Abstract
Objective:
People with diabetic peripheral neuropathy (DPN) often exhibit deteriorations in motor-performance mainly due to lack of plantar-sensation. The study explored effectiveness of plantar electrical-stimulation therapy to enhance motor-performance among people with DPN.
Design and methods:
Using a double-blinded model, 28 volunteers with DPN (age: 57.8 ± 10.2 years) were recruited and randomized to either intervention (IG: n = 17) or control (CG: n = 11) group. Both groups received identical plantar-stimulation devices for six weeks of daily use at home; however, only the IG devices were set to deliver stimulation. Balance (ankle, hip, and center of mass [COM] sway) and gait (stride velocity [SV], stride time [ST], stride length [SL], and cadence) were measured using validated wearable sensors. Outcomes were assessed at baseline and at six-week. Clinical assessment including vascular as measured by ankle-brachial-index (ABI) and plantar-sensation as quantified by vibratory plantar threshold (VPT) were also measured at baseline and six weeks.
Results:
No difference were observed between groups for baseline characteristics (P > .050). Posttherapy, ankle and COM sway with eyes open were significantly improved (P < .05, Cohen’s effect size d = 0.67-0.76) in the IG with no noticeable changes in CG. All gait parameters were significantly improved in the IG with highest effect size observed for cadence (d = 1.35, P = .000). Results revealed improvement in VPT (P = .004, d = 1.15) with significant correlation with stride velocity improvement (r = .56, P = .037). ABI was improved in the IG in particulate among those with ABI>1.20 (P = .041, d = 0.99)
Conclusion:
This study suggests that daily home use of plantar electrical-stimulation may be a practical means to enhance motor-performance and plantar-sensation in people with DPN.
Keywords: diabetes, electrical stimulation, peripheral neuropathy, balance, gait, skin perfusion, intervention, wearable
Around the world more than 347 million people suffer from diabetes with 90% of them having Type 2 diabetes.1,2 The most common factors contributing toward diabetes include excessive body weight and poor physical activity. Among other countries around the world, Qatar has also been the victim of this world pandemic with a high prevalence in nearly 23% of its population;3,4 thereby increasing the associated health care cost.5 One of common predictors of metabolic syndrome in Qatar was found to be increased body mass index,5 which is usually associated with dietary and sedentary behavior. Management of diabetes is therefore of utmost importance in Qatar to reduce the negative impact of complications related to diabetes. One big challenge in encouraging people with diabetes and older people in particular is poor balance control.6-8 This is often the primary comorbidity that limits their ability to be engaged in daily physical activities and seems to have a significant impact on quality of life.9-12
Balance is a fundamental ability for humans, and its impairment dramatically reduces an individual’s ability to perform activities essential to daily living (walking, turning, change of posture, and so on).13 In particular, diabetic peripheral neuropathy (DPN), which impacts 60-70% of older people with diabetes,14,15 significantly deteriorates postural control system mainly due to lack of plantar sensation, which alters both local and central postural control systems.8 Furthermore, DPN has also been found to be one of the independent risk factors for increased risk of falls and fear of falls in older adults with diabetes.16-19 Furthermore, fall-related injuries in diabetes are often assumed to trigger a vicious circle as they have potentially detrimental influence on the physical activity levels. This vicious circle of low physical activity, functional deficits and high fall risk further increases health care and economic costs. Among other factors sedentary behavior has been reported to be associated with the risk of developing type 2 diabetes.20-22 The importance of decreasing sedentary time, as well as increasing time spent in physical activity, for metabolic health has also been identified.20 If health continues to deteriorate without appropriate interventions, foot ulcers can develop which are the most common cause of hospitalization and can ultimately lead to limb loss.23-26 Improved postural balance thus could break this viscous cycle and reduce the risk of diabetes related complications.
Exercise is probably the best evidence based intervention to enhance balance in older adults. But unfortunately exercising among people with diabetes is limited in particular among older people with DPN, who have high concern for falls. Thus, an alternative intervention, which may effectively address loss of plantar sensation, may assist in enhancing balance, reducing fear of falling, and encouraging individuals to become more active.
Recently, a large body of basic science and clinical work has shown conclusively that low-level mechanical or electrical noise presented directly to sensory neurons can significantly enhance their ability to detect weak signals, enhance skin perfusion, even assist in recovery of damaged nerve cells.27-35 This study has been designed to clinical validate the effectiveness and feasibility of a daily home use of plantar electrical stimulation to enhance postural balance and plantar sensation among people with DPN and loss of protective sensation.
Methods
Subjects
Twenty-eight subjects with type 2 diabetes and confirmed DPN were enrolled. The inclusion and exclusion criteria are described in Table 1. DPN was defined as insensitivity of a 10 gram monofilament at 1-3 sites in any following locations in either foot: hallux, 1st, 3rd, and 5th metatarsal heads.36 Eligibility of subjects were confirmed by a podiatrist or clinical coordinator with relevant training in diabetic foot and wound care. Eligible and willing patients signed a local institutional review board approved informed consent prior to screening. All subjects were recruited from the Wound and Diabetic Foot outpatient clinic at Hamad General Hospital (Doha-Qatar).
Table 1.
Patient Inclusion/Exclusion Criteria.
| Inclusion | Exclusion |
|---|---|
| Men or women (non pregnant) 18 years old or above | Amputation and active ulcers or infection |
| Diagnosed for diabetes mellitus (type 2)* and ADA criteria diabetes52 |
Cognitive deficits MMSE score of 24 or lower |
| Evidence of peripheral neuropathy on neurologic examination, identified by our clinical staff examination and based on the criteria explained in ADA statement (Boulton et al)36 | Unable to stand for more than 5 minutes (including symptomatic orthostatic hypotension or pain) |
| Any clinically significant orthopedic, muscular, or peripheral vascular disorders that affect balance | |
| Alcohol or substance abuse within 6 months or major psychiatric disorder | |
| Agreed to participate in this study and comply with instruction | Significant vision problem Less than 20/100 vision after correction |
| Any other neurological or medical disorders that may significantly affect balance based on clinical judgment (eg, CVA, asymmetric neuropathy, etc) | |
| Refusing or lacking medical decisional capacity to provide informed consent | |
| Use of medications that is likely to affect cognition or balance (based on physician review) within 14 days |
Device
For the purpose of this study, we have used an FDA cleared wearable electrical stimulation system, named SENSUS® (NeuroMetrix Inc, Waltham, MA, USA), which is a transcutaneous electrical nerve stimulator (TENS) and has been designed for management of painful neuropathy. However, the system was modified to provide electrical stimulation (30 milliapms) to plantar area via two electrodes placed on hind and forefoot area instead of leg (Figure 1). The device has a 60-minute run cycle after which it automatically turns off. Placebo units had an electrical stimulation unit programmed not to provide any electrical current. However, all the lights and programming indicators were functional. SENSUS is wearable and fully wireless thus providing the user the freedom to be active while they are receiving therapy. SENSUS is also FDA cleared for use during sleep, which we found that patients may prefer to use the system when going to bed and for sleep. The device is activated using a single button without the need of any electrical stimulation adjustment making it easier for the purpose of home therapy under unsupervised conditions. SENSUS was used with FDA cleared TENS electrodes that provide an electrically conductive interface between the device and subject’s skin. The electrodes are nonsterile and are designed and intended for single use only and to be disposable. For the purpose of this study, enough electrodes were provided to each subject and he/she was requested to replace the electrode with a new one each day. We didn’t control for the exact time of TENS treatment. To simplify the protocol for the participants, all subjects were asked to use the system at night before sleeping for a duration of one hour. They were also asked to log if they couldn’t use the device for any reason. The device was programed to deliver 1-hour treatment when the start bottom was pressed.
Figure 1.
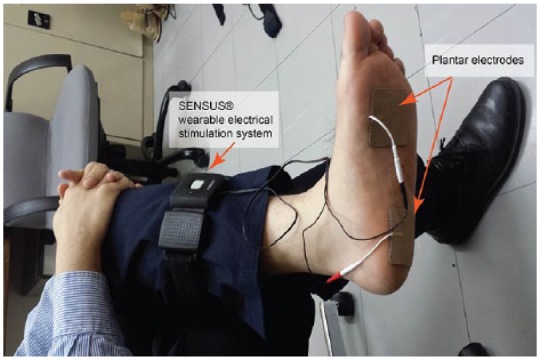
An illustration of application of the therapeutic device on patients.
Randomization and Removal of Study Blind
This study was designed as a double-blind randomized clinical model. Both patients and the clinical coordinator who evaluated and monitored study patients were blinded to electrical stimulation application. The study coordinator site (University of Arizona) was the only site aware of assignment (active device or sham device). Each unit was coded with a unique identification number, and the coordinating site revealed their status, placebo or electric stimulation, only at the end of data collection from the last patient. Subsequently, the investigators could match the status of the identification numbers with the corresponding units to start analyzing the data. Patients that receive an activated electrical stimulation unit received a standard dose of 30 milliamps as described in the following. Since patients that participated in this project had moderate to severe peripheral sensory neuropathy, they were entirely unable to “feel” if they were receiving electrical stimulation. Furthermore, credibility of this was enhanced due to all enrolled patients being therapeutically naïve to the sensation of electrical stimulation.
Protocol and Outcomes Measures
The study consisted of a six-week treatment phase of daily use of plantar electrical stimulations. There were a total of five study visits required for each participant. There was one scheduled visit preintervention for baseline evaluation, and four additional visits at 2 weeks, 4 weeks, 6 weeks during treatment, and 2 weeks post–stopping the treatment. The baseline visit involved completion of a standardized intake form, which were included pertinent demographics such as age, height, weight, education level, occupation, and comorbidities of the study patient. Several clinical assessments were also performed at baseline and conclusion of intervention including severity of neuropathy as assessed by VPT and peripheral vascular status as assessed by the ankle brachial index (ABI). VPT was evaluated at the distal great toe and 5th metatarsal head using a Biothesiometer (Bio-Medical Instrument Co, Newbury, OH, USA) using the protocol described in details in our previous study.37
At baseline, the research coordinator collected participant characteristics including age, gender, BMI, cognitive status (MMSE), ADL-status (Barthel Index38), comorbidity (number of diagnoses), medications (number), depressive symptoms (Center for Epidemiologic Studies Depression Scale, CES-D39), pain (Visual Analogue Scale ranging from 0-10 cm), frailty status (Fried Frailty Criteria40), concern for fall (Falls Efficacy Scale–International, FES-I), and history of falls (past year). In addition, health status and clinical history such as duration and type of diabetes, previous history of foot ulcers, amputation, lower extremity bypass, type of diabetes medication, cardiac angioplasty, lower extremity angioplasty, coronary artery bypass surgery, arthritis, liver disease, osteoporosis, malignancy, bone tumors, number of medication used, and any other disease that may impact postural controls, were collected.
Assessments of depression, frailty, ADL level, and concerns for fall were repeated at 6-weeks including depression, frailty, ADL level, and concerns for fall.
The CES-D short-version scale was used for measuring self-reported depression symptoms. A cut-off of CES-D score of 16 or greater was used to identify subjects with depression.41 The Fried Frailty Criteria, including unintentional weight loss, self-reported exhaustion, weakness (grip strength), slow gait speed (5-meter gait test), and self-reported low physical activity, were used for assessing prefrailty and frailty.40 Subjects with 1 or 2 positive Fried criteria were considered prefrail, and those with 3 or more positive Fried criteria were considered frail. Subjects with all negative Fried criteria were considered nonfrail.
Fear of falling was assessed using the FES-I questionnaire.42 In this scale, scores are treated as continuous variables ranging from 16-64, where 16 indicates no concern and 64 indicates severe concern for falling. Participants in our study were further classified as having low concern (score between 16-19), moderate concern (score between 20 and 27), or high concern (score ≥28) for falling according to the prior works.19,43
Balance was measured using two wearable sensors (BalanSens™, BioSensics, Watertown, MA, USA) attached to dominant leg and the lower back. Participants were instructed to stand for 30 seconds with feet close together (without touching), with eyes open (EO), and eyes closed (EC). Anterior-posterior (AP, cm) sway, medial-lateral (ML, cm) sway, and total sway area (cm2) of the center of mass (CoM) was quantified using validated algorithms.7
Gait performance was measured using wearable sensors (Figure 2) attached to both the left and right lower legs (LegSys™, BioSensics, Watertown, MA, USA). Participants walked ten meters at normal and fast pace. Gait speed and variability (coefficient of variation of stride velocity) were calculated using validated algorithms.44
Figure 2.

Patients during assessment of postural balance and gait in a clinical setting. Body-worn sensors attached to shank, thigh, and lower back for assessment of postural balance.
At each visit the participants were questioned about potential adverse experiences; response were recorded in the source documentation. The adverse events defined as any sign of burning because of use of electrical stimulation, edema, skin breakdown, and any other skin damage due to use of eletrical stimulation. In addition, subjects were interviewed about potential problems in using the system at home as well as their adherence to use the stimulation on daily basis. The SENSUS system was also kept the log each time the system was activated.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (SD). Unpaired t-tests, Mann-Whitney U-tests, and chi-square tests were used for baseline comparison according to the scale of the investigated variable and the distribution of the data. Repeated-measures ANOVA tests were used for examining the effect of intervention in each group after adjusting by age and BMI. To account for missing data (prevent entire subject data removal due to lack of a data point), a linear mixed-effect model was selected instead of univariate general linear model repeated-measures analysis. The effect size to discriminate between groups was estimated using Cohen’s d effect size and denoted as d in the results section. Values ranging from 0.20 to 0.49 indicate small, from 0.50 to 0.79 indicate medium, from 0.80 to 1.29 indicate large, and above 1.30 indicate very large effects.45 Values less than 0.20 are considered as having no noticeable effect.45 Spearman correlation of coefficient was used to examine association between changes in motor parameters and changes in plantar sensation postintervention. All analyses were performed using SPSS statistics (version 24; IBM, Armonk, NY, USA), with a significance level of P < .05.
Results
Twenty-eight subjects met the inclusion and exclusion criteria of this study. According to the randomization allocation, seventeen (n = 17) subjects were assigned to active and eleven (n = 11) subjects were assigned to control group. Table 2 summarizes subjects’ demographic and clinical characteristics. All participants had loss of protective plantar sensation as confirmed by VPT test (VPT of 25 volts or greater at either foot). The average of VPTmax values (maximum value between right and left foot) was 41 ± 7 volts and 40 ± 10 in the intervention and control group, respectively. The average of HbA1C was relatively high (9.1 ± 2.0%, range from 6.2% to 13.2%) suggesting poor blood glucose control in our sample. Symptom of foot pain was reported in 37% of participants (VAS score of 4 to 10 on scale of 10), while overall pain was reported in 78% of participants. According to CES-D criteria, 19% of participants were depressed. According to Fried frailly criteria, almost all participants (expect one) were prefrail (74%) or frail (22%). According to Mobility Tiredness Scale, except one, all participants had symptom of fatigue during activities of daily living with average score of 3.0 ± 2.1 on scale of 6. Peripheral arterial disease was reported for 26% of participants. According to FES-I, 85% of participants had fall concerns including 63% with high concerns for fall (FES-I of 28 or greater). According to the Barthel ADL index, only one subject (4%) was scored less than 80 and is considered as dependent.
Table 2.
Participant Demographics and Baseline Clinical Characteristics.
| Intervention group (N = 17) | Control group (N = 11) | P value | |
|---|---|---|---|
| Age, years | 56 ± 11 | 64 ± 10 | .096 |
| BMI, kg/m2 | 28.7 ± 5.9 | 31.5 ± 8.0 | .323 |
| Sex, % male | 73 | 82 | .638 |
| VPTmax, volts | 41 ± 7 | 40 ± 10 | .629 |
| ABI_max | 1.1 ± 0.2 | 1.1 ± 0.2 | .628 |
| HbA1C | 8.8 ± 1.9 | 9.6 ± 2.2 | .398 |
| CES-D | 8.3 ± 11.4 | 7.3 ± 7.5 | .832 |
| % with depression | 24 | 25 | .612 |
| Foot pain max, cm | 2.2 ± 3.0 | 3.4 ± 4.0 | .416 |
| Overall pain, cm | 3.9 ± 2.7 | 4.6 ± 0.8 | .187 |
| Barthel ADL | 98.2 ± 3.5 | 96.4 ± 9.4 | .486 |
| Mobility Tiredness Scale | 3.1 ± 2.0 | 2.9 ± 2.5 | .789 |
| FES-I | 33.0 ± 12.1 | 41.9 ± 14.7 | .124 |
ABI_max, maximum ABI measured from right and left foot; ES, effect size Cohen’s d; EO, eyes open; foot pain max, maximum foot pain reported from left and right foot; SD, standard deviation; VPTmax, maximum value of VPT measured from right and left foot.
No difference were observed between the groups for baseline characteristics or for motor performance including postural sway and spatiotemporal parameters of gait (P > .050; Table 2 and Table 3). However, the majorities of measurable metrics were improved post-treatment in the intervention group with no significant changes in the control group (P > .050; Table 4 and Table 5). The highest effect size in balance parameters was observed for ankle sway during eyes-open condition, where the sway was reduced on average by 34.4% (P = .001, d = 0.76; Figure 3, Table 4). The highest effect size for gait parameters were observed for cadence, where the number of steps per minute was improved on average by 37.8% (P = .000, d = 1.35; Table 5). The improvement for both gait and balance parameters was noticeable from week 2 with little to no further improvement from week 2 to week 6 (P > .050). Gait parameters were also improved in the CG but the effect sizes were small (d = 0.27-0.48, Table 5).
Table 3.
Baseline Balance and Gait Data.
| Intervention group (N = 17) | Control group (N = 11) | P value | |
|---|---|---|---|
| Ankle sway-EO, deg2 | 1.54 ± 0.71 | 1.72 ± 1.40 | .669 |
| Hip sway, EO, deg2 | 1.76 ± 0.93 | 1.94 ± 1.45 | .696 |
| COM sway-EO, cm2 | 3.49 ± 2.36 | 3.60 ± 2.89 | .917 |
| Ankle sway-EC, deg2 | 2.94 ± 1.94 | 2.79 ± 1.72 | .854 |
| Hip sway, EC, deg2 | 3.23 ± 2.13 | 3.17 ± 0.97 | .949 |
| COM sway-EC, cm2 | 6.37 ± 4.60 | 5.59 ± 5.57 | .915 |
| Stride velocity, m/s | 0.87 ± 0.22 | 0.82 ± 0.21 | .439 |
| Stride time, s | 1.26 ± 0.14 | 1.35 ± 0.25 | .271 |
| Stride length, m | 1.05 ± 0.15 | 1.03 ± 0.19 | .623 |
| Cadence | 74.1 ± 27.9 | 72.4 ± 24.3 | .872 |
EC, eyes closed; EO, eyes open.
Table 4.
Effect of Intervention for Active and Control Group for Balance Parameters.
| Parameter | Active group | P value | Effect size* | Control group | P value | Effect size* | ||
|---|---|---|---|---|---|---|---|---|
| Eyes open | Ankle sway, deg2 | Baseline | 1.54 ± 0.71 | .001 | 0.76 | 1.72 ± 1.40 | .637 | 0.11 |
| Week 6 | 1.01 ± 0.69 | 1.57 ± 1.11 | ||||||
| Hip sway, deg2 | Baseline | 1.76 ± 0.93 | .298 | 0.30 | 1.95 ± 1.45 | .638 | 0.12 | |
| Week 6 | 1.54 ± 1.01 | 1.78 ± 1.30 | ||||||
| COM sway, cm2 | Baseline | 3.49 ± 2.36 | .038 | 0.67 | 3.60 ± 2.89 | .716 | 0.08 | |
| Week 6 | 2.42 ± 1.18 | 3.36 ± 2.25 | ||||||
| Eyes closed | Ankle sway, deg2 | Baseline | 2.94 ± 1.94 | .047 | 0.55 | 2.79 ± 1.72 | .264 | 0.41 |
| Week 6 | 2.09 ± 1.00 | 4.18 ± 4.42 | ||||||
| Hip sway, deg2 | Baseline | 3.23 ± 2.13 | .307 | 0.25 | 3.17 ± 0.67 | .197 | 1.96 | |
| Week 6 | 2.77 ± 1.41 | 5.53 ± 1.96 | ||||||
| COM sway, cm2 | Baseline | 6.37 ± 4.60 | .255 | 0.32 | 6.59 ± 5.57 | .453 | 0.25 | |
| Week 6 | 5.23 ± 2.04 | 8.00 ± 5.58 | ||||||
Results have been adjusted by age and BMI.
Effect size Cohen’s d.
Table 5.
Between-Group Comparisons for Gait Parameters Postintervention.
| Parameters | Active group | P value | Effect size* | Control group | P value | Effect size* | |
|---|---|---|---|---|---|---|---|
| Stride velocity, m/s | Baseline | 0.87 ± 0.26 | .001 | 0.41 | 0.82 ± 0.21 | .129 | 0.38 |
| Week 6 | 0.97 ± 0.23 | 0.90 ± 0.21 | |||||
| Stride time, s | Baseline | 1.26 ± 0.14 | .001 | 0.64 | 1.35 ± 0.25 | .024 | 0.420 |
| Week 6 | 1.18 ± 0.11 | 1.26 ± 0.21 | |||||
| Stride length, m | Baseline | 1.05 ± 0.15 | .000 | 0.49 | 1.03 ± 0.19 | .064 | 0.269 |
| Week 6 | 1.14 ± 0.21 | 1.14 ± 0.21 | |||||
| Cadence | Baseline | 74 ± 28 | .000 | 1.35 | 72 ± 24 | .012 | 0.482 |
| Week 6 | 102 ± 9 | 97 ± 14 | |||||
Results have been adjusted by age and BMI.
Effect size Cohen’s d.
Figure 3.
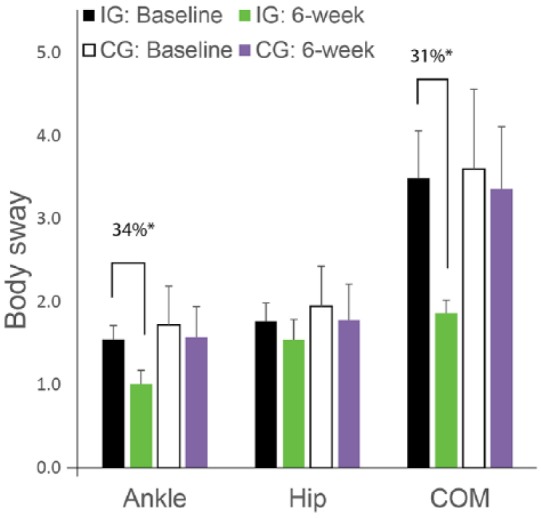
Body sway changes over 6 weeks treatment compared to baseline in the intervention group (IG) and in the control group (CG). The improvement is significant for ankle sway and center of mass (COM) sway in the IG with no noticeable changes in the CG.
*p<0.050.
Interestingly, results revealed a significant reduction in VPT value in the IG on average by 27% (VPT: 41.5 at baseline v. 30.3 postintervention, P = .004, 95% CI = [4.9, 17.7] volt) with large effect size (d = 1.15) indicating significant improvement in plantar sensation in the intervention group. The observed improvement in VPT was correlated with improvement in the stride velocity (r = .56, P = .037). ABI as an indicator of lower extremity vascular health was also reduced on average by 6.4% (ABI: 1.10 at baseline vs 1.03 at follow-up). However it didn’t achieved statistical significant level in our sample (P = .385). Interestingly, when participants with ABI of 1.20 or greater (n = 7) were selected, the reduction in ABI was significant and reached to 16.5% (ABI:1.34 at baseline v. 1.12 postintervention, P = .041, d = 0.99).
While foot pain was reduced in the intervention group, the reduction was not meaningful (average pain reduction less than 1 cm on scale of 10 cm) nor statistically significant (P = .504). However, when overall pain was assessed, the reduction was on average 2.9 cm on scale of 10 cm and achieved statistical significance (P = .043, 95% CI = [0.11, 4.8], d = 1.19). The improvement in other measured health related parameters including CES-D, FES-I, Barthel ADL, and mobility tiredness scale, was not statistically significant (P > .050).
Discussion
This randomized controlled study demonstrated that daily use of plantar electrical stimulation is practical, feasible and improves motor performance in people with DPN. No adverse events were reported due to daily use of electrical stimulation and only two subjects from control group were dropped out from the study. While, the participant perception of benefit and acceptability were not controlled in this study, no complain was reported from the daily home use of electrical stimulation or its burden.
While many studies suggested that electrical stimulation is effective to enhance balance,34,35 to our knowledge this is the first randomized control trial that examined therapeutic effectiveness of daily home use of plantar electrical stimulation in enhancement of motor performance, vascular health, and plantar sensation in patients suffering from DPN and poor plantar sensation.
This study confirmed that daily basis electrical stimulation therapy could be effective to enhance plantar sensation as quantified by significant reduction in VPT values on average by 27% in the intervention group. This is aligned with previous animal models in which it has been revealed that neurological stimulation could improve the performance of mechanoreceptor cells that provide protective sensation in the feet.46,35 Previous clinical studies have also demonstrated significant reduction in vibration perception threshold and an increase in monofilament detection after electrical stimulation treatment in people with DPN.37,47
The recovery of plantar sensation could be explained by improvement in plantar skin perfusion in response to daily use of plantar electrical stimulation. Diminished local blood flow can initiate oxidative stress and the release of factors that impede the normal passage of neurological signals as described by Malik and colleagues.48 A recent systematic review35 by assessing 21 randomized clinical trials that used electrical stimulation for wound healing confirmed that electrical stimulation increases cutaneous perfusion possibly through a release of vasoendothelial growth factor (VEGF).46,49 The increase in VEGF may counter the pathways to DPN through stimulation of angiogenesis for increase perfusion of endoneurial microvessels.37 In addition, VEGF has been shown to induce Schwann cell proliferation, stimulate axonal outgrowth and promote survival of neurons and Schwann cells in cultured animal cells.50
Poor postural balance is a major concerns in people with DPN and leads to increasing risk of falling, reduced mobility, and increased fall concerns. This study suggests that daily use of plantar electrical stimulation, could enhance balance and gait, thus may reduce risk of falling and enhance motilities. Interestingly, the magnitude of improvement in balance after six weeks of daily use of electrical stimulation is comparable with magnitude of improvement in balance post–balance exercise therapy program among people with DPN.51 However, usually adherence to regular exercise therapy is not high among people with diabetes and in addition, such balance training programs are often expensive and required regular clinical visits, which may not affordable for all people with diabetes. In these cases, plantar electrical stimulation seems to be an alternative therapy to enhance balance. However, another study is required to confirm the results in larger study. In addition another study merits to explore long term lasting of effectiveness of plantar electrical stimulation to improve balance.
The improvement in center of mass sway, as surrogate of postural control,13 in response to plantar electrical stimulation seems to be due to enhancement in ankle stability. While hip stability has been also improved in response to plantar stimulation, the improvement compared to ankle stability was negligible. The enhancement in ankle stability could be due to recovery of somatosensory feedback as described above and confirmed by enhancement in vibratory perception threshold test. The magnitude of improvement in balance was however small during eyes-closed condition probably due to the fact that postural control in people with DPN are highly dependent on visual feedback as well as central sensory feedback.8 This study was however unable to show any significant improvement in fear of falling, depression, and activities of daily living survey in response to intervention though. This could be explained by the fact that perceived psychosocial changes take longer than 6 weeks to demonstrate that an intervention has resulted in and maintained behavior change. Another study merits to examine long term benefit of plantar electrical stimulation in perceived psychosocial factors.
This study has few limitations. The sample size is underpowered to detect changes in vascular health, activity parameters, and psychosocial parameters. In addition, we had an unbalance distribution of sample size between two groups, which is due to the fact that randomization was done based on an anticipated list of 50 subjects. However, no between-group differences were observed at baseline for the parameters of interest, suggesting successful randomization of participants. Further studies with larger sample size and better randomization protocol are required to confirm these results and address potential long term retention of planar electrical stimulation intervention. According to participants’ log report, they had 100% adherence in daily use of the system during treatment phase. The log of the SENSU system also confirmed the system was activated on daily basis. However, we couldn’t objectively control, whether the system has been worn during the entire electrical-stimulation treatment. In addition, our method in assessing skin perfusion may not be accurate in particular among those with peripheral vascular diseases. In addition, other studies are warranted to examine long term benefit of plantar electrical stimulation to reduce consequences of DPN such as prospective falls and prevention of plantar ulcers.
Conclusions
This study suggests opportunities for better patient care, enhancement of plantar sensation, and improvement of motor performance among people suffering from diabetic peripheral neuropathy using a simple, inexpensive approach that has no obvious adverse effects and is practical for daily use. In a setting where no therapeutic agents or interventions effectively address loss of protective sensation and where affected individuals life with a heightened risk of developing a debilitating foot ulcer and quite possibly a disabling amputation, the effects seen with the plantar electrical stimulation system may offer the potential for significant clinical benefit, with very low risk.
Acknowledgments
We wish to thank Dr Saman Parvaneh, Dr Nima Toosizadeh, Ms Jacqueline Lee-Eng, and Mrs Samira Abdulla, who contributed in part in data analysis, data collection, or patient recruitment.
Footnotes
Abbreviations: ABI, ankle brachial index; ADL, activity of daily living; ANOVA, analysis of variance; BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression CI, confidential interval; DPN, diabetic peripheral neuropathy; FES-I, Falls Efficacy Scale–International; IRB, institutional review board; SD, standard deviation; TENS, transcutaneous electrical nerve stimulator.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported in part by a grant from the Qatar National Research Foundation (Award Numbers NPRP 5-117-3-028, http://www.qnrf.org/). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Qatar National Research Foundation.
References
- 1. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31-40. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. November 19, 1999. Available at: http://www.who.int/mediacentre/factsheets/fs312/en/.
- 3. Bener A, Zirie M, Janahi IM, Al-Hamaq AO, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract. 2009;84:99-106. [DOI] [PubMed] [Google Scholar]
- 4. International Diabetes Federation. Diabetes: facts and figures. October 8, 2013. Available at: http://www.idf.org/worlddiabetesday/toolkit/gp/facts-figures.
- 5. Bener A, Zirie M, Musallam M, Khader YS, Al-Hamaq AO. Prevalence of metabolic syndrome according to Adult Treatment Panel III and International Diabetes Federation criteria: a population-based study. Metab Syndr Relat Disord. 2009;7:221-229. [DOI] [PubMed] [Google Scholar]
- 6. Morrison S, Colberg SR, Parson HK, Vinik AI. Relation between risk of falling and postural sway complexity in diabetes. Gait Posture. 2012;35:662-668. [DOI] [PubMed] [Google Scholar]
- 7. Najafi B, Horn D, Marclay S, Crews RT, Wu S, Wrobel JS. Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J Diabetes Sci Technol. 2010;4:780-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toosizadeh N, Mohler J, Armstrong DG, Talal TK, Najafi B. The influence of diabetic peripheral neuropathy on local postural muscle and central sensory feedback balance control. PLOS ONE. 2015;10:e0135255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wrobel JS, Najafi B. Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Technol. 2010;4:833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Najafi B, Armstrong DG, Mohler J. Novel wearable technology for assessing spontaneous daily physical activity and risk of falling in older adults with diabetes. J Diabetes Sci Technol. 2013;7:1147-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armstrong DG, Abu-Rumman L, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91:451-455. [DOI] [PubMed] [Google Scholar]
- 12. Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev. 2001;17:246-249. [DOI] [PubMed] [Google Scholar]
- 13. Najafi B, Bharara M, Talal TK, Armstrong DG. Advances in balance assessment and balance training for diabetes. Diabetes Management. 2012;2:293-308. [Google Scholar]
- 14. Rogers LC, Andros G, Armstrong DG. Update from the Diabetic Foot Global Conference (DFCon) 2007 Int Wound J. 2007;4:295-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Armstrong DG, Lavery LA. Clinical Care of the Diabetic Foot. American Diabetes Association; 2005. [Google Scholar]
- 16. Lord SR, Caplan GA, Colagiuri R, Colagiuri S, Ward JA. Sensori-motor function in older persons with diabetes. Diabet Med. 1993;10:614-618. [DOI] [PubMed] [Google Scholar]
- 17. van Schie CH. Neuropathy: mobility and quality of life. Diabetes Metab Res Rev. 2008;24(suppl 1):S45-S51. [DOI] [PubMed] [Google Scholar]
- 18. Tilling LM, Darawil K, Britton M. Falls as a complication of diabetes mellitus in older people. J Diabetes Complications. 2006;20:158-162. [DOI] [PubMed] [Google Scholar]
- 19. Kelly C, Fleischer A, Yalla S, et al. Fear of falling is prevalent in older adults with diabetes mellitus but is unrelated to level of neuropathy. J Am Podiatr Med Assoc. 2013;103:480-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care. 2008;31:369-371. [DOI] [PubMed] [Google Scholar]
- 21. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population-health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilmot E, Edwardson C, Achana F, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895-2905. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong DG, Lavery LA, Quebedeaux TL, Walker SC. Surgical morbidity and the risk of amputation due to infected puncture wounds in diabetic versus nondiabetic adults. South Med J. 1997;90:384-389. [DOI] [PubMed] [Google Scholar]
- 24. Lavery LA, Ashry HR, van Houtum W, Pugh JA, Harkless LB, Basu S. Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care. 1996;19:48-52. [DOI] [PubMed] [Google Scholar]
- 25. Lavery LA, Van Houtum WH, Armstrong DG. Institutionalization following diabetes-related lower extremity amputation. Am J Med. 1997;103:383-388. [DOI] [PubMed] [Google Scholar]
- 26. Miller AD, Van Buskirk A, Verhoek-Oftedahl W, Miller ER. Diabetes-related lower extremity amputations in New Jersey, 1979 to 1981. J Med Soc N J. 1985;82:723-726. [PubMed] [Google Scholar]
- 27. Dhruv NT, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Enhancing tactile sensation in older adults with electrical noise stimulation. NeuroReport. 2002;13:597-600. [DOI] [PubMed] [Google Scholar]
- 28. Priplata AA, Patritti BL, Niemi JB, et al. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol. 2006;59:4-12. [DOI] [PubMed] [Google Scholar]
- 29. Gravelle DC, Laughton CA, Dhruv NT, et al. Noise-enhanced balance control in older adults. Neuroreport. 2002;13:1853-1856. [DOI] [PubMed] [Google Scholar]
- 30. Reed BV. Effect of high voltage pulsed electrical stimulation on microvascular permeability to plasma proteins. A possible mechanism in minimizing edema. Phys Ther. 1988;68:491-495. [DOI] [PubMed] [Google Scholar]
- 31. Reich JD, Cazzaniga AL, Mertz M, Kerdel FA, Eaglstein WH. The effect of electrical stimulation on the number of mast cells in healing wounds. J Am Acad Dermatol. 1991;25:40-46. [DOI] [PubMed] [Google Scholar]
- 32. Najafi B, Crews R, Wrobel JS. A novel plantar stimulation technology for improving postural control in patients with diabetic peripheral neuropathy—a double-blinded, randomized study. Paper presented at: 6th International Symposium on the Diabetic Foot; 2001; Netherlands. [DOI] [PubMed] [Google Scholar]
- 33. Gooneratne NS. Complementary and alternative medicine for sleep disturbances in older adults. Clin Geriatr Med. 2008;24:121-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thakral G, Kim J, LaFontaine J, Menzies R, Najafi B, Lavery LA. Electrical stimulation as an adjunctive treatment of painful and sensory diabetic neuropathy. J Diabetes Sci Technol. 2013;7:1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thakral G, LaFontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle. 2013;4. doi: 10.3402/dfa.v4i0.22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Najafi B, Crews RT, Wrobel JS. A novel plantar stimulation technology for improving protective sensation and postural control in patients with diabetic peripheral neuropathy: a double-blinded, randomized study. Gerontology. 2013;59:473-480. [DOI] [PubMed] [Google Scholar]
- 38. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61-65. [PubMed] [Google Scholar]
- 39. Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385-401. [Google Scholar]
- 40. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [DOI] [PubMed] [Google Scholar]
- 41. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203-214. [DOI] [PubMed] [Google Scholar]
- 42. Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34:614-619. [DOI] [PubMed] [Google Scholar]
- 43. Delbaere K, Close JC, Mikolaizak AS, Sachdev S, Brodaty H, Lord SR. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing. 2010;39:210-216. [DOI] [PubMed] [Google Scholar]
- 44. Aminian K, Najafi B, Büla C, Leyvraz PF, Robert P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech. 2002;35:689-699. [DOI] [PubMed] [Google Scholar]
- 45. Ellis P. Thresholds for interpreting effect sizes. 2009. Available at: http://www.polyu.edu.hk/mm/effectsizefaqs/thresholds_for_interpreting_effect_sizes2.html. Accessed January 13, 2014.
- 46. Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bosi E, Conti M, Vermigli C, et al. Effectiveness of frequency-modulated electromagnetic neural stimulation in the treatment of painful diabetic neuropathy. Diabetologia. 2005;48:817-823. [DOI] [PubMed] [Google Scholar]
- 48. Malik RA, Newrick G, Sharma AK, et al. Microangiopathy in human diabetic neuropathy: relationship between capillary abnormalities and the severity of neuropathy. Diabetologia. 1989;32:92-102. [DOI] [PubMed] [Google Scholar]
- 49. Kanno S, Oda N, Abe M, et al. Establishment of a simple and practical procedure applicable to therapeutic angiogenesis. Circulation. 1999;99:2682-2687. [DOI] [PubMed] [Google Scholar]
- 50. Schratzberger P, Schratzberger G, Silver M, et al. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med. 2000;6:405-413. [DOI] [PubMed] [Google Scholar]
- 51. Grewal GS, Schwenk M, Lee-Eng J, et al. Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: a randomized controlled trial. Gerontology. 2015;61:567-574. [DOI] [PubMed] [Google Scholar]
- 52. World-Health-Organization. Diagnosis and classification of diabetes mellitus. Department of Non Communicable Disease Surveillance; Geneva: 1999. Available at: http://apps.who.int/iris/handle/10665/66040. Accessed February 15, 2017. [Google Scholar]


