Abstract
Background:
For decades, the major source of information used to make therapeutic decisions by patients with diabetes has been glucose measurements using capillary blood samples. Knowledge gained from clinical studies, for example, on the impact of metabolic control on diabetes-related complications, is based on such measurements. Different to traditional blood glucose measurement systems, systems for continuous glucose monitoring (CGM) measure glucose in interstitial fluid (ISF). The assumption is that glucose levels in blood and ISF are practically the same and that the information provided can be used interchangeably. Thus, therapeutic decisions, that is, the selection of insulin doses, are based on CGM system results interpreted as though they were blood glucose values.
Methods:
We performed a more detailed analysis and interpretation of glucose profiles obtained with CGM in situations with high glucose dynamics to evaluate this potentially misleading assumption.
Results:
Considering physical activity, hypoglycemic episodes, and meal-related differences between glucose levels in blood and ISF uncover clinically relevant differences that can make it risky from a therapeutic point of view to use blood glucose for therapeutic decisions.
Conclusions:
Further systematic and structured evaluation as to whether the use of ISF glucose is more safe and efficient when it comes to acute therapeutic decisions is necessary. These data might also have a higher prognostic relevance when it comes to long-term metabolic consequences of diabetes. In the long run, it may be reasonable to abandon blood glucose measurements as the basis for diabetes management and switch to using ISF glucose as the appropriate therapeutic target.
Keywords: physiology of glucose regulation, continuous glucose monitoring, glucose variability, blood glucose, interstitial glucose
Treatment of patients with diabetes has undergone drastic improvements over the last decades, driven by the introduction of new diagnostic and therapeutic options. Today, patients with diabetes not only can measure blood glucose (BG) relatively easily and with good measurement reliability whenever they deem it necessary, they can also monitor changes in glucose levels in their body continuously by using systems for continuous glucose monitoring (CGM). The glucose sensors of these CGM systems are constructed as needle sensors that are inserted in the subcutaneous adipose tissue to access to interstitial fluid (ISF). It is important to note that CGM systems monitor glucose changes in ISF and not in blood, thus glucose is measured in two different compartments; in addition, the frequency of measurements in the ISF is performed continuously every few minutes whereas self-monitoring of blood glucose (SMBG) is only done a few times every day. Blood and ISF differ in a number of aspects with respect to glucose: blood transfers glucose to all body sites while ISF transfers glucose to cells; blood is (relatively) easily accessible whereas ISF is tricky to access; BG levels can be measured with a high reliability—something that is more difficult with ISF glucose levels.1-5
Measurement using BG values only provides limited information about dynamic glucose changes in the human body and no information is given about the direction in which the glucose level will change. This is not the case for CGM measurement. Systematic analysis of CGM data stored in the CGM systems not only enables visualization of glucose changes over time, but also helps identify the factors driving such changes and provide indications for optimization of diabetes therapy in CGM patients. Assumptions exist that state that the compartment in which glucose levels are measured is irrelevant, that is, in blood or in ISF, and that the numbers measured are more or less the same and can be used interchangeably as therapeutic target glucose (TTG) values.
The aim of this article is to challenge this assumption by presenting data from individual CGM recordings in certain clinical situations that exist in all patients with diabetes (= clinical entities). Our hypothesis is that in these situations, certain physiologic factors (and not measurement artefacts) induce clinically relevant differences between glucose levels measured in blood and ISF, which means that therapeutic decisions based on BG values might be inappropriate or even dangerous. In the long run, it might appropriate to switch from BG measurements to ISF measurements as the primary source for therapeutic decisions.
Relevance of Measuring Glucose in Two Different Compartments: BG Versus ISF
While the blood stream is the transport system of the body for transferring substances such as glucose over longer distances, the ISF is the compartment in which substances such as glucose diffuse to the tissues/cells on a local level. Therefore, the BG levels represent the summary of all glucose uptakes from the intestines or glucose storages as well as the glucose transfer across the capillary walls toward the ISF. The BG concentration is a marker of the total currently available glucose in blood. The glucose concentration in the ISF depends much more on the local conditions, that is, on how much glucose diffuses from the blood into the ISF and on how much is metabolized by the more or less metabolically active tissues nearby. This means that there are basic differences between the two measurement approaches: ISF glucose changes are measured by CGM systems in a relatively small volume of tissue around the tip of the glucose sensor which undergoes relatively slow changes in glucose levels, whereas BG measurement is performed in a compartment in which differences in glucose levels are rapidly eliminated by instant “mixing.”
Comparing BG measurement results with CGM recordings shows more or less identical glucose values (assuming CGM recordings are adequately calibrated; see below) in the case of stable glucose levels, that is, rates of change of glucose levels below 1 or 2 mg/dl per min. However, when more rapid changes in glucose levels are induced in either compartment (eg, by exercise, see below), glucose measurement results can differ considerably between BG and ISF (known as physiologic time delay). The assumption by most users of CGM systems (and diabetologists) is that these differences are short-lived and not pronounced enough to be of significant therapeutic relevance. In reality refilling of, for example, glycogen stores takes some time and glucose levels in ISF measured by a CGM system are lower than those in blood. In this case, BG levels do not represent the correct TTG values. Dynamic changes in glucose levels can be induced by numerous situations; here the focus is on certain clinical “entities” that are of therapeutic relevance. Using the measurement result from the correct compartment to determine the insulin dose is assumed to be clinically relevant in such situations.
Clearly, such differences have been observed and described by other colleagues before. However, what has not yet been presented is the systematic analysis of CGM profiles (called ISF glucose measurements subsequently) versus conventional BG measurements and the defining of certain clinical situations in which the observed differences are of therapeutic relevance.
In addition to the physiologic differences between ISF and blood, it is important to acknowledge the influence on the measurement results of both the measurement technology itself and the algorithms implemented into the CGM systems (physical or technical time lag).6 The calibration of the ISF signal to BG by means of capillary BG measurements may have an impact on the maximal glucose levels recorded.
Entity 1: Physical-Activity-Related Glucose Differences Between Blood and ISF
Intriguing differences between glucose levels in blood and ISF arise during physical exercise and the hours thereafter (Figures 1a and 1b).7 ISF glucose measurements in a patient who participated in a cycling marathon (on day 2) showed differences between the CGM profile and the BG measurement results on marathon day in contrast to the recordings on day 1 and day 3, that is, before and after the physical activity. Under day 1 and 3 conditions, glucose levels in both compartments are comparable, that is, regulated schedules provide a homeostatic picture. The differences in glucose levels during the cycling marathon arose despite the fact that the patient had multiple snacks during the period of time; these caused an elevation of the BG levels but not a corresponding one the ISF glucose levels. It has to be pointed out, that discrepancies between CGM and SMBG only show up after carbohydrate uptake; in this case blood is used as transport system for glucose.
Figure 1a.
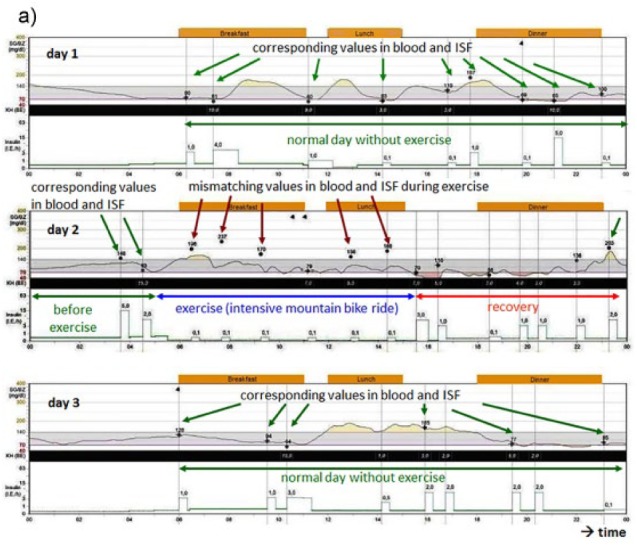
Differences in glucose excursions in ISF monitored by a glucose sensor and blood glucose measurements before (day 1, upper graph), during (day 2, middle graph), and after (day 3, lower graph) a cycling marathon (day 2). During physical activity, the differences in the glucose values measured in blood (black dots, red arrows) and the black line (representing ISF values measured by a CGM system) are higher than on the other days with normal physical activity (green arrows) and low glucose dynamics. The most probable explanation is the pronounced and quick glucose utilization during the marathon.7 These effects can get visible when carbohydrates are resorbed.
Figure 1b.
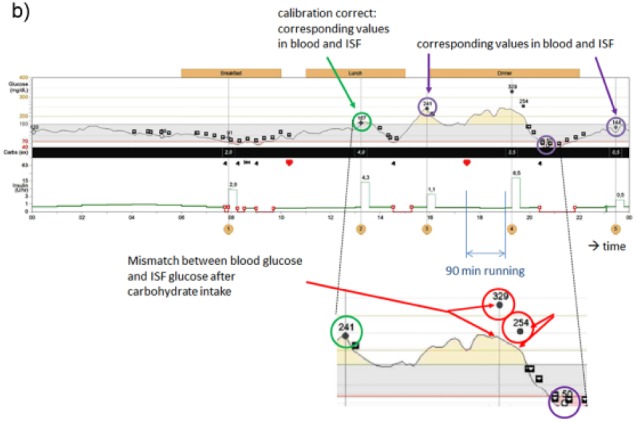
Difference between blood glucose and interstitial glucose values during/after exercise: the differences occur if (1) food is ingested and (2) the glycogen stores of the liver are emptied after exercise and therefore must be restored.
What is the appropriate TTG value in such a situation; to which glucose signal should patients adjust their diabetes therapy? Is the correct TTG value the glucose level measured in blood or that in ISF? If the patient had administered a correction dose of insulin at 7:30 am when his BG level was 237 mg/dl, he might have encountered a hypoglycemic event as this dose was not appropriate taking into consideration the lower ISF glucose level measured at this point in time. Fortunately, the patient—due his experience in sports and his trust in the CGM measurement result—did not alter his insulin therapy and completed the cycling marathon without relevant hypoglycemic episodes. Figure 1b shows a similar example for difference between BG and interstitial glucose values during/after exercise. The presented cases/figures were registered in patients using an Enlite CGM system. Nevertheless, we have observed similar discrepancies with other CGM systems as well.
Entity 2: Hypoglycemia-Related Glucose Differences Between Blood and ISF
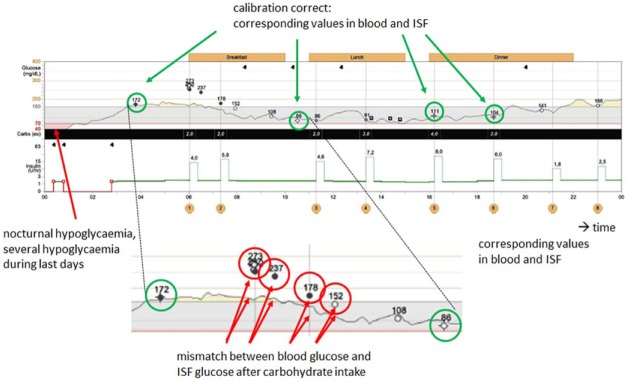
Glucose dynamics are also high during hypoglycemic events, that is, such acute metabolic deteriorations induce strong glucose fluctuations (Figure 2). After hypoglycemic events, a pronounced period of glycemic instability is quite often observed. Such glucose swings might be even more pronounced after severe hypoglycemic events; however, to a certain extent they can also be seen after mild or moderate hypoglycemic events.
Figure 2.
Difference between blood glucose and interstitial glucose values for a previous hypoglycemia.
The question of the appropriate TTG used in such a situation can also be discussed for the data shown in Figure 2 (time: 8 am): The hypoglycemic event that took place in the middle of the night induced differences between BG and ISF between 6 and 8 am after ingestion of carbohydrates, reflecting the restoration of glycogen stores.
Entity 3: Meal-Related Glucose Differences Between BG and ISF
After a meal, a rise in glycemia is induced by glucose uptake in the intestines, but this is not accompanied by a rise in ISF glucose levels at the same point in time. The reported time lag between glucose changes in both compartments is about 5 to 25 minutes.4,6,8-10 This range of time lag can be explained by the fact that the gradient in glucose increase in blood differs depending on the type and amount of carbohydrates in a given meal; that is, the increase in glycemia is steeper with a high glucose load and rapidly absorbable carbohydrates. Differences in prandial glucose profiles between blood and ISF most likely also depend on the glucose utilization rate in the given compartment: If the glycogen storage capacity in the liver is different in a given patient on different days, this might have an impact on how rapidly glucose is taken up by this organ and subsequently how rapidly ISF glucose levels decline. If patients use the ISF glucose value 60 minutes after starting a meal and adjust a correction dose to this value, this might be not adequate in relation to the BG value, which would be significantly higher if measured.
Are the Differences Between Blood and ISF Glucose Levels Simply Measurement Errors?
One might argue that the observed discrepancies between blood and ISF glucose levels are measurement “errors” in this specific patient or situation. Clearly it cannot be completely ruled out that in the specific examples shown technical issues, such as insufficient analytical measurement quality (especially in the lower glycemic range) and calibration mistakes, contribute to the observed differences. However, such differences between blood and ISF glucose levels when analyzing CGM profiles were observed over and over again. The assumption has thereby materialized is that these results derive from the underlying physiological mechanisms and not from insufficient measurement quality of the current CGM systems. Over the past years, the analytical measurement quality of CGM systems has improved drastically. The examples presented were obtained using current CGM systems.
For some reason, new technology is often blamed as the source of such differences and the established approach is regarded as the more trustworthy one. From our point of view, differences between the blood and ISF profiles are not “bad”; they are simply the result of the given physiological situation. In the human body, ISF glucose in the cells and in the brain is likely a more relevant physiological parameter than BG values. This would mean that the ISF glucose values are not of lower relevance than the BG values, but rather just the opposite. This, in turn, also means that the improvements observed over the last decade with the parameter used most often to characterize the analytical performance of CGM systems,11,12 the mean (or median) absolute relative difference (MARD), will never be as low as when two BG measurement systems (system refers to the combination of the meter and the test strips) are compared. The MARD will reach a certain limit due to basic differences between the two measurement approaches (see above). It is important to understand that a study protocol which induces only a small degree of deviation between blood and ISF glucose levels (ie, no high dynamic glucose changes) might result in a better MARD value even if the analytical performance of one CGM system is worse in comparison to a different one when it is challenged with more dynamic changes in glycemia.13,14
Consequences for Diabetes Management
Therapeutic decisions for diabetes management by patients with diabetes are usually based on BG measurements. In situations with no or low glucose dynamics, insulin therapy based on BG measurements results would be appropriate for an optimal glucose regulation (balancing exogenous glucose uptake from diet with exogenous insulin). However, in the case of a biological emergency, such as a hypoglycemic episode, glucose is shifted from glycogen stores that—until that point—are not included in the existing “basic diabetological concept” (adjustment between glucose absorption and pharmacodynamics of insulin). The consequence of this way of thinking is that the optimization of diabetes therapy should no longer be based on BG measurements but on CGM data.
According to the regulatory approval of CGM systems, patients using these systems are required to perform BG measurements to make their therapeutic decisions (insulin dose adjustments); in practice, patients often ignore this and base their decision on CGM data. In the near future, CGM systems may well receive approval for nonadjunctive usage, which would mean that CGM measurements could replace or substitute BG values. This could have helped avoid a situation such as the one presented above where the patient would have made the wrong decision during the cycling marathon had he based his decision on the BG measurement.
In daily practice, patients base their therapeutic decisions not only on the current glucose value being displayed but also the availability of the trend information and glucose profile of the last hours. Informed and well-trained patients can take such additional information into account for dose determination, especially when they see that rapid changes in glucose have taken place.
Need for Scientific Proof for the Hypothesis That ISF Values Should Be Used as TTG
From our point of view, clinical studies should be performed under standardized conditions to enable a systematic evaluation of all factors of relevance. Such studies would require frequent measurements of BG and ISF glucose in parallel to monitor the time point and course of differences in the glucose profiles. The findings obtained from these studies would confirm the relevance and more importantly the degree of correlation between ISF glucose in different compartments and capillary BG. It is possible that these kinds of studies were performed already but the analysis was not focused on differences between compartments during dynamic changes in glycemia. In the future, when analyzing CGM profiles obtained under daily life conditions, it would be beneficial to take into consideration information collected by the activity tracker built into smartphones. This might help detect certain patterns in the differences between blood and ISF glucose.
Clinical Proof of the Concept That ISF Glucose Should Be Used as TTG
From a historical point of view, BG measurements were introduced because they were relatively easy to perform. From a clinical standpoint, all evidence that optimization of metabolic control reduces the risk of developing diabetes-related complications is based on BG and HbA1c measurements. Clinical proof for the ISF glucose concept is vital to be able to make the switch from BG values to ISF glucose values as TTG. Thus, long-term clinical studies should be performed during which patients in one study arm use the CGM glucose values as TTG for insulin dosing decisions to optimize their therapy. The other study arm, or control group, should use BG values as TTG for optimization of glucose control. One would assume that in such patients (which should have some dynamics in their glucose profiles), relevant differences in frequency of hypoglycemic events, glucose variability, time in range, and so on would show up. Such studies would prove the hypothesis that CGM data are more relevant than BG values for the safety and efficacy of diabetes therapy. The question then becomes who might be willing to finance studies with a target of proving the hypothesis that diabetes management decisions should be based on CGM values and not on BG values? Another approach might be to initiate such a switch in a large group of patients over the span of several years and follow certain outcome parameters by means of a register.
In various clinical trials, varying rates of hypoglycemic events were observed when measurements were performed with a BG meters versus CGM devices:15 With BG measurements, one can only roughly estimate the number of low glucose values during the day and night, which indeed have their impact on the patients, might they perceive them or not.15-19 By contrast, CGM detects not only all hypoglycemic events but also makes it possible to evaluate the intensity of each hypoglycemic event. The strength and duration of a given hypoglycemic event (which might be clinically more important than the sheer number of such events) can be calculated as the area under the curve (AUC) for values below a defined hypoglycemic level (eg, 55 or 70 mg/dl) and the time spent below that threshold. From a physiological point of view, it is clear that the intensity of hypoglycemic events has an impact on the stability of glucose regulation in the hours after the event and probably even for longer periods of time. This is another reason why diabetes management in the future should be based on CGM recordings,7 just recently the FDA announced the approval of dosing of insulin based on CGM data.
Studies of even longer duration are needed to answer the question: what is more relevant to the long-term outcome with respect to developing diabetes-related late complications?
Discussion
CGM recordings provide a more complete picture of all factors influencing glucose profiles, uncovering conditions that would have remained hidden without CGM. Much important information does not become apparent solely using BG measurements and acting toward an inappropriate TTG might be the cause of a good part of the unpredictability of diabetes therapy—and this unpredictability is in turn a source of massive frustration for patients and health care providers. At first glance, the idea of switching from the very well-established standard BG monitoring to a different approach for therapeutic decisions might seem far-fetched and irrelevant as periods with high dynamic changes in glucose level readings represent only a certain period of time in a given patient. Nonetheless, precisely these entities presented above are relevant ones for therapeutic decisions.
Clearly there are a number of reasons why the proposed switch from BG measurement to CGM recordings can be viewed critically: Not least because the number of patients using CGM regularly (mainly patients with type 1 diabetes) is still small and varies extensively between countries, driven by the different reimbursement situations. However, CGM systems will become available for larger patients groups, either due to a price reduction and/or better reimbursement coverage. In this case, the question becomes crucial as to which measurement result will form the basis for diabetes management. Such a switch would also require patients and all members of the diabetes team to learn an at least somewhat different approach to diabetes management. It would also be necessary to reconsider current therapeutic strategies and definitions, for example, definitions of hypoglycemia. It is still unclear whether an optimization of diabetes management based on ISF glucose measurements will also be relevant for the long-term prognosis of patients with diabetes.
CGM delivers the opportunity to overcome the widespread “clinical inertia” in diabetes management, the data density of CGM will help to overcome the underlying dilemma that physicians still appear to favor error-prone, episodic measurements of glucose over reliable, accurate and verifiable continuous measurements of glucose. Clearly one would like to have more data about the reality of such discrepancies between CGM and SMBG, that is, how often do these show up? From our clinical experience, this depends critically on the situation of the given patient. In other words, if a patient underwent strenuous exercise several times on a given day, the discrepancies might show up each time. The same holds true if a patient has recurrent hypoglycemic events. However, a quantitative assessment of the frequency of discrepancies between CGM and SMBG would require the performance of respective clinical trials with a clearly defined experimental framework. It is our understanding, that the timing of these discrepancies varies to a given extent form event to event/patient to patient. Such a variability is to be expected, depending on the amount of glucose stored, insulinemia, and so on. In the future, when artificial pancreas systems become available, the relevance of using the appropriate TTG will further increase and more CGM systems will receive approval for nonadjunctive usage.
Footnotes
Abbreviations: AUC, area under the curve; BG, blood glucose; CGM, continuous glucose monitoring; DT, diabetes technology; ISF, interstitial fluid; MARD, mean (or median) absolute relative difference; SMBG, self-monitoring of blood glucose; TTG, therapeutic target glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TS has received lecture fees, consultancy fees or assistance on scientific projects from Abbott, Astra Zeneca/BMS, Becton Dickinson, Berlin-Chemie, Boehringer Ingelheim, Eli Lilly, Medtronic, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi. LH is a partner and scientific consultant of Profil Institute for Clinical Research, Inc, San Diego, CA, USA and Profil Institut für Stoffwechselforschung Neuss, Germany and is a consultant for a range of companies that develop new diagnostic and therapeutic options for the treatment of diabetes. RK has received lecture fees from Medtronic. AT is a full-time employee of Medtronic.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Rebrin K, Steil GM. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther. 2000;2(3):461-472. [DOI] [PubMed] [Google Scholar]
- 2. Rebrin K, Steil GM, Van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol. 1999;277:E561-571. [DOI] [PubMed] [Google Scholar]
- 3. Muchin S, Borzov A, Dreval A. Mathematical modeling of insulin and glucose transport on a vessel graph as potential new approach in diabetes modelling. Diabetes Technol Ther. 2013;15(suppl 1):A-140. [Google Scholar]
- 4. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes. 2013;62:4083-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davey RJ, Low C, Jones TW, Fournier PA. Contribution of an intrinsic lag of continuous glucose monitoring systems to differences in measured and actual glucose concentrations changing at variable rates in vitro. J Diabetes Sci Technol. 2010;4:1393-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keenan DB, Mastrototaro JJ, Voskanyan G, Steil GM. Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. J Diabetes Sci Technol. 2009;3:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siegmund T, Kolassa R, Thomas A. Continuous glucose monitoring (CGM) and sensor-augmented pump therapy (SAP). 2011. Unimed Science Verlag Bremen. ISBN 978-3-8374-1321-1. [Google Scholar]
- 8. Mazze RS, Strock E, Stout P, Racchini J, Wesley D, Borgman S. A novel methodology to evaluate continuous glucose monitoring accuracy and clinical representation of glucose exposure and variability. Diabetes. 2007;56 (suppl 1):A107. [Google Scholar]
- 9. Kulcu E, Tamada JA, Reach G, Potts OR, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26:2405-2409. [DOI] [PubMed] [Google Scholar]
- 10. Kovatchev BP, Shields D, Breton M. Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol Ther. 2009;11:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laffel L. Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther. 2016;18(suppl 2):S223-S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andelin M, Kropff J, Matuleviciene V, et al. Assessing the accuracy of continuous glucose monitoring (CGM) calibrated with capillary values using capillary or venous glucose levels as a reference. J Diabetes Sci Technol. 2016;10(4):876-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiterer F, Polterauer P, Schoemaker M, et al. Significance and reliability of MARD for the accuracy of CGM systems. J Diabetes Sci Technol. 2017;11(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pleus S, Haug C, Link M, et al. Rate-of-change dependence of the performance of two CGM systems during induced glucose excursions. Diabetes. 2014;63(suppl 1):A216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hay LC, Wilmshurst EG, Fulcher G. Unrecognized hypo- and hyperglycemia in well-controlled patients with type 2 diabetes mellitus: the results of continuous glucose monitoring. Diabetes Technol Ther. 2003;5:19-26. [DOI] [PubMed] [Google Scholar]
- 16. Sibenaler A, Boullu-Sanchiz S, Jean-Didier N, Pinget M, Meyer L. Detection of hypoglycemia with the continuous glucose monitoring system. Diabetes. 2003;52 (suppl 1):A99. [Google Scholar]
- 17. Gandrud LM, Dongyuan X, Kollman C, et al. The Medtronic MiniMed gold continuous glucose monitoring system: an effective means to discover hypo- and hyperglycemia in children under 7 years of age. Diabetes Technol Ther. 2007;9:307-316. [DOI] [PubMed] [Google Scholar]
- 18. Moreno C, Barros L, Baptista C, et al. Importance of retrospective continuous glucose monitoring in poorly controlled diabetic patients: a system that still has clinical usefulness. Diabetes Technol Ther. 2013;15(suppl 1):A67. [Google Scholar]
- 19. Calhoun PM, Buckingham BA, Maahs DM, et al. Efficacy of an overnight predictive low-glucose suspend system in relation to hypoglycemia risk factors in youth and adults with type 1 diabetes. J Diabetes Sci Technol. 2016;10(6):1216-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]