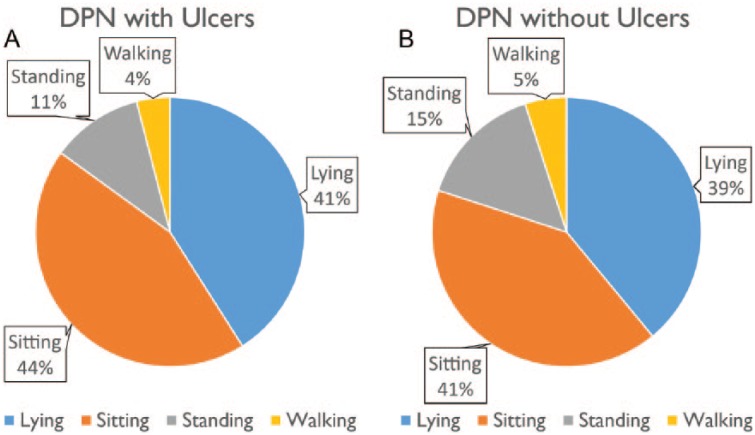
No noticeable difference in daily physical activities were observed between DPN with and without ulcers, when results of this study was retrospectively compared with prior data collected from individuals with DPN and without foot ulcers.23 In average, the duration of lying, sitting, standing, and walking in DPN with foot ulcers were, respectively, 41%, 44%, 11%, and 4% of 24-hour daily physical activities (Figure 7A), which were almost the same distribution as previously reported in DPN without foot ulcers (Figure 7B).7 Similar to DPN without ulcer, those who have active ulcers spent almost 3 times longer in standing posture compared to walking posture. On average, the group with active foot ulcers took 15% fewer steps per day compared to group without ulcer, but overall duration of daily walking was almost the same between two groups.
Figure 7.
Physical activities in diabetes with peripheral neuropathy (DPN) with (A) and without (B) diabetic foot ulcers. Duration of standing in both groups was almost 3-fold longer than duration of walking. Although DPN without ulcers are in average more active than DPN with ulcers, the difference was not noticeable.
Discussion
The results from this study suggest significant differences in activity behavior between patients who are offloading using a removable device (RCW) and those who were offloaded using the same device but is rendered irremovable (iTCC). To our knowledge, this is the first study to demonstrate these detailed profiles of physical activity between the two treatment groups including not only steps but also body posture/position. These data may ultimately help in addressing the missing gaps in clinically dosing of physical activity in the target population for better wound healing outcomes. The group treated with the RCW was more active than the iTCC group in particular during follow-up monitoring. We assumed that the irremovable offloading device should limit activities—in particular walking. Thus, these results may indicate lack of adherence in RCW group in wearing the prescribed footwear during everyday conditions. This is supported by previous works by our group that reported fewer than 30% of daily activity in removable devices is taken with the device on the patient.10
While no noticeable differences were observed between groups at baseline for the main postures and weight-bearing activities (ie, lying, sitting, standing, and walking), the number of postural transition (sit to stand) was significantly higher in the RCW group at baseline. In addition, total number of steps were 35% higher in the RCW group, but didn’t achieve statistical significant level in our sample. The high number of postural transition in RCW group could be due to the fact that when patients are at home or sitting, they may remove their heavy offloading boot,10 which may increase the level of comfort to frequently rise from a chair and walk more. While the number of taken steps, postural transitions, and weight-bearing posture continue to increase over time in the RCW group, they continue to reduce in the iTCC group. This could be due to deconditioning, muscle atrophy and weakness caused by prolonged immobilizing patients’ foot in the iTCC group. This is aligned with previous studies, which suggest that prolonged immobilization via using irremovable casts will lead to muscle wasting and weakness.17,18 Additional study need to be addressed to confirm this hypothesis.
In this study, we have also observed a change in sleep posture pattern in the iTCC group. Specifically, we observed that the iTCC group spent nearly twice as much time lying on their sides than did the group in the RCW, while the between-group difference at baseline was not noticeable. This may indicate an alteration in sleep posture pattern to tolerate irremovable offloading. While patients in the RCW group could freely remove their offloading boot while sleeping thus had least alteration in their sleep posture pattern. Further study should be addressed to confirm whether wearing offloading while sleeping may alter sleep quality and change the posture behavior while sleeping.
Results of this study revealed that the activity behaviors are changing over wound healing period and patients become more active compared to baseline in RCW group. This may indicate potential diminish in adherence over time for RCW and highlights the importance of continued and frequent patient education during wound healing process. Considering, that activity behavior appeared to change significantly from week 4, future works might consider intervening with education at least every 4 weeks. On the other hand, patients in iTCC become less active compared to baseline. This may indicate the sign of muscle weakness and loss of mobility ability. Thus appropriate and safe exercise program is recommended to retain mobility ability in those patients who are wearing irremovable offloading.
The total number of steps had a significant negative correlation with weekly rate of wound healing independent on the type of offloading. While no significant correlation was found between the duration of standing and weekly rate of wound healing in iTCC (irremovable offloading) group, a relatively high negative correlation was observed in RCW. Together, these results suggest that although offloading may be efficient in reducing pressure during standing, it may fail to successfully suppress pressure during walking in particular in highly active participants. In addition, the results suggest unprotected standing could possibly contribute to retarded wound healing.
Interestingly, although baseline wound size was significantly larger in nonhealers’ group, time spent standing came as significant predictor for successful wound healing in the multivariable model. Our instructions to subjects with RCW were only to “walk with the RCW in place and to leave it on at all times.” Unfortunately, we failed to emphasize wearing offloading during standing as well. It is conceivable that patients believed that a few steps plus standing still wouldn’t deleteriously affect wound. Such loading could be damaging and in particular for a group that has the potential to remove their off-loading device.
Results suggest that despite having ulcers, duration of foot loading condition (standing and walking) were not reduced when are compared with DPN without ulcers. Interestingly, while the number of steps were reduced by 15% in DPN patients with active ulcers compared to those without ulcers, presence of ulcers do not necessary reduce the duration of walking. This finding is similar to Grewal et al study35 in which they have demonstrated that presence of ulcers do not necessary deteriorate walking ability in diabetic patients with plantar ulcers. In particular, similar to what was observed in DPN without ulcers, the duration of standing posture, is almost 3-fold longer than the duration of walking. While standing is an important foot loading condition, which as demonstrated in this study could delay wound healing in diabetic patients with lose of protective sensation. Thus further attention should be allocated to the risk of standing when treating and preventing foot ulcers.
The study has several limitations. First, our sample size is modest and may not have sufficient power to confirm all the observations in this study. Second, we didn’t have true baseline for activity monitoring (activity monitoring prior using each offloading modality) thus couldn’t evaluate how much each offloading may alter daily physical activities. However, when we retrospectively compared the results with DPN patients with no active plantar ulcers and no offloading, we didn’t observe noticeable limitation in weight-bearing activities and postures due to wound and offloading. Third, we didn’t control adherence to offloading. We believe that this is a very important issue contributed to some of the key findings in the study as regards differences in activity. Future studies might address with higher fidelity specific activity patterns with and without worn devices and their interplay with wound healing and other subsequent complications. We also believe that data derived from such work might be used to create more intelligent activity management systems to alert a patient and his or her caregiver about potentially dangerous activity signatures.
Conclusions
The results from this study suggest significant differences in activity patterns between removable and irremovable offloading devices. These patterns appear to start diverging at week 4 (ie, increase in level of activity in the RCW group and reduction in level of activity of iTCC group), which may indicate a decline in adherence to offloading in the group who are using removable cast walker and muscle wasting and weakness caused by prolonged immobilization in the group who are using irremovable offloading. Results suggest that while walking may delay wound healing, unprotected standing might be an even more unrealized and sinister culprit. In particular, this study revealed that standing duration is the only significant predictor of healing at 12 weeks and is almost 3-times greater than walking duration in neuropathic foot ulcers.
Footnotes
Abbreviations: ABI, ankle brachial index; ANOVA, analysis of variance; BMI, body mass index; CI, confidential interval DFU, diabetic foot ulcer; DPN, diabetic peripheral neuropathy; HMC, Hamad Medical Corporation; IRB, institutional review board; iTCC, instant total contact cast; PHI, projected health information; PN, peripheral neuropathy; RCW, removable cast walker; SALSA, Southern Arizona Limb Salvage Alliance; TBI, toe brachial index; VPT, vibratory perception threshold.
Author contribution: BN, DA, and TT designed the study and supervised its milestones. BN and GG researched and analyzed the data. BN and DA wrote manuscript, reviewed/edited manuscript, contributed to discussion. TT and RM researched data and contribute in subject recruitment. MB coordinated the study, evaluate source document, and contributed in data analysis. All authors reviewed/edited manuscript and contributed to discussion.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported in part by a grant from the Qatar National Research Foundation (Award Number NPRP 4-1026-3-277, http://www.qnrf.org/). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Qatar National Research Foundation. None of the authors employed or contracted by the funder.
References
- 1. International Diabetes Federation. Diabetes Atlas. 7th ed. 2015. Available at: http://www.diabetesatlas.org/. Accessed April 24, 2016.
- 2. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217-228. [DOI] [PubMed] [Google Scholar]
- 3. Apelqvist J, Armstrong DG, Lavery LA, Boulton AJ. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg. 2008;195(6):782-788. [DOI] [PubMed] [Google Scholar]
- 4. Barshes NR, Sigireddi M, Wrobel JS, et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabet Foot Ankle. 2013;4:PMC3796020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bus SA, van Netten JJ. A shift in priority in diabetic foot care and research: 75% of foot ulcers are preventable. Diabetes Metab Res Rev. 2016;32(suppl 1):195-200. [DOI] [PubMed] [Google Scholar]
- 6. Wrobel JS, Najafi B. Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Technol. 2010;4(4):833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Najafi B, Crews RT, Wrobel JS. Importance of time spent standing for those at risk of diabetic foot ulceration. Diabetes Care. 2010;33(11):2448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armstrong DG, Boulton AJM. Activity monitors: should we begin dosing activity as we dose a drug? J Am Podiatr Med Assoc. 2001;91:152-153. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong DG, Lavery LA, Holtz-Neiderer K, et al. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004;27:1980-1984. [DOI] [PubMed] [Google Scholar]
- 10. Armstrong DG, Lavery LA, Kimbriel HR, Nixon BP, Boulton AJ. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care. 2003;26(9):2595-2597. [DOI] [PubMed] [Google Scholar]
- 11. Armstrong DG, Lavery LA. Evidence-based options for off-loading diabetic wounds. Clin Podiatr Med Surg. 1998;15(1):95-104. [PubMed] [Google Scholar]
- 12. Armstrong DG, Lavery LA, Holtz-Neiderer K, et al. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004;27(8):1980-1984. [DOI] [PubMed] [Google Scholar]
- 13. Flahr D. The effect of nonweight-bearing exercise and protocol adherence on diabetic foot ulcer healing: a pilot study. Ostomy Wound Manage. 2010;56(10):40-50. [PubMed] [Google Scholar]
- 14. Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88(11):1385-1398. [DOI] [PubMed] [Google Scholar]
- 15. Emery CF, Kiecolt-Glaser JK, Glaser R, Malarkey WB, Frid DJ. Exercise accelerates wound healing among healthy older adults: a preliminary investigation. J Gerontol A Biol Sci Med Sci. 2005;60(11):1432-1436. [DOI] [PubMed] [Google Scholar]
- 16. Mueller MJ, Tuttle LJ, Lemaster JW, et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94(5):829-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Oliveira AL, Moore Z. Treatment of the diabetic foot by offloading: a systematic review. J Wound Care. 2015;24(12):560, 562-570. [DOI] [PubMed] [Google Scholar]
- 18. Appell HJ. Muscular atrophy following immobilisation. A review. Sports Med. 1990;10(1):42-58. [DOI] [PubMed] [Google Scholar]
- 19. Maluf KS, Mueller MJ. Novel Award 2002. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech (Bristol, Avon). 2003;18(7):567-575. [DOI] [PubMed] [Google Scholar]
- 20. Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30(2):203-209. [DOI] [PubMed] [Google Scholar]
- 21. Tudor-Locke CE, Bell RC, Myers AM, Harris SB, Lauzon N, Rodger NW. Pedometer-determined ambulatory activity in individuals with type 2 diabetes. Diabetes Res Clin Pract. 2002;55(3):191-199. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong DG, Abu Rumman P, Nixon B, Boulton A. Continuous activity monitoring in persons at high risk for diabetes-related lower extremity amputation. J Am Podiatr Med Assoc. 2001;91:451-455. [DOI] [PubMed] [Google Scholar]
- 23. Najafi B, Crews RT, Wrobel JS. The importance of time spent standing for those at risk of diabetic foot ulceration. Diabetes Care. 2010;33(11):2448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keylock KT, Vieira VJ, Wallig MA, DiPietro LA, Schrementi M, Woods JA. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R179-184. [DOI] [PubMed] [Google Scholar]
- 25. Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45(10):1563-1569. [DOI] [PubMed] [Google Scholar]
- 26. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001;24(6):1019-1022. [DOI] [PubMed] [Google Scholar]
- 27. Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39(7):885-910. [DOI] [PubMed] [Google Scholar]
- 28. Armstrong DG, Hussain SK, Middleton J, Peters EJ, Wunderlich RP, Lavery LA. Vibration perception threshold: are multiple sites of testing superior to single site testing on diabetic foot examination? Ostomy Wound Manage. 1998;44(5):70-76. [PubMed] [Google Scholar]
- 29. Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158:289-292. [DOI] [PubMed] [Google Scholar]
- 30. Katz IA, Harlan A, Miranda-Palma B, et al. A randomized trial of two irremovable off-loading devices in the management of plantar neuropathic diabetic foot ulcers. Diabetes Care. 2005;28(3):555-559. [DOI] [PubMed] [Google Scholar]
- 31. Armstrong DG, Short B, Nixon BP, Boulton AJM. Technique for fabrication of an “instant” total contact cast for treatment of neuropathic diabetic foot ulcers. J Am Podiatr Med Assoc. 2002;92:405-408. [DOI] [PubMed] [Google Scholar]
- 32. Najafi B, Armstrong DG, Mohler J. Novel wearable technology for assessing spontaneous daily physical activity and risk of falling in older adults with diabetes. J Diabetes Sci Technol. 2013;7(5):1147-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Najafi B, Aminian K, Loew F, Blanc Y, Robert PA. Measurement of stand-sit and sit-stand transitions using a miniature gyroscope and its application in fall risk evaluation in the elderly. IEEE Trans Biomed Eng. 2002;49(8):843-851. [DOI] [PubMed] [Google Scholar]
- 34. Najafi B, Aminian K, Paraschiv-Ionescu A, Loew F, Bula CJ, Robert P. Ambulatory system for human motion analysis using a kinematic sensor: monitoring of daily physical activity in the elderly. IEEE Trans Biomed Eng. 2003;50(6):711-723. [DOI] [PubMed] [Google Scholar]
- 35. Grewal GS, Bharara M, Menzies R, Talal TK, Armstrong D, Najafi B. Diabetic peripheral neuropathy and gait: does footwear modify this association? J Diabetes Sci Technol. 2013;7(5):1138-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]