Abstract
Insulin remains indispensable to the treatment of diabetes, but its availability in injectable form only has hampered its timely and broader use. The development of an oral insulin remains an ultimate goal to both enhance ease of use, and to provide therapeutic advantages rooted in its direct delivery to the portal vein and liver. By mimicking the physiological path taken by pancreatic insulin, oral insulin is expected to have a distinct effect on the hepatic aspect of carbohydrate metabolism, hepatic insulin resistance, and, at the same time, avoid hyperinsulinemia and minimize the risk of hypoglycemia. With oral insulin approaching late stages of development, the goal of this review is to examine oral insulin in a physiological context and report on recent progress in its development.
Keywords: oral insulin, type 1 diabetes, type 2 diabetes, hypoglycemia, glycemic stability
Despite the wide selection of available antidiabetic pharmaceuticals and new classes of agents, since its discovery, insulin has remained the mainstay drug for treating type 1 diabetes mellitus (T1DM) and advanced type 2 diabetes mellitus (T2DM), and as such, has served to save a countless number of lives and prevent serious complications.1,2,3-5 Although insulin remains the single therapy with unlimited potential to safely achieve glucose control in most patients with T2DM,6 for many patients and providers, it remains a last resort venue, with enormous negative connotations, this owing to the discomfort and adverse effects associated with the most commonly administered injectable forms. An exciting alternative to parenteral administration of insulin delivery via the pulmonary route has been clinically available since June 2014.7
While still theoretical, yet compelling nonetheless, an oral route of insulin delivery may bear physiologic implications, which could significantly reduce the risk of hypoglycemia, while eliciting salient metabolic effects without weight gain. Moreover, oral insulin, devoid of the apprehension and distress associated with insulin injections, may bring this drug from last resort therapy to the forefront. At the present time, evidence suggesting the possible advantages of oral insulin can mostly be inferred from data generated from studies with intraperitoneal8-12 and intraportal insulins,13-16 which follow a similar route of absorption through the portal vein, and more recently, hepato-preferential insulins.17-19 Yet, insulin’s molecular mass of 5808 Da, and its physicochemical properties, hinder its intestinal absorption, posing a challenge for its oral delivery. Advances in the understanding of intestinal drug absorption and in drug delivery science may overcome the challenges of insulin absorption through the gastrointestinal tract in the not too distant future. While the topic of oral insulin has been extensively reviewed including in this journal,20,21 the purpose of this review is to present and emphasize the potential physiological advantages of an oral insulin preparation and to provide a current state of affairs in regard to oral insulin.
Potential Clinical Benefits of Oral Insulin
Oral Insulin and Its Relevance to Diabetic Hyperglycemia
Insulin secreted from pancreatic β-cells promotes glucose disposal through stimulation of glucose uptake and subsequent intracellular oxidative and nonoxidative metabolism in insulin-sensitive tissues and organs. Ingestion of a meal containing carbohydrates, elicits a prompt rise in insulin and a decrease in glucagon concentrations, both potentiated by intestinal L cell-secreted glucagon-like peptide 1 (GLP-1). In parallel, intestinal K cell-secreted gastric inhibitory polpeptide (GIP) stimulates glucagon release. From the portal vein, insulin passes through the liver, where up to 80% is extracted on first-pass, giving rise to a significantly (2.5- to 3-fold) higher insulin concentration in the portal vein as compared to the systemic circulation (Figure 1).22,23 This portal–peripheral gradient is maintained both in the fasting state (basal) and postprandial state,24 leaving the liver constantly exposed to significantly higher insulin concentrations as compared with other organs/tissues. The fraction of insulin extracted by the liver is dynamic, varying in accordance with metabolic demands to maintain optimal peripheral insulinization, while also securing sufficient insulinization of the liver.25 This dynamic process is further reinforced by insulin’s short half-life of 4-6 min in the circulation, which simplifies fine-tuning of insulin release into the systemic circulation, avoiding peaks and troughs in insulin concentrations and in glycemic excursions.
Figure 1.
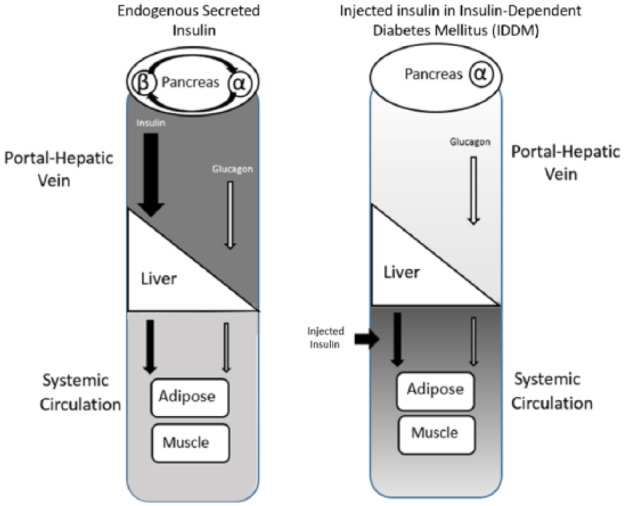
The pathways and targets of secreted versus subcutaneously injected insulin. The differences in routes taken by and targets of secreted versus subcutaneously delivered insulin may play a role in iatrogenic hypoglycemia, weight gain and glycemic variability. Left panel: The physiologic pathway and hepatic clearance of endogenous insulin. Following caloric intake, insulin (black arrows) is secreted from β-cells at concentrations sufficient to partially suppress secretion of glucagon (white arrows) from α-cells (paracrine action). The insulin and glucagon flow into the portal-hepatic vein at a ratio that allows for glucose disposition by the liver and peripheral tissue. Up to 80% of the secreted insulin is taken up by the liver and the rest reaches the systemic circulation, creating a portal/peripheral insulin gradient. Only a small fraction of glucagon is taken up by the liver. Due to receptor binding and its short plasma half-life, insulin is rapidly cleared from the circulation. Right panel: Insulin delivered by injection is ferried to the systemic circulation, with equal distribution in tissues; a portal/peripheral insulin gradient is absent. Lack or insufficient insulin levels at the islet level lead to inadequate suppression of glucagon secretion, resulting in hyperglucagonemia, and a perturbed insulin/glucagon ratio in the portal vein. In consequence, the balance between hepatic glucose production and storage of hepatic glycogen is disrupted, yielding hyperglycemia. Attempts to control the resulting hyperglucagonemia and resulting hyperglycemia with intensification of treatment by increasing the doses of injected insulin, may cause hypoglycemia.40
The liver maintains plasma glucose concentrations within a narrow range, by sequestering ingested glucose after a meal (postprandial) and releasing glucose in response to low glucose levels (e.g., fasting state). Fasting hyperglycemia is the sequela of increased or unconstrained hepatic glucose release resulting from insufficient insulin secretion in T1DM or in the context of relative hepatic insulin resistance in T2DM,26-29 whereas postprandial hyperglycemia is mainly due to disproportionately high endogenous glucose production resulting from its reduced suppression and/or decreased glucose clearance in tissues.30 Thus, sufficient hepatic insulinization is indispensably needed to suppress hepatic glucose production and to reduce both fasting and postprandial hyperglycemia.
Hepatic glucose production is discerningly sensitive to changes in insulin levels and thus can be controlled by a minute increase in hepatic insulinization.31-33
An oral insulin product is predicted to have therapeutic advantages in the management of hepatic glucose production, via its potential to mimic the natural route of endogenous insulin secreted by the pancreas. After reaching the portal vein, the oral insulin is directly delivered to the liver and then to the peripheral circulation, thereby reestablishing the physiologic portal–peripheral insulin gradient and providing for adequate hepatic insulinization. In contrast, parenteral or inhaled insulin is absorbed directly into the peripheral circulation without initial hepatic extraction, and fails to restore the portal-peripheral insulin gradient and physiologic hepatic insulinization. In addition, these routes expose peripheral targets to greater insulin concentrations relative to the liver, predisposing patients to a high risk of hypoglycemia, and the deleterious effects of hyperinsulinemia.
Mitigating Risk of Hypoglycemia
Hypoglycemia is one of the most common and feared iatrogenic side effects faced by individuals with diabetes, especially in those receiving intensive therapy, and is a known barrier to the glycemic management of diabetes.34 The serious morbidity associated with hypoglycemia, and its fatal potential, apply to both T1DM and T2DM patients, as the pathophysiology of glucose counterregulation is the same in both. Furthermore, recurring episodes of hypoglycemia can impair defenses against subsequent incidents, by causing hypoglycemia-associated autonomic failure and hypoglycemia unawareness, thereby perpetuating a vicious cycle of recurrent hypoglycemia.35 In addition, the concern over hypoglycemia often precludes maintenance of ideal glycemic control and in many individuals, triggers overeating and weight gain, and thus thwarts full realization of the therapeutic value of exogenous insulin.
Glucagon acts as a counterregulatory hormone to insulin by rapidly promoting hepatic glucose output via conversion of liver glycogen into circulating glucose. In healthy individuals, falling glucose levels triggers glucagon secretion, a response that is often blunted or absent in T1DM and in advanced T2DM and, as a result, increases the risk of hypoglycemia.36
Under normal conditions, insulin and glucagon operate in concert to maintain the glucose level within a narrow physiological range.37 The insulin/glucagon ratio the hepatocyte is exposed to will dictate if it will be induced to store or supply nutrients such as glucose and lipids.38 The ratio is determined by the rate of secretion of these hormones from the α-cells and β-cells, which reside contiguously within the islets of Langerhans (islets) (Figure 1) and share the same interstitial space. By a paracrine action, insulin secreted from β-cells reciprocally regulates α-cell glucagon secretion, creating a secretory alliance, and generating the insulin/glucagon ratio optimal for maintained glycemic balance.39 When exogenous insulin is necessary, injected forms distribute evenly throughout the body, in contrast to secreted insulin, which displays acute peaks (almost ~400 times higher) in the islets compared with the systemic concentration.40 Therefore, suppression of glucagon secretion by injected insulin is impractical and exposes patients to the risk of hypoglycemia.40 However, improving the ratio is attainable by increasing the insulin concentration in the portal vein.41,42 There is strong evidence that such a strategy can significantly reduce the occurrence of hypoglycemic events.13,43,44 Alternatively, the insulin/glucagon ratio can be increased by lowering glucagon levels, an approach that is drawing much attention and has spurred drug development research.40,45
Glycemic Stability
Much attention has recently been paid to the impact of glycemic variability, independent of hyperglycemia, on risk for diabetic complications.46,47 Studies in animal models and in humans suggest that oscillating glucose levels are more detrimental than stable high glucose concentrations, and have been correlated with production of free radicals, accompanied by an insufficient increase in intracellular antioxidant defenses.48,49
While glycemic variability appears to result from the complex interplay between behavioral, psychological, and treatment-related factors, its pathophysiology remains unclear. It is posited that the pathophysiology of glycemic variability hinges on α-cell and/or β-cell dysfunction, resulting in an imbalance of the portal insulin-to-glucagon ratio.50,51 More specifically, both insulin deficiency and impaired glucagon signaling, both of which affect hepatic glucose production and modify hepatic glucose uptake and storage, translate to glycemic swings.52,53 The net result is that more glucose (endogenous + ingested) enters the circulation at a faster rate than the body can assimilate, yielding prolonged elevation of plasma glucose levels.54,55 In parallel, glucose nadirs can result from malabsorption, interactions with concomitant drugs, defective insulin degradation, and delayed gastric emptying as a result of autonomic neuropathy. In addition, low glucose levels are often attributed to defective glucagon signaling, which adversely affects glycogenolysis and gluconeogenesis. Consequently, hepatic glycogen stores are lacking, limiting the ability of patients with diabetes to appropriately respond to low glucose levels.56-58 Studies involving direct portal administration of insulin have demonstrated a significant attenuation of glycemic swings and stabilization of glycemia.59-62 In the first recorded pilot study assessing the effect of oral insulin as an add-on therapy, on glycemic stability in eight uncontrolled T1DM patients on subcutaneous insulin therapy, a significant reduction in the amplitude of glycemic excursions and glucose area under the curve was observed. Nevertheless, due to the small sample size and short follow-up period, the clinical relevance of these findings remains speculative (Clinicaltrials.gov NCT00867594).63
Mitigating Weight Gain
The association between insulin therapy and weight gain is well known64,65 and its magnitude is influenced by the intensity and duration of the insulin regimen, level of the initial glycemic control, the glycemic control achieved with treatment, and the combination of oral agents concomitantly used.66 Many patients opt to delay treatment initiation or fail to exhibit long-term compliance due to this adverse effect of insulin.67 Furthermore, weight gain is, by itself, associated with increased risk of cardiovascular disease, worsening insulin resistance and dyslipidemia, and can also fuel a vicious cycle of beta-cell dysfunction, further aggravating insulin resistance, increasing insulin requirement and leading to further weight gain.68 Several mechanisms have been proposed to explain the insulin-weight gain correlation associated with the nonphysiologic route of insulin administration as the result of systemic hyperinsulinemia leading to a disproportional anabolic effect on muscle and adipose tissue.64,65 Adequate hepatic insulinization without systemic hyperinsulinization, achieved by means of sulfonylureas,69 peritoneally delivered insulin,70 or with hepatoselective insulin,71 has shown that the route of insulin delivery has a strong bearing on weight control.
Potential Benefits of Portal Insulin Administration Beyond Glycemic Control
Insulin delivery directly to the liver has demonstrated salient effects on a wide range of processes, extending beyond glycemic control. Such effects have been observed with intraperitoneal insulin infusions,10,11,72 direct intraportal insulin administration,13 and hepatoselective insulins, as well as with long-acting parenteral insulin with a circulating depot, such as insulin detemir, which appears to possess an increased liver specificity.18,71,73 Insulin increases the sensitivity of the liver to growth hormone (GH) by upregulating GH receptor expression, thereby augmenting insulin-like growth factor-1 (IGF-1) production.74 In addition, it downregulates IGF binding protein-1 (IGFBP-1) production in the liver, thereby increasing circulating IGF-1 bioactivity.75 Thus, portal insulinopenia, as seen in diabetes, is implicated by perturbations in GH bioactivity, worsening glucose intolerance and disrupted lipid metabolism.76 Studies have shown that delivering insulin by continuous intraperitoneal insulin infusion (CIPII) or intraportally, as opposed to subcutaneously (SC), in patients with T1DM, had a beneficial effect on the GH–IGF1–IGFBP axis.15,77-79
Another example pertains to sex hormone–binding globulin (SHBG), which is produced in the liver and regulates the concentrations of freely circulating sex hormones. High SHBG levels lower the proportion of bioavailable sex hormones, such as estradiol and testosterone, and influences the relative balance of estradiol to testosterone through bidirectional feedback.80 In male children and young adults with T1DM, SHBG and total testosterone levels appear to be significantly higher than in controls.81 Moreover, adult men with controlled T1DM have a higher risk for hypogonadism, as reflected by lower free testosterone and higher SHBG levels.82 In T2DM, a reduction in total testosterone, including both bioavailable and free testosterone, is observed.83,84 Portal insulin has been shown to downregulate SHBG, irrespective of glycemic control.85-87
The Challenges of Polypeptide Absorption Following Oral Delivery
The major challenges in the oral delivery of peptide and protein (p/p) drugs is their susceptibility to acid hydrolysis in the stomach, proteolytic degradation in the intestine, limited permeability across membranes and their tendency to complex and adsorb to the gut.88 The process of proteolysis is a physiologic and efficient mechanism that enables the digestion of proteins in food and also plays a role in the inactivation of some organisms. Efforts to enhance p/p drug absorption have concentrated on methods which protect the drug during transit in the gastric environment and/or inhibition of proteolysis in the gut. Other technologies involve micronization, absorption enhancement and carrier-mediated transport enhancement, all of which allow for the sizable molecules to cross the epithelium either via the paracellular or transcellular route.89,90 The current status of insulin development, in general, and of oral insulin, in particular, has recently been reviewed.21,91,92 Two oral insulin development programs have gained visibility of recent.91 Oramed Pharmaceuticals Inc. (Jerusalem, Israel) has developed its proprietary Protein Oral Delivery (POD™) technology, which employs a three-pronged approach composed of encapsulation, protease inhibitors and a chelating agent. The pH-sensitive capsule shields the insulin from hydrolysis in the stomach and ascertains that the protein and other additives within the formulation are contemporaneously released in the small intestines, where the pH is close to neutral. The protease inhibitors serve to protect the insulin from degradation by the ubiquitous proteases in the brush border zone of the small intestines. The chelating agent scavenges calcium, an important cofactor for many proteases, and thereby inhibits intestinal enzyme activities, while also increasing paracellular permeability. In subjects with T1DM, Oramed’s oral insulin has been shown to reduce postprandial glucose concentrations63 and when administered preprandially, to reduce both fasting blood glucose levels and the requirement for fast-acting insulin doses.93 In subjects with T2DM, Oramed’s oral insulin led to a reduction in fasting blood glucose levels94 and a decrease of inflammatory marker (c-reactive protein; CRP) levels in response to a six-week, once-daily, bedtime oral insulin regimen.63 In a recently completed phase IIb trial with oral insulin capsules in adults with T2DM, a significant lowering of mean nighttime glucose levels as compared to their average levels during the run-in period, was observed as well as a reduction in mean 24-hour glucose, fasting glucose and daytime glucose (unpublished data).
Novo Nordisk A/S (Denmark) has conducted five phase I clinical trials (NCT02470039, NCT02304627, NCT01931137, NCT01796366, and NCT01334034) with oral insulins (NN1953, NN1954, and NN1956) to treat T1DM and T2DM95 and has completed a phase 2 study with an oral insulin (unpublished). The drug delivery technology used is Merrion Pharmaceuticals’ proprietary formulations, collectively referred to as gastrointestinal permeation enhancement technology (GIPET™). The technology is based on microemulsions of oil and surfactant or a mixture of fatty-acid derivatives in an enteric-coated gel capsule. This absorption enhancer system has shown to safely increase the oral bioavailability of several types of low-permeability compounds in man.96
The Future
The realization of a safe and effective oral insulin dosage form will undoubtedly be a major advance in the field of diabetology. Yet, there remain aspects requiring further studies and evaluations.97 Due to its potency and narrow therapeutic window, to be effective and safe, insulin doses must be reasonably titratable, with a consistent and reproducible absorption index, both within and among subjects. Even now, current parenteral insulin therapies continue to suffer from serious deficiencies owing to inconsistency of therapeutic action from dose to dose and from patient to patient, and thus only infrequently normalizes blood glucose in chronic use.98,99 To a large extent, its pharmacodynamic effect reflects the significant variability in its absorption, that can range from 20% to over 55% with injectable insulin.100,101 Absorption variability, inherent to oral ingested medications, is likely to be amplified when bioavailability is low. Current oral peptides and proteins delivery technologies are typified by relatively low bioavailability, estimated at 5-8% for Oramed’s oral insulin.102 Furthermore, considerations and surveillance of the effects of the large amounts of unabsorbed drug lingering in the intestines, particularly regarding insulin, a growth factor with mitogenic potential103 and a recognized modulator of gastrointestinal physiology will be required.104 Moreover, given that the effect of oral insulin will be mainly on the liver, such a preparation is unlikely to have a potent effect on glucose disposal in the periphery and on controlling free fatty acid lipolysis in adipose tissue. While it is improbable that it will replace injectable insulin in insulin-dependent individuals, an oral insulin dosage form may serve as a stand-alone drug for patients in the early stages of T2DM with impaired fasting blood glucose, largely the outcome of excess hepatic glucose production. In addition, it may be advantageous as an adjuvant to antihyperglycemic drugs (e.g., insulin sensitizers), prescribed to contend with insulin insufficiency, or when hepatic insulin resistance is driven by a pathogenic mechanism. Oral insulin may also be effective in reducing glucose instability, such as often seen in unstable T1DM, by modifying the insulin-to-glucagon ratio in the portal vein to favor hepatic glucose sequestration and restoration of glycogen stores. In such a case, oral insulin will not only dampen glucose excursions, but may also replenish glycogen stores in the liver, which is important in hypoglycemia, to allow for rapid glycogenolysis and glucose infusion into the circulation.
Given the magnitude of diabetes and the heterogeneity of its pathophysiology, it is likely that no single drug or delivery method will meet the needs of all patients. It is therefore essential that oral insulin will be optimally positioned to address the specific pathophysiologic aspects of glucose intolerance, where its use may have the greatest impact to improve outcome.
Conclusion
Insulin remains the one therapy with unlimited potential to safely achieve glucose control in most patients with diabetes. Acceptance and compliance of insulin therapy in patients with T2DM is wanting. The oral dosage form is generally the preferable and safest route for drug delivery. An oral insulin, often referred to as the holy grail, being patient friendlier and with theoretical physiologic advantages, may foster adherence and compliance, to result in superior outcomes. However, its relatively low bioavailability and consequently high absorption variability, remain a challenge to be overcome. While the first century of insulin therapy focused on supply, purification, and improved pharmacokinetics, the future will likely focus on development of more user-friendly and physiologic formulations that will minimize the risk of hypoglycemia, weight gain and other insulin therapy-associated complications. Early-stage development programs of oral insulin are under way and appear promising; challenges remain, but seem surmountable.
Acknowledgments
The authors would like to acknowledge and thank Yehudit Posen, PhD for her invaluable editorial assistance.
Footnotes
Abbreviations: CIPII, continuous intraperitoneal insulin infusion; CRP, c-reactive protein; GH, growth hormone; GIP, gastric inhibitory polpeptide; GIPET, gastrointestinal permeation enhancement technology; GLP-1, glucagon-like peptide 1; IGFBP-1, IGF binding protein-1; IGF-1, insulin-like growth factor-1; POD, Protein Oral Delivery; p/p, peptide and protein; SC, subcutaneous; SHBG, sex hormone–binding globulin; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MK is an employee of Oramed Pharmaceuticals Inc. and holds shares in the company. EA is not affiliated with Oramed and holds no shares in the company.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group: intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53(3):298-304. [DOI] [PubMed] [Google Scholar]
- 4. Turnbull F, Abraira C, Anderson R, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288-2298. [DOI] [PubMed] [Google Scholar]
- 5. Zhang C-Y, Sun A-J, Zhang S-N, et al. Effects of intensive glucose control on incidence of cardiovascular events in patients with type 2 diabetes: a meta-analysis. Ann Med. 2010;42(4):305-315. [DOI] [PubMed] [Google Scholar]
- 6. Leahy JL. Insulin therapy in type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2012;41(1):119-144. [DOI] [PubMed] [Google Scholar]
- 7. Segal AR, Vootla T, Beaser RS. Insulin: making sense of current options. Endocrinol Metab Clin North Am. 2016;45(4):845-874. [DOI] [PubMed] [Google Scholar]
- 8. Spaan NA, Teplova AE, Renard E, Spaan JA. Implantable insulin pumps: an effective option with restricted dissemination. Lancet Diabetes Endocrinol. 2014;2(5):358-360. [DOI] [PubMed] [Google Scholar]
- 9. Spaan N, Teplova A, Stam G, Spaan J, Lucas C. Systematic review: continuous intraperitoneal insulin infusion with implantable insulin pumps for diabetes mellitus. Acta Diabetologica. 2014;51(3):339-351. [DOI] [PubMed] [Google Scholar]
- 10. van Dijk PR, Logtenberg SJ, Gans RO, Bilo HJ, Kleefstra N. Intraperitoneal insulin infusion: treatment option for type 1 diabetes resulting in beneficial endocrine effects beyond glycaemia. Clin Endocrinol (Oxf). 2014;81(4):488-497. [DOI] [PubMed] [Google Scholar]
- 11. Schade DS, Eaton RP, Friedman NM, Spencer WJ. Normalization of plasma insulin profiles with intraperitoneal insulin infusion in diabetic man. Diabetologia. 1980;19(1):35-39. [DOI] [PubMed] [Google Scholar]
- 12. Logtenberg SJ, Kleefstra N, Houweling ST, et al. Improved glycemic control with intraperitoneal versus subcutaneous insulin in type 1 diabetes: a randomized controlled trial. Diabetes Care. 2009;32(8):1372-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shishko PI, Kovalev PA, Goncharov VG, Zajarny IU. Comparison of peripheral and portal (via the umbilical vein) routes of insulin infusion in IDDM patients. Diabetes. 1992;41(9):1042-1049. [DOI] [PubMed] [Google Scholar]
- 14. Dijk PRv, Logtenberg SJJ, Chisalita SI, et al. Different effects of intraperitoneal and subcutaneous insulin administration on the GH-IGF-1 axis in type 1 diabetes. J Clin Endocrinol Metab. 2016;101(6):2493-2501. [DOI] [PubMed] [Google Scholar]
- 15. Hanaire-Broutin H, Sallerin-Caute B, Poncet MF, et al. Effect of intraperitoneal insulin delivery on growth hormone binding protein, insulin-like growth factor (IGF)-I, and IGF-binding protein-3 in IDDM. Diabetologia. 1996;39(12):1498-504. [DOI] [PubMed] [Google Scholar]
- 16. Jeandidier N, Selam JL, Renard E, et al. Decreased severe hypoglycemia frequency during intraperitoneal insulin infusion using programmable implantable pumps. Evadiac Study Group. Diabetes Care. 1996;19(7):780. [DOI] [PubMed] [Google Scholar]
- 17. Herring R, Jones RH, Russell-Jones DL. Hepatoselectivity and the evolution of insulin. Diabetes Obes Metab. 2014;16(1):1-8. [DOI] [PubMed] [Google Scholar]
- 18. Herring R, Shojaee-Moradie F, Umpleby A, Jones R, Jackson N, Russell-Jones D. Effect of subcutaneous insulin detemir on glucose flux and lipolysis during hyperglycaemia in people with type 1 diabetes. Diabetes Obes Metab. 2015;17(5):459-467. [DOI] [PubMed] [Google Scholar]
- 19. Edgerton DS, Moore MC, Winnick JJ, et al. Changes in glucose and fat metabolism in response to the administration of a hepato-preferential insulin analog. Diabetes. 2014;63(11):3946-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heinemann L, Jacques Y. Oral insulin and buccal insulin: a critical reappraisal. J Diabetes Sci Technol. 2009;3(3):568-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fonte P, Araújo F, Reis S, Sarmento B. Oral insulin delivery: how far are we? J Diabetes Sci Technol. 2013;7(2):520-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eaton RP, Allen RC, Schade DS. Hepatic removal of insulin in normal man: dose response to endogenous insulin secretion. J Clin Endocrinol Metab. 1983;56(6):1294-1300. [DOI] [PubMed] [Google Scholar]
- 23. Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes. 1984;33(5):486-494. [DOI] [PubMed] [Google Scholar]
- 24. Cherrington AD. Control of glucose production in vivo by insulin and glucagon. Comprehensive Physiology. 2011;759-785. doi: 10.1002/cphy.cp070225 [DOI] [Google Scholar]
- 25. Bergman R. Non-esterified fatty acids and the liver: why is insulin secreted into the portal vein? Diabetologia. 2000;43(7):946-952. [DOI] [PubMed] [Google Scholar]
- 26. DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982;31(9):795-801. [DOI] [PubMed] [Google Scholar]
- 27. Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59(11):2697-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dunning BE, Gerich JE. The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007;28(3):253-283. [DOI] [PubMed] [Google Scholar]
- 29. Vaag A, Alford F, Henriksen F, Christopher M, Beck-Nielsen H. Multiple defects of both hepatic and peripheral intracellular glucose processing contribute to the hyperglycaemia of NIDDM. Diabetologia. 1995;38(3):326-336. [DOI] [PubMed] [Google Scholar]
- 30. Firth R, Bell P, Marsh H, Hansen I, Rizza RA. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986;77(5):1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edgerton DS, Cardin S, Emshwiller M, et al. Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes. 2001;50(8):1872-82. [DOI] [PubMed] [Google Scholar]
- 32. Edgerton DS, Lautz M, Scott M, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest. 2006;116(2):521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes. 2004;53(8):2042-2050. [DOI] [PubMed] [Google Scholar]
- 34. Cryer P. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937-948. [DOI] [PubMed] [Google Scholar]
- 35. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54(12):3592-3601. [DOI] [PubMed] [Google Scholar]
- 36. Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2011;153(3):1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maruyama H, Hisatomi A, Orci L, Grodsky G, Unger R. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74(6):2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Satake S, Moore MC, Igawa K, et al. Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes. 2002;51(6):1663-71. [DOI] [PubMed] [Google Scholar]
- 39. Wang Q, Liang X, Wang S. Intra-islet glucagon secretion and action in the regulation of glucose homeostasis. Frontiers Physiol. 2013;3:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davidson JA, Holland WL, Roth MG, et al. Glucagon therapeutics, dawn of a new era for diabetes care. Diabetes Metab Res Rev. 2016;32(7):660-665. [DOI] [PubMed] [Google Scholar]
- 41. Gregory JM, Kraft G, Scott MF, et al. Insulin delivery into the peripheral circulation: a key contributor to hypoglycemia in type 1 diabetes. Diabetes. 2015;64(10):3439-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Evans ML. Too much or too little? The double jeopardy of subcutaneous insulin therapy in diabetes. Diabetes. 2015;64(10):3353-3354. [DOI] [PubMed] [Google Scholar]
- 43. Oskarsson P, Lins P, Backman L, Adamson U. Continuous intraperitoneal insulin infusion partly restores the glucagon response to hypoglycaemia in type 1 diabetic patients. Diabetes Metab. 2000;26(2):118-124. [PubMed] [Google Scholar]
- 44. Liebl A, Hoogma R, Renard E, et al. A reduction in severe hypoglycaemia in type 1 diabetes in a randomized crossover study of continuous intraperitoneal compared with subcutaneous insulin infusion. Diabetes Obes Metab. 2009;11(11):1001-1008. [DOI] [PubMed] [Google Scholar]
- 45. Cho YM, Merchant CE, Kieffer TJ. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol Ther. 2012;135(3):247-278. [DOI] [PubMed] [Google Scholar]
- 46. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c–independent risk factor for diabetic complications. JAMA. 2006;295(14):1707-1708. [DOI] [PubMed] [Google Scholar]
- 47. Monnier L, Colette C, Leiter L, et al. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes: response to Kilpatrick et al. Diabetes Care. 2007;30(1):185-186. [DOI] [PubMed] [Google Scholar]
- 48. Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349-1354. [DOI] [PubMed] [Google Scholar]
- 49. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681-1687. [DOI] [PubMed] [Google Scholar]
- 50. Gillard P, Hilbrands R, Van de, Velde U, et al. Minimal functional beta-cell mass in intraportal implants that reduces glycemic variability in type 1 diabetic recipients. Diabetes Care. 2013;36(11):3483-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA. 2010;107(37):16009-16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D’Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab. 2011;13(1):126-132. [DOI] [PubMed] [Google Scholar]
- 53. Hughes DS, Narendran P. Alpha cell function in type 1 diabetes. Br J Diabetes. 2014;14(2):45-51. [Google Scholar]
- 54. Meyer C, Woerle HJ, Dostou JM, Welle SL, Gerich JE. Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287(6):E1049-E1056. [DOI] [PubMed] [Google Scholar]
- 55. Woerle HJ, Szoke E, Meyer C, et al. Mechanisms for abnormal postprandial glucose metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;290(1):E67-E77. [DOI] [PubMed] [Google Scholar]
- 56. Krssak M, Brehm A, Bernroider E, et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53(12):3048-3056. [DOI] [PubMed] [Google Scholar]
- 57. Hwang J-H, Perseghin G, Rothman DL, et al. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest. 1995;95(2):783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012;3(3):286-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DeVries JH, Snoek FJ, Kostense PJ, Masurel N, Heine RJ. A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic control. Diabetes Care. 2002;25(11):2074-2080. [DOI] [PubMed] [Google Scholar]
- 60. Catargi B, Meyer L, Melki V, Renard E, Jeandidier N. Comparison of blood glucose stability and HbA1C between implantable insulin pumps using U400 HOE 21PH insulin and external pumps using lispro in type 1 diabetic patients: a pilot study. Diabetes Metab. 2002;28(2):133-137. [PubMed] [Google Scholar]
- 61. van Dijk PR, Groenier KH, DeVries JH, et al. Continuous intraperitoneal insulin infusion versus subcutaneous insulin therapy in the treatment of type 1 diabetes: effects on glycemic variability. Diabetes Technol Ther. 2015;17(6):379-384. [DOI] [PubMed] [Google Scholar]
- 62. Logtenberg SJ, Kleefstra N, Houweling ST, et al. Improved glycemic control with intraperitoneal versus subcutaneous insulin in type 1 diabetes: a randomized controlled trial. Diabetes Care. 2009;32(8):1372-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eldor R, Arbit E, Corcos A, Kidron M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLOS ONE. 2013;8(4):e59524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heller S. Weight gain during insulin therapy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2004;65:S23-S27. [DOI] [PubMed] [Google Scholar]
- 65. Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab. 2007;9(6):799-812. [DOI] [PubMed] [Google Scholar]
- 66. Khan R. Weight gain and insulin therapy. Br J Diabetes Vasc Dis. 2004;4(4):264-267. [Google Scholar]
- 67. Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin. 2011;27(sup3):13-20. [DOI] [PubMed] [Google Scholar]
- 68. Purnell JQ, Hokanson JE, Cleary PA, et al. The effect of excess weight gain with intensive diabetes treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127(2):180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wright A, Cull C, Holman R, et al. United Kingdom Prospective Diabetes Study 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann Intern Med. 1998;128(3):165-175. [DOI] [PubMed] [Google Scholar]
- 70. Freyse EJ, Fischer U, Knospe S, Ford GC, Nair KS. Differences in protein and energy metabolism following portal versus systemic administration of insulin in diabetic dogs. Diabetologia. 2006;49(3):543-51. [DOI] [PubMed] [Google Scholar]
- 71. Rosenstock J, Bergenstal RM, Blevins TC, et al. Better glycemic control and weight loss with the novel long-acting basal insulin LY2605541 compared with insulin glargine in type 1 diabetes: A randomized, crossover study. Diabetes Care. 2013;36(3):522-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hanaire-Broutin H, Sallerin-Caute B, Poncet MF, et al. Insulin therapy and GH-IGF-I axis disorders in diabetes: impact of glycaemic control and hepatic insulinization. Diabetes Metab. 1996;22(4):245-250. [PubMed] [Google Scholar]
- 73. Ma Z, Christiansen JS, Laursen T, Lauritzen T, Frystyk J. Short-term effects of NPH insulin, insulin detemir, and insulin glargine on the GH–IGF1–IGFBP axis in patients with type 1 diabetes. Eur J Endocrinol. 2014;171(4):471-479. [DOI] [PubMed] [Google Scholar]
- 74. Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85(12):4712-4720. [DOI] [PubMed] [Google Scholar]
- 75. Brismar K, Fernqvist-Forbes E, Wahren J, Hall K. Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. J Clin Endocrinol Metab. 1994;79(3):872-878. [DOI] [PubMed] [Google Scholar]
- 76. Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41(2):425-443, vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shishko PI, Dreval AV, Abugova IA, Zajarny IU, Goncharov VC. Insulin-like growth factors and binding proteins in patients with recent-onset type 1 (insulin-dependent) diabetes mellitus: influence of diabetes control and intraportal insulin infusion. Diabetes Res Clin Pract. 1994;25(1):1-12. [DOI] [PubMed] [Google Scholar]
- 78. van Dijk PR, Logtenberg SJ, Groenier KH, Kleefstra N, Bilo HJ, Arnqvist HJ. Effect of i.p. insulin administration on IGF1 and IGFBP1 in type 1 diabetes. Endocr Connect. 2014;3(1):17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hedman CA, Frystyk J, Lindstrom T, Oskarsson P, Arnqvist HJ. Intraperitoneal insulin delivery to patients with type 1 diabetes results in higher serum IGF-I bioactivity than continuous subcutaneous insulin infusion. Clin Endocrinol (Oxf). 2014;81(1):58-62. [DOI] [PubMed] [Google Scholar]
- 80. Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf). 1974;3(1):69-96. [DOI] [PubMed] [Google Scholar]
- 81. Danielson KK, Drum ML, Lipton RB. Sex hormone-binding globulin and testosterone in individuals with childhood diabetes. Diabetes Care. 2008;31(6):1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van Dam EW, Dekker JM, Lentjes EG, et al. Steroids in adult men with type 1 diabetes: a tendency to hypogonadism. Diabetes Care. 2003;26(6):1812-1818. [DOI] [PubMed] [Google Scholar]
- 83. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288-1299. [DOI] [PubMed] [Google Scholar]
- 84. Corona G, Monami M, Rastrelli G, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34(6 pt 1):528-540. [DOI] [PubMed] [Google Scholar]
- 85. Yki-Jarvinen H, Makimattila S, Utriainen T, Rutanen EM. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab. 1995;80(11):3227-3232. [DOI] [PubMed] [Google Scholar]
- 86. Lassmann-Vague V, Raccah D, Pugeat M, Bautrant D, Belicar P, Vague P. SHBG (sex hormone binding globulin) levels in insulin dependent diabetic patients according to the route of insulin administration. Horm Metab Res. 1994;26(9):436-437. [DOI] [PubMed] [Google Scholar]
- 87. Boering M, van Dijk PR, Logtenberg SJJ, et al. Effects of intraperitoneal insulin versus subcutaneous insulin administration on sex hormone-binding globulin concentrations in patients with type 1 diabetes mellitus. Endocr Connect. 2016;5(3):136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Amidon GL, Lee H. Absorption of peptide and peptidomimetic drugs. Ann Rev Pharmacol Toxicol. 1994;34(1):321-341. [DOI] [PubMed] [Google Scholar]
- 89. Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chin J, Mahmud KF, Kim SE, Park K, Byun Y. Insight of current technologies for oral delivery of proteins and peptides. Drug Discov Today Technol. 2012;9(2):e105-e112. [DOI] [PubMed] [Google Scholar]
- 91. Zaykov AN, Mayer JP, DiMarchi RD. Pursuit of a perfect insulin. Nat Rev Drug Discov.2016;15(6):425-439. [DOI] [PubMed] [Google Scholar]
- 92. Zijlstra E, Heinemann L, Plum-Morschel L. Oral insulin reloaded: a structured approach. J Diabetes Sci Technol. 2014;8(3):458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Eldor R, Kidron M, Arbit E. Open-label study to assess the safety and pharmacodynamics of five oral insulin formulations in healthy subjects. Diabetes Obes Metab. 2010;12(3):219-223. [DOI] [PubMed] [Google Scholar]
- 94. Neutel J, Kidron M, Arbit E, Homer K. Bedtime oral insulin lowers fasting blood glucose levels in T2DM patients. Poster presented at: San Fransisco, CA, USA American Diabetes Association. 2014. [Google Scholar]
- 95. Aguirre T, Teijeiro-Osorio D, Rosa M, Coulter I, Alonso M, Brayden D. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv Drug Deliv Rev. 2016; 106(Pt B): 223-241. [DOI] [PubMed] [Google Scholar]
- 96. Walsh EG, Adamczyk BE, Chalasani KB, et al. Oral delivery of macromolecules: rationale underpinning Gastrointestinal Permeation Enhancement Technology (GIPET). Ther Deliv. 2011;2(12):1595-1610. [DOI] [PubMed] [Google Scholar]
- 97. Moroz E, Matoori S, Leroux J-C. Oral delivery of macromolecular drugs: Where we are after almost 100years of attempts. Adv Drug Deliv Rev. 2016;101:108-121. [DOI] [PubMed] [Google Scholar]
- 98. Cryer P. The Physiology of Glucose Regulation in Cryer P, Hypoglycemia in diabetes: pathophysiology, prevalence, and prevention. 3rd ed. Alexandria, American Diabetes Association. 2016;63-90. [Google Scholar]
- 99. Vora J, Heise T. Variability of glucose-lowering effect as a limiting factor in optimizing basal insulin therapy: a review. Diabetes Obes Metab. 2013;15(8):701-712. [DOI] [PubMed] [Google Scholar]
- 100. Gin H, Hanaire-Broutin H. Reproducibility and variability in the action of injected insulin. Diabetes Metab. 2005;31(1):7-13. [DOI] [PubMed] [Google Scholar]
- 101. Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673-682. [DOI] [PubMed] [Google Scholar]
- 102. Li YZ, Wu WD, Zeng R, Greenberg-Shushlav Y, Kidron M. Pharmacokinetic and pharmacodynamic profiles of orally, duodenally and subcutaneously delivered insulin in beagle canines. Paper presented at: ADA; June 10-14, 2016; New Orleans, LA. [Google Scholar]
- 103. Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology. 2011;152(7):2546-2551. [DOI] [PubMed] [Google Scholar]
- 104. Saffran M, Pansky B, Budd GC, Williams FE. Insulin and the gastrointestinal tract. J Controlled Release. 1997;46:89-98. [Google Scholar]


