Abstract
Background:
We assessed the effect of structured self-monitoring of blood glucose (SMBG), in combination with intensive education, on metabolic control, SMBG frequency, hospitalizations, cardiovascular risk factors, and quality-of-life parameters in patients with insulin-treated diabetes in primary health care settings in Serbia.
Methods:
This 6-month, observational, noninterventional study, followed 346 insulin-treated diabetes patients (type 1 diabetes [T1D], n = 57; type 2 diabetes [T2D], n = 289) from 28 primary care centers. Patients attended a 10-day course at the specialized educational center and were followed monthly by their primary care physicians. Patients used a simple paper tool to document 3-day, 7-point glucose profiles prior to each monthly clinic visit. Physicians reviewed the completed forms at each visit and used a standardized education program to provide remedial training. Changes in HbA1c levels, SMBG frequency, metabolic risk factors, and Diabetes Distress Scale (DDS) were assessed.
Results:
Mean (± SD) HbA1c within the full cohort was significantly improved from baseline at 6 months (8.85 ± 1.17% vs 7.91 ± 1.24%, P < .01). Significant increases in average SMBG frequency per week were seen at 6 months versus baseline (14.6/week vs 4.3/week, P < .001). The mean (± SE) number of hospitalizations due to metabolic conditions was significantly lower during the 6-month study compared to the 6-month period prior to the study (0.14 ± 0.04 vs 0.59 ± 0.09). DDS scores decreased from 39.6 ± 13.9 to 33.9 ± 14.5, P < .01.
Conclusion:
The use of structured SMBG combined with intensive education was associated with clinically significant reductions in HbA1c, increased SMBG frequency, and improved quality of life.
Keywords: structured SMBG, type 1 diabetes, type 2 diabetes, HbA1c
Maintaining optimal glycemic control has been shown to improve clinical outcomes in both type 1 (T1D) and type 2 diabetes (T2D).1,2 However, achieving desired glycemic control is particularly challenging for individuals with insulin-treated diabetes and their health care providers. Individuals with diabetes spend an average of approximately 8750 hours per year self-managing their disease,3 which can eventually lead to reduced adherence due to treatment fatigue for many individuals.4
Conversely, health care providers must often contend with constraints on the time they can spend evaluating and counseling their patients with diabetes; approximately 50% of physicians spend less than 16 minutes with each patient.5 The impact of these time constraints is compounded when accurate blood glucose data are not available in formats that allow for identification and/or interpretation of meaningful glycemic patterns.6 Lack of time and useful glucose data can hinder timely initiation and/or intensification of therapy, a condition often referred to as “clinical inertia.”7-9
Because most of the daily tasks associated with diabetes self-management are handled by patients (or caregivers), reliable and valid measures are needed to support adherence to therapy.10 Self-monitoring of blood glucose (SMBG) provides this metric. However, effective use of this technology requires that testing regimens are structured to identify patterns of glycemic control, and that patients and their health care providers possess the knowledge, skills, and willingness to accurately interpret the data and make appropriate therapy changes.11,12 This highlights the strong need for comprehensive education and training in diabetes self-management concepts and skills.13
Several recent studies have shown that use of structured SMBG in combination with education and clinician support, promotes desired behavioral changes, enhances patient motivation and empowerment, increases patient understanding of their treatment regimens and facilitates therapy intensification, leading to improved clinical outcomes and quality of life.14-21 Among these was the Structured Testing Program (STeP) study, a cluster-randomized, controlled, multicenter trial, in which poorly controlled, non-insulin-treated T2D patients used a simple paper tool to collect 7-point glucose profiles on 3 consecutive days.15 At 12 months, patients who utilized the structured testing tool showed significant improvements in glycemic control, HbA1c depression, and diabetes-related distress,22 and patient self-efficacy and autonomous motivation in managing their diabetes.23
Although well-designed randomized controlled trials allow for assessment of treatment interventions under controlled conditions, they may not represent the impact and/or feasibility of those interventions in real world clinical settings. In 2012, we reported findings from a 12-week, multinational observational study that showed use of a modified version of the STeP study intervention is associated with improvements in glycemic control and markers of metabolic risk in poorly controlled T1D and T2D patients treated by diabetes specialists in everyday practice.24 In the current study, we sought to confirm the clinical and quality-of-life benefits associated with the modified STeP intervention in primary care settings throughout Serbia.
Methods
This was a 6-month, prospective, multicenter, noninterventional, observational study of people with T1D and insulin-treated T2D who used the Accu-Chek 360° View tool, supported by use of the Accu-Chek Assist materials (Roche Diagnostics GmbH, Mannheim, Germany), a standardized educational program, to assess the impact of structured SMBG on glycemic status, metabolic markers of diabetic complications, and diabetes-related distress when used in primary care practice settings.
Participants
Patients were recruited from by 32 physicians from 28 primary health care centers across Serbia. Physicians were asked to identify at least 10 poorly controlled subjects with T1D and insulin-treated T2D from their own practices for inclusion in the study. Study physicians received training in using the structured testing tool and education module prior to study initiation.
Four hundred patients were screened; 349 met inclusion criteria and were enrolled in the study. Inclusion criteria were age 18-70 years; HbA1c ≥8%; treated with insulin (monotherapy or in combination with oral antidiabetes agents); and willing/capable to perform SMBG measurements. Exclusion criteria were recent myocardial infarction, stroke, or diabetic ketoacidosis; pregnant or breast feeding; chronic infection; malignancy; recent emotional trauma; dementia; psychiatric disorders; treated with steroid therapy ≥14 days during the last 3 months; parenteral therapy with medications containing maltose (or metabolized into maltose); recent D-xylose test for malabsorption; and congenital galactosemia.
All recruited patients provided signed written consent. The study protocol was approved by the Ethics Committee in each primary health care center and by the central regulatory body of the country (Agency for Drugs and Medical Devices of Serbia).
Study Materials
The structured testing tool is a simple paper form that patients can use to generate 7-point blood glucose profiles over 3 consecutive days. The tool allows patients to document meal sizes and energy levels and to comment on their SMBG experiences (Figure 1).
Figure 1.
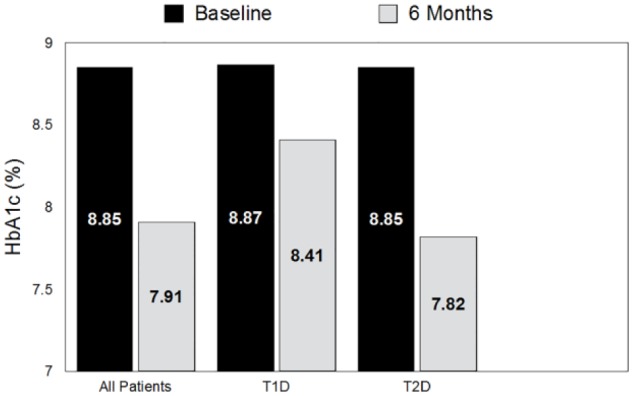
HbA1c levels at baseline and study end in all patients, type 1 diabetes (T1D) and type 2 diabetes (T2D) patients. All patients: P < .01; T1D patients: P = .07; T2D patients: P < .001.
The standardized educational program comprised 9 modules covering the main topics in diabetes. Education content incorporating cartoons is made available through easy-to-teach and easy-to-learn modules.
Procedures
At the baseline visit (visit 0), investigators used a 1-page form to collect and document patient name/number; type of diabetes; diabetes duration; gender; age; diabetes therapy (diet, noninsulin drug, insulin); average number of blood glucose test strips used per week. Measurements of weight, height, lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides), HbA1c, and blood pressure were taken and documented. The Diabetes Distress Scale (DDS) questionnaire25 was administered to assess psychosocial distress in diabetes.
All patients were referred to a specialized educational hospital (Merkur Hospital/Vrnjacka Spa, Serbia) to participate in a 10-day, in-patient diabetes education program in accordance with current social security regulations of the country. All patients received a free blood glucose meter (Accu-Chek Active, Roche Diagnostics GmbH, Mannheim, Germany) and testing supplies. Patients were trained to operate the blood glucose meter and use the structured testing tool to document their SMBG results and adjust diet, physical activity and therapy as needed.
After completing the educational program, patients were seen monthly for the next 5 months (visits 1-5) by their primary care physicians. Patients were asked to complete the structured testing tool 1 week prior to each monthly visit, and bring the completed tool to each clinic visit for review and discussion. Physicians used a module of the educational program to provide remedial training as needed. to active group subjects. The module focused on improving glycemic control through structured glucose monitoring and lifestyle adjustments (eg, exercise, diet, prevention/treatment of hypoglycemia/hyperglycemia and other self-management behaviors). At the 6-month visit, physicians obtained physical and metabolic measurements and readministered the DDS questionnaire.
Clinical Measures
The primary endpoint for the study was change in HbA1c from baseline to study end. Secondary endpoints included weekly frequency of SMBG; number of hospitalizations due to metabolic disorders of diabetic complications during the study period compared to hospitalization during the 6 months prior to the study; changes in waist circumference, blood pressure, lipid levels (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides), and albumin excretion (24-hour urinary albumin excretion [UAE] rate); and changes in diabetes-related distress. Samples and measurements for comparison were obtained at the baseline (visit 0) and study end (visit 5).
All laboratory analyses were conducted by a central laboratory (Konzilijum referent biochemical laboratory, Belgrade, Serbia), using commercially available kits on paired samples. The HbA1c values were determined using immunoturbidimetric assay. Determination of lipid parameters, total cholesterol, HDL cholesterol, and triglycerides concentrations were analyzed using commercial enzymatic kit (Roche Diagnostics GmbH, Mannheim, Germany). LDL cholesterol levels were calculated by using standard Friedewald formula. UAE in 24-hour urine collection was determined by immunonephelometry method. Microalbuminuria was defined as UAE 30-300 mg/24h. Macroalbuminuria was defined as UAE >300 mg/24h. Frequency of SMBG was determined according to downloaded data from blood glucose meters and patient blood glucose logbooks. The number of hospitalization and reasons for hospitalizations were determined from patients’ individual medical history records.
Diabetes-related distress was assessed using a modified Diabetes Distress Scale (DDS) questionnaire, a validated instrument.25 From modified DDS questionnaire, 4 preestablished domains of diabetes related distress were computed: total DDS score as a score of all questions; emotional burden (EB) as a score of 5 items; physician-related distress (PRD) as a score of 4 items; and regimen-related distress (RRD) as a score of 5 items.
Statistical Analysis
Descriptive statistics were used to summarize the baseline characteristics of the study population. Categorical variables were summarized counting frequency and percentages, while means and standard deviations were used for continuous variables. The Kolmogorov-Smirnov test was used for testing normality of the data distribution and variable not normally distributed were log-transformed. The significance of variable differences between visit 0 and visit 5 was tested by 2-sided Student’s t-test for paired samples (continuous variables) or by the McNemar’s test (categorical variables). Differences between the groups were tested by 2-sided Student’s t-test, Mann–Whitney t-test or χ2 test. The Pearson correlation coefficient or the Spearman’s rank correlation was used to test the relationship between the variables. The statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc, Chicago, IL, USA), and data are presented as mean ± SD. Differences were defined statistically significant if P < .05.
Results
All 346 enrolled patients completed the study. Patient characteristics are presented in Table 1. Patients with T1D were significantly younger than T2D patients group, while both groups (T1D and T2D) did not differ in respect to gender. However, T2D patients had higher BMI (P < .05) and waist circumference (P < .01) in comparison to T1D patients. The frequency of retinopathy was similar in both groups, but patients with T2D had more cardiovascular and ischemic brain disease, as well as peripheral artery disease and diabetic foot complications. However, the frequency of previously diagnosed nephropathy was higher in T1D patients, while the incidence and levels of microalbuminuria (UAE 30-300 mg/24h) were similar in both groups, respectively. Also, the investigated T1D and T2D patient groups did not differ in the incidence of macroalbuminuria (UAE > 300 mg/24h). Simultaneously, T2D patients had higher both systolic and diastolic blood pressure than T1D patients.
Table 1.
Baseline Patients Characteristics.
| All patients | T1D patients | T2D patients | |
|---|---|---|---|
| Number (m/f) | 346 (172/174) | 57 (34/23) | 289 (138/151) |
| Age (years) | 56.7 ± 11.1 | 39.8 ± 11.8 | 60.1 ± 7.3a |
| BMI (kg/m2) | 28.7 ± 5.0 | 24.7 ± 3.8 | 29.5 ± 4.8a |
| Waist (cm) | 99.4 ± 13.6 | 88.7 ± 11.9 | 101.7 ± 12.8b |
| Retinopathy (% pts) | 39.2 | 40.4 | 38.7 |
| Nephropathy (% pts) | 9.0 | 16.1 | 7.7a |
| Cardiovascular disease (% pts) | 20.5 | 7.0 | 23.4a |
| Ishemic brain disease (% pts) | 5.8 | 3.5 | 6.3 |
| Peripheral artery disease (% pts) | 31.0 | 21.1 | 32.3a |
| Diabetic foot (% pts) | 21.1 | 12.3 | 23.1a |
| UAE 30-300 (% pts) | 32.3 | 26.3 | 33.4 |
| UAE 30-300 mg/24h | 92.6 ± 6.3 | 91.8 ± 21.9 | 92.5 ± 6.6 |
| UAE > 300 (% pts) | 8.9 | 5.3 | 9.8 |
| Systolic BP (mmHg) | 133.6 ± 19.8 | 120.5 ± 16.2 | 136.3 ± 19.5 |
| Diastolic BP (mmHg) | 81.4 ± 9.9 | 75.9 ± 8.2 | 82.6 ± 9.8 |
Data are expressed as mean ± SD, unless otherwise noted. BP, blood pressure; pts, patients; T1D, type 1 diabetes; T2D, type 2 diabetes; UAE, urinary albumin excretion.
P < .05 vs T1D.
P < .01 vs T1D.
HbA1c Levels
Mean (± SD) HbA1c within the full cohort was significantly improved at 6 months versus baseline (8.85 ± 1.17% vs 7.91 ± 1.24%, P < .01). Improvements were more profound among T2D patients (8.85 ± 1.14 vs 7.82 ± 1.24%, P < .001) than T1D patients (8.87 ± 1.33 vs 8.41 ± 1.09%, P = .07) (Figure 1).
SMBG Frequency
Significant increases in average SMBG frequency per week were seen within the full cohort and T1D and T2D patients at 6 months versus baseline (14.6/week vs 4.3/week, P < .001) (Figure 2). The last visit (visit 5), a high percentage of all patients (95.7%) recorded at least 2 out of 3 daily blood glucose profile using the structured testing tool.
Figure 2.
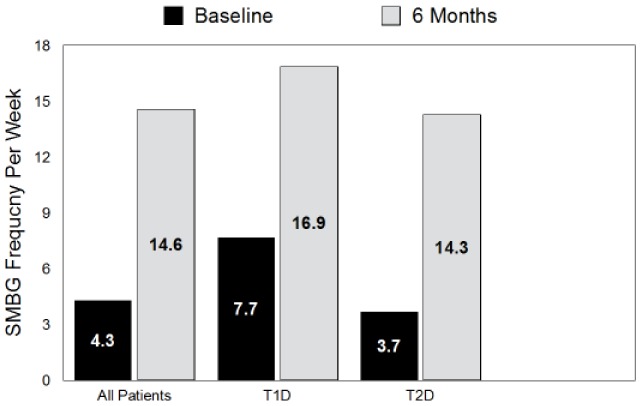
Change in SMBG frequency expressed as the number of blood glucose (bG) test strips used during the 1 week prior to visits 0 and visit 5. All changes P < .001.
Hospitalization Rates
The mean (± SE) number of hospitalizations due to metabolic conditions was significantly lower during the 6-month study compared to the 6-month period prior to the study (0.14 ± 0.04 vs 0.59 ± 0.09). Similarly, the rate of hospitalizations due to metabolic problems significantly decreased in both T1D (1.32 ± 0.46 vs 0.35 ± 0.23, P < .05) and T2D patients (0.46 ± 0.07 vs 0.10 ± 0.03, P < .001) during the study period. The number of hospitalizations due to diabetic complications significantly decreased only in T2D patients (0.28 ± 0.06 vs 0.11 ± 0.03, P < .05), but not in T1D patients (0.12 ± 0.09 vs 0.07 ± 0.03, P = ns) at month 6.
Cardiovascular Risk Factors
No significant changes in waist circumference, systolic and diastolic BP during the study period (Table 2). A small but statistically significant increase in total and LDL cholesterol levels at the end of the study period was observed, while the levels of HDL cholesterol and triglycerides remained the same. In T2D patients, total and LDL cholesterol significantly increased; however, a significant reduction in triglycerides and increase in HDL cholesterol was seen during the study period. Microalbuminuria levels decreased in T1D group and increased in T2D group but without statistical significance in either group. The percentage of patients with macroalbuminuria slightly increased in the T1D group and decreased in T2D group but without statistical significance.
Table 2.
Changes in Cardiovascular Risk Factors During the Study.
| T1D patients |
T2D patients |
|||
|---|---|---|---|---|
| V0 | V5 | V0 | V5 | |
| Waist (cm) | 88.7 ± 11.9 | 87.4 ± 11.9 | 101.7 ± 12.8 | 101.3 ± 11.9 |
| Systolic BP (mmHg) | 120.5 ± 16.2 | 121.4 ± 14.8 | 136.3 ± 19.5 | 136.6 ± 15.6 |
| Diastolic BP (mmHg) | 75.9 ± 8.2 | 77.1 ± 5.9 | 82.6 ± 9.8 | 82.3 ± 8.6 |
| Total chol (mmol/l) | 5.04 ± 0.89 | 5.55 ± 1.02a | 5.68 ± 1.35 | 5.84 ± 1.19a |
| LDL chol (mmol/l) | 2.92 ± 0.73 | 3.36 ± 0.91a | 3.31 ± 0.99 | 3.52 ± 0.98a |
| HDL chol (mmol/l) | 1.41 ± 0.38 | 1.49 ± 0.35 | 1.21 ± 0.38 | 1.26 ± 0.39a |
| Triglycerides (mmol/l) | 1.48 ± 0.79 | 1.56 ± 0.98 | 2.91 ± 2.44 | 2.52 ± 2.01a |
| UAE 30-300 mg/24h | 91.8 ± 21.9 | 65.3 ± 31.5 | 92.5 ± 6.6 | 120.4 ± 13.7 |
| UAE > 300 (% of pts) | 5.3 | 7.8 | 9.8 | 8.8 |
Data are expressed as mean ± SD, unless otherwise noted. BP, blood pressure; chol, cholesterol; pts, patients; T1D, type 1 diabetes; T2D, type 2 diabetes; UAE, urinary albumin excretion.
P < .01 vs V0.
Diabetes Distress Scale (DDS) Score
Total DDS scores in the full cohort were significantly lower at study end compared to baseline (P < .01) (Table 3). Significant improvements in emotional burden (EB) score (P < .01), physician-related distress (PRD) score (P < .01) and regimen-related distress (RRD) score (P < .01) were also seen. Similar favorable effects in all 4 scores were seen in T2D patients but significant improvements were not seen in T1D patients.
Table 3.
The Diabetes Distress Scale (DDS) Scores Before and at Study End.
| All patients |
T1D patients |
T2D patients |
||||
|---|---|---|---|---|---|---|
| V0 | V5 | V0 | V5 | V0 | V5 | |
| Total Diabetes Distress Scale (DDS) | 39.6 ± 13.9 | 33.9 ± 14.5a | 37.5 ± 13.1 | 35.4 ± 13.9 | 40.1 ± 14.1 | 33.7 ± 14.6a |
| Emotional burden (EB) score | 17.5 ± 7.0 | 15.3 ± 7.3a | 16.0 ± 6.8 | 15.6 ± 7.1 | 17.8 ± 7.0 | 15.3 ± 7.4a |
| Physician-related distress (PRD) score | 6.7 ± 4.1 | 5.9 ± 3.4a | 7.0 ± 3.5 | 5.8 ± 3.2 | 6.7 ± 4.2 | 6.0 ± 3.5a |
| Regimen-related distress (RRD) score | 15.4 ± 6.1 | 12.8 ± 6.0a | 14.2 ± 5.6 | 13.8 ± 5.9 | 15.6 ± 6.1 | 12.6 ± 6.0a |
P < .01 vs V0.
Analysis of Correlations
In T2D patients, baseline SMBG frequency negatively correlated with initial total DDS score (r = −.182, P < .01), EB score (r = −.135, P < .05), and RRD score (r = −.267, P < .001). In T1D patients, correlation was found only with PRD score (r = −.343, P < .01). In both T1D and T2D, baseline SMBG frequency at the study end (visit 5) correlated with initial hospitalization rate due to metabolic problems, in both T1D (r = .329, P < .05) and T2D patients (r = .198, P < .01).
In T2D, the number of the education models used for patient education, both at first and at last visit, significantly correlated with the final SMBG frequency (r = .202, P < .01 and r = .148, P < .05, respectively), but not in T1D patients.
In T2D patients, HbA1c level at the end of the study positively correlated with initial total DDS score (r = .168, P < .01), EB score (r = .149, P = .05), and RRD score (r = .212, P < .001), as well as total DDS score (r = .242, P < .001), EB score (r = .255, P = .001), and RRD score (r = .258, P < .001) at the study end. In contrast, significant correlation between final HbA1c level and the most of the DDS scores, except with RRD score were not seen in T1D patients (r = .403, P < .01).
Final HbA1c levels significantly correlated with hospitalization rates due to metabolic problems at the study end, both in T1D (r = .403, P < .01) and T2D patients (r = .154, P < .05).
Discussion
Our study showed that use of structured SMBG in combination with comprehensive diabetes education and self-management training is associated with improved glycemic control and fewer hospitalizations and reduced diabetes-related distress. Moreover, our results showed that this intervention reduced diabetes-related distress and increased adherence to treatment regimens. In addition, the high percentage of patients who utilized the structured testing tool suggests an increased level of patient engagement with their treatment.
Several limitations of this study are noteworthy. First, patients’ participation in the in-hospital educational program likely impacted both metabolic and quality-of-life measures. Therefore, it is not possible to parse out the individual effects of either in-hospital education or structured SMBG. It is noteworthy, however, that results from meta-analyses of studies that assessed the impact of structured diabetes education on glycemic control showed improvements in HbA1c ranging from −0.32%26 to −0.76%.27 In our analysis, HbA1c improvements ranged from −0.46% in T1D patients to 1.03% in T2D patients, which suggests that structured SMBG in combination with education had an additive effect. Another limitation is the provision of free blood glucose meters and test strips to the T2D study patients. In Serbia, T1D patients receive free meters and test strips with a limit of 50 strips per month for adults and 150 test strips per month for children and pregnant women. T2D patients are responsible for 100% of blood glucose monitoring costs. In addition, use of a randomized, controlled study design would have allowed a more conclusive assessment of the impact of structured SMBG in combination with education.
A key strength of our study is that it was conducted in primary care practices, which suggests that the intervention is practical in real world clinical settings and can be used effectively in both T1D and T2D populations by clinicians nonspecialized in diabetes. In addition, our results are consistent with findings from several recent studies of both insulin-treated and non-insulin-treated populations in which SMBG data were gathered in a structured manner and then utilized to make appropriate treatment changes,14-21 thereby suggesting a high level of external validity.
Conclusions
Use of structured SMBG in combination with intensive education was associated with clinically significant reductions in HbA1c, increased SMBG frequency, fewer hospitalizations, and improved quality of life. Given the increasing worldwide prevalence of diabetes worldwide, it is important that treatment tools and approaches are utilized effectively to facilitate improved clinical outcomes and reduce the costs associated with poorly managed diabetes. Contrary to random or unfocussed glucose monitoring, structured SMBG has been shown to be a valuable, practical component of effective diabetes management in real-world clinical settings. Additional studies are needed to elucidate the how structured SMBG can be used most effectively in various patient populations and practice settings.
Acknowledgments
The authors wish to thank the SPA-EDU Study Group for their assistance in recruting participants and coordinating the study at their research centers: * SPA-EDU Study Group: D. Radivojević, N. Lazarević Pejović, Vrnjacka Spa; J. Radin, Novi Sad; S. Stošić, Indjija; R. Kormanjoš, Zrenjanin; A. Mitrić, Pančevo; R. Mihajlović, Valjevo; S. Simić, Kruševac; Lj. Nozinić Vilus, Šabac; B. Manić, Požarevac; Ž. Tutunović Čačak; M. Barlov, Novi Pazar; M. Marković, Užice; S. Zlatković, Niš; S. Mišić, Vranje; S. Mitić, Leskovac; M. Petković Košćal, T. Kojić, B. Djurić Pejović, D. Vasić, D. Ilić, Lj. Čukvas, Z. Cvijan, M. Lapčević, Lj. Marković, I. Damjanović, Belgrade; M. Aleksić, Zemun; V. Jelenić, Barajevo; D. Belović, Lazarevac; Lj. Janošević Nešić, Mladenovac; M. Nedeljković, Obrenovac.
Footnotes
Abbreviations: bG, blood glucose; EB, emotional burden; HbA1c, glycated hemoglobin; PRD, physician-related distress; RRD, regimen-related distress; SMBG, self-monitoring of blood glucose; STeP, Structured Testing Program; T1D, type 1 diabetes; T2D, type 2 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NL reports no disclosures. CP has received consulting fees from Roche Diagnostics GmbH.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the study was provided by Roche Diagnostics GmbH, Mannheim, Germany.
References
- 1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853. [PubMed] [Google Scholar]
- 3. Lange K. Therapieadhärenz bei typ 1 Diabetes. Herausforderung, Barrieren und Chancen. Der Diabetologe. 2012;1:1-10. [Google Scholar]
- 4. Pyatak EA, Florindez D, Weigensberg MJ. Adherence decision making in the everyday lives of emerging adults with type 1 diabetes. Patient Prefer Adherence. 2013;7:709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medscape physician compensation report: 2012 results. Available at: http://www.medscape.com/features/slideshow/compensation/2012/public. Accessed May 5, 2014.
- 6. Berg J, Dodd SD. The role of a community pharmacist in diabetes education: practice matters. SA Pharmaceutical J. 2010;55:55-57. [Google Scholar]
- 7. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834. [DOI] [PubMed] [Google Scholar]
- 8. Peyrot M, Rubin RR, Lauritzen T, et al. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. 2005;22:1379-1385. [DOI] [PubMed] [Google Scholar]
- 9. Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28:337-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNabb WL. Adherence in diabetes: can we define it and can we measure it? Diabetes Care 1997;20:215-218. [DOI] [PubMed] [Google Scholar]
- 11. Parkin CG, Buskirk A, Hinnen DA, Axel-Schweitzer M. Results that matter: structured vs. unstructured self-monitoring of blood glucose in type 2 diabetes. Diabetes Res Clin Pract. 2012;97(1):6-15. [DOI] [PubMed] [Google Scholar]
- 12. International Diabetes Federation Clinical Guidelines Taskforce, SMBG International Working Group. Global guideline on self-monitoring of blood glucose in non-insulin treated type 2 diabetes. 2009. Available at: www.idf.org.
- 13. International Diabetes Federation. Position statement: self-management education. Available at: http://www.idf.org/education/self-management-education. Accessed January 21, 2015.
- 14. Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2:203-211. [DOI] [PubMed] [Google Scholar]
- 15. Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34:262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabet Med. 2011;28:789-796. [DOI] [PubMed] [Google Scholar]
- 17. Bonomo K, De Salve A, Fiora E, et al. Evaluation of a simple policy for pre- and post-prandial blood glucose self-monitoring in people with type 2 diabetes not on insulin. Diabetes Res Clin Pract. 2010;87:246-251. [DOI] [PubMed] [Google Scholar]
- 18. Kempf K, Kruse J, Martin S. ROSSO-in-praxi: a self-monitoring of blood glucose-structured 12-week lifestyle intervention significantly improves glucometabolic control of patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2010;12:547-553. [DOI] [PubMed] [Google Scholar]
- 19. Mohan V, Ravikumar R, Poongothai S, et al. A single-center, open, comparative study of the effect of using self-monitoring of blood glucose to guide therapy on preclinical atherosclerotic markers in type 2 diabetic subjects. J Diabetes Sci Technol. 2010;4:942-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kato N, Cui J, Kato M. Structured self-monitoring of blood glucose reduces glycated hemoglobin in insulin-treated diabetes. J Diabetes Investigation. 2013;4:450-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shiraiwa T, Takahara M, Kaneto H, et al. Efficacy of occasional self-monitoring of postprandial blood glucose levels in type 2 diabetic patients without insulin therapy. Diabetes Res Clin Pract. 2010;90:e91-e92. [DOI] [PubMed] [Google Scholar]
- 22. Fisher L, Polonsky W, Parkin CG, et al. The impact of blood glucose monitoring on depression and distress in insulin-naive patients with type 2 diabetes. Curr Med Res Opin. 2011;27(Suppl 30:39-46. [DOI] [PubMed] [Google Scholar]
- 23. Fisher L, Polonsky WH, Parkin CG, et al. The impact of structured blood glucose testing on attitudes toward self-management among poorly controlled, insulin-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;96(2):149-155. [DOI] [PubMed] [Google Scholar]
- 24. Lalic N, Tankova T, Nourredine M, et al. Value and utility of structured self-monitoring of blood glucose in real world clinical practice: findings from a multinational observational study. Diabetes Technol Ther. 2012;14:338-343. [DOI] [PubMed] [Google Scholar]
- 25. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631. [DOI] [PubMed] [Google Scholar]
- 26. Ellis SE, Speroff T, Dittus RS, et al. Diabetes patient education: a meta-analysis and meta-regression. Patient Educ Couns. 2004;52:97-105. [DOI] [PubMed] [Google Scholar]
- 27. Norris SL, Lau J, Smith SJ, et al. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159-1171. [DOI] [PubMed] [Google Scholar]


