Abstract
Background:
Poor healing is an important contributing factor to amputation among patients with diabetic foot ulcers (DFUs). Physiological stress may slow wound healing and increase susceptibility to infection.
Objectives:
The objective was to examine the association between heart rate variability (HRV) as an indicator of physiological stress response and healing speed (HealSpeed) among outpatients with active DFUs.
Design and Methods:
Ambulatory patients with diabetes with DFUs (n = 25, age: 59.3 ± 8.3 years) were recruited. HRV during pre–wound dressing was measured using a wearable sensor attached to participants’ chest. HRVs were quantified in both time and frequency domains to assess physiological stress response and vagal tone (relaxation). Change in wound size between two consecutive visits was used to estimate HealSpeed. Participants were then categorized into slow healing and fast healing groups. Between the two groups, comparisons were performed for demographic, clinical, and HRV derived parameters. Associations between different descriptors of HRV and HealSpeed were also assessed.
Results:
HealSpeed was significantly correlated with both vagal tone (r = –.705, P = .001) and stress response (r = .713, P = .001) extracted from frequency domain. No between-group differences were observed except those from HRV-derived parameters. Models based on HRVs were the highest predictors of slow/fast HealSpeed (AUC > 0.90), while models based on demographic and clinical information had poor classification performance (AUC = 0.44).
Conclusion:
This study confirms an association between stress/vagal tone and wound healing in patients with DFUs. In particular, it highlights the importance of vagal tone (relaxation) in expediting wound healing. It also demonstrates the feasibility of assessing physiological stress responses using wearable technology in outpatient clinic during routine clinic visits.
Keywords: diabetes foot ulcers, wound healing, stress, wearable sensor, heart rate variability, wound dressing
Diabetic foot ulcers (DFUs), a leading cause of approximately 80% of lower-limb amputations,1 develop in at least 25% of patients with diabetes2 largely due to diabetic peripheral neuropathy.3 In the US adult population reported estimates of the prevalence of diagnosed diabetes have increased from 5.9% in 20004 to 6.4% (~28.5 million) in 2010.5 Whereas in 2005 a limb was lost to diabetes every 30 seconds, the frequency of limb loss has increased to every 20 seconds in 2011.5,6 Chronic DFUs also dramatically compromise physical activity pattern which is significantly associated with falls and can lead to serious complications, such as fractures, hospital admissions and poor quality of life.7,8 The economic costs associated with diabetic foot care, including amputation care, represent the single largest category of excess medical expenses related to diabetes. In the United States, the total annual cost for diabetic limb complications is estimated to be $17 billion, which exceeds the annual costs of expenses related to breast cancer and to colorectal cancer.9
To avoid the adverse outcomes associated with DFUs and reduce the risk of amputation, it is necessary to expedite the wound healing process. Wound healing is a dynamic process,10 and multiple factors including local (e.g. oxygenation, infection, etc) and systematic (e.g. demographics, nutrition, medication, stress, etc). Both local and systematic factors can impair the process of wound healing.11-14
Physiological stress is a systematic response to a stressor that facilitates adaptation to meet the challenge. The autonomic responses involved in modulating physiological stress include activation of the sympathetic and parasympathetic nervous systems, which work in tandem to keep the body in a state of homeostasis. During a stressful event, the sympathetic nervous system predominates, resulting in the fight-or-flight response. Because the body cannot maintain this state for extended periods of time, the parasympathetic system returns the body’s physiological conditions to the normal rest-and-digest state. Although the sympathetic physiological response is essential to protect the body and adapt to the stressors, prolonged exposure to stressors, referred to as episodic acute stress, can have an adverse impact on both psychological and physiological health and may affect the wound healing process.15
Wound healing is a complex and fragile process, and stress may interrupt or lead to the formation of nonhealing chronic wounds in DFU patients. Stress disrupts the wound healing process primarily by mediating the hypothalamic-pituitary-adrenal and sympathetic-adrenal medullary axes as well as the psychological response, inducing unhealthy behaviors such as sedentary lifestyle, smoking, and so on.11 Several studies confirmed the association between poor wound healing and stress by quantifying the presence of physiological stress.11-14
Several approaches have been proposed to quantify the presence of stress. These approaches include use of both subjective and objective tools. Questionnaires are a subjective method used to measure the patient’s perceived stress or mental strain. Objective methods record measurements induced stress via vital signs or biomarkers, such as cortisol sampling, heart rate, heart rate variability (HRV), blood pressure, sweating, and galvanic skin response.13,16-22
The effect of physiological stress on the wound healing process has not been explored specifically within a population with diabetes at high risk of limb amputation. However, studies have demonstrated that management of stress has a positive effect in diabetes patients.23 Studies have also measured pain and stress during dressing changes in patients with a variety of chronic wounds using objective measures such as heart rate, blood pressure, and respiration rate. It has been suggested that wound dressing is a painful and stressful condition. Applying interdisciplinary approaches may help to reduce adverse outcomes such as pain and lower limb amputations in DFUs.9
Advances in technology allow assessing phsyiological stress response by wearable wireless sensors. These sensors have few important advantages over wired alternatives including ease of use, comfort, enhanced freedom of mobility, and long term monitoring irrespective of setting (e.g. in home, in clinic, or in research laboratory).24-28 Our previous research assessed the level of physiological stress response in patients with DFUs during outpatient clinical visits and found the existence of high stress during wound dressing and wound care (e.g debridement, dressing change, etc).29 In this exploratory study of patients with DFUs, we examined the physiological stress response in an outpatient wound care setting to determine the association between wound healing speed and phsyiological stress responses. This was done through quantification of HRV in time and frequency domains, which allow quantification of sympathetic regulation (indicator of stress) and vagal tone activity (indicator of relaxation).
Method
Participants’ Recruitment and Instruments
A convenience sample of ambulatory patients with non-infected and non-ischemic DFUs was recruited from The Diabetic Foot and Wound Center at the Hamad Medical Corporation, Doha, Qatar. Informed consent in agreement with the procedure of Hamad Medical Corporation was collected from each participant. The existence of neuropathy was confirmed via medical records and by Semmes-Weinstein Monofilament testing. Real-time ECG and heart rate data were collected using a body-worn device, BioHarness3 (Zephyr Technology Corp, Annapolis, MD) using the procedure described in our previous study.29 Briefly, the device was attached to the chest using two electrocardiography (ECG) patches, and it recorded uni-channel ECG (250 Hz), respiration speed, accelerations and approximate core body temperature. ECG data were collected for approximately 10-15 minutes during the waiting period before wound dressing. The time was logged by a trained research assistant to synchronize with the bio-patch clock. The sensor allows for recording ECG during the entire duration of outpatient clinic visit—from the waiting room period (pre–wound dressing change [preWD]) to the completion of the wound care process, including debridement and wound dressing. The ECG measurements were recorded only during the first visit, but wound healing outcomes were assessed at baseline and the next clinic visits. Specifically, the wound area at the first and the second clinical visit were measured (21 ± 4 days apart) and the percentage of change was used to determine the wound healing speed.
Clinical Assessment
Along with demographic information, several questionnaires were used to assess participants’ characteristics including self-reported Mobility-Tiredness (Mob-T) Scale,30 Barthel index,31 depression scale (CES-D),32 perceived stress scale,33 Falls Self-Efficacy Scale (FES-I),34 Short Health Survey (SF12),35 and 0-10 Numeric Pain Rating Scale.36 In addition, we collected the glycated hemoglobin or HbA1c level.
HRV Analysis and Quantification of Physiological Stress Response
The recorded sensor ECG data were transferred to a computer and fed into an open source software, Kubios, for HRV analysis.37 The R-waves were extracted from the ECG signal and manually inspected for correction to estimate R-wave-to R-wave (NN) intervals. The time and frequency domain parameters were obtained for each nonoverlapping five-minute windows following the recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology.38 Average NN intervals and average heart rate (HR-wave) were reported. The standard deviation of NN intervals (SDNN), which represents the variations in the R-to-R waves, was calculated with lower SDNN indicating the presence of physiological stress. Root mean squared of successive NN intervals (RMSSD), another time domain parameter commonly used as an index of vagally (vagal nerve) mediated cardiac control (relaxation), was calculated.38 To measure frequency domain HRV parameters, we used the recommendation provided by the European Society of Cardiology guideline.38 In summary, the power spectrum density (PSD) of time series representing R-wave-to-R-wave intervals was calculated. The frequency band of the PSD was divided into four bands: very low frequency (VLF, 0-0.04 Hz), low frequency (LF, 0.04-0.15 Hz), and high frequency (HF, 0.15-0.4 Hz). Then, the area under the curve for each frequency band, was calculated to estimate the energy localized at each frequency band. The normalized energy value at each frequency band was calculated using the following formulas: normalized LF (nLF) = LF/(LF+HF) × 100, normalized HF (nHF) = HF/(LF+HF)x100, and LF-to-HF ratio (LF/HF) = LF/HF.38 The nLF represents the sympathetic regulation (stress indicator). The nHF represents the parasympathetic regulations (relaxation indicator) of the heart. LF/HF ratio, which indicates the balance between the sympathetic and parasympathetic activity of heart, was also estimated with higher values representing a more stressful condition.38
Statistical Analysis
Correlations between various measured parameters and HRV were determined using Pearson correlation coefficients (r). Based on the level of correlation, three levels were defined as follows: (1) weak (r = ±.10 to ±.29), (2) moderate (r = ±.30 to ±.49), (3) strong (r = ±.50 to ±1.0).39
Based on the distribution of the “speed of wound healing” variable, the median split (–46.7%; the negative value indicates reduction in wound size in the follow-up visit)13 was chosen as a cutoff for categorizing participants into two groups: fast healing and slow healing. Independent t-test was performed to compare baseline differences between the two groups. A further univariate analysis was carried out to identify independent predictors between the two groups and adjusted to wound size at the baseline. To quantify the difference between two fast healing and slow healing groups, the effect size was reported based on the partial eta-squared (η2) for each parameter. The η2 was interpreted as small (~.02), medium (~.13), and large (~.26) effect size.40 Two different discriminant analysis models were tested to predict wound healing rate classification group. Model 1 used the long-term blood glucose level marker (HbA1c) and years of diabetes. Model 2 used HRV parameters from the preWD period. The receiver operating curve (ROC), the area under the curve (AUC), along with sensitivity, specificity, and accuracy were reported. Because of the small sample size, we used leave-one-out cross-validation and the performance of classifiers was reported for the validation set.41 The canonical correlation of coefficient was used to report the goodness of classification to discriminate fast- and slow- healers (dependent variables) based on independent variables such as physiological stress values, HbA1c, and years of diabetes. A high correlation of coefficient indicates a better goodness of classification.42 A P value of .05 or less was considered as statistical significant level. All the analyses were performed in SPSS v22 (IBM Corp, Armonk, NY, USA).
Results
A total of 25 eligible participants recruited and completed baseline and follow-up visits. However, the data from 19 subjects (age = 59.3 ± 8.3 years, 83% male), who had valid recorded data included in the final analysis. The reasons for excluding six subjects were, observed arrhythmias and/or low signal quality (e.g. sensor frequent disconnections, poor sensor attachment, etc), which make difficult to accurantly estimate HRV (retention = 76%). Ten participants classified as slow-healers and 9 participants classified as fast-healers. Table 1 summarizes demographic and clinical data for each group. The speed of wound healing was 80% higher in the fast-healing group compared to the slow-healers (P < .000, 95% CI = [34, 65]%, η2 = .726). None of the demographics (eg, age, BMI) nor baseline clinical characteristics (eg, years with diabetes, HbA1c, baseline wound sizes) were different between groups (P > .050). On the same note, none of the standard questionnaires, which describe the quality of life, depression, mobility, the perception of pain, and fear of falling were different between groups (P > .050). Surprisingly, the perception of stress (i.e. psychological stress) as assessed using questionnaire, was not different between groups (P = .764). However, several HRV derived parameters were significantly different between groups.
Table 1.
Univariate Analysis of Descriptive Variables and Heart Rate Variability (HRV) Between Slow Healing and Fast Healing Groups (n = 19).
| Slow healing (mean ± SD), n = 10 | Fast healing (mean ± SD), n = 9 | Difference (%) | 95% CI | P value | Partial η2 | |
|---|---|---|---|---|---|---|
| Age, years | 60.2 ± 8.6 | 58.9 ± 8.6 | 6.3 | −7.2; 9.4 | .784 | .005 |
| Body mass index (BMI), kg/m3 | 25.1 ± 3.2 | 25.5 ± 1.8 | −0.3 | −3.3; 2.5 | .762 | .008 |
| Years in diabetes | 13.3 ± 6.3 | 13.4 ± 6.0 | −30.6 | −6.1; 5.9 | .982 | .000 |
| Baseline wound area, mm2 | 12.0 ± 22.1 | 14.6 ± 41.3 | −2.61 | −33.4; 28.5 | .826 | .002 |
| Speed of wound healing, % | −15.4 ± 18.7 | −64.9 ± 12.9 | 79.5 | 34.4; 64.6 | .000* | .726 |
| Glycated hemoglobin (HbA1c),% | 8.4 ± 1.0 | 9.4 ± 3.3 | −15.5 | −4.2; 2.1 | .472 | .053 |
| Mobility tiredness scale | 3.2 ± 3.1 | 3.0 ± 2.8 | 5.3 | −3.9; 4.3 | .929 | .001 |
| Barthal index | 94.2 ± 14.3 | 95.0 ± 11.2 | −0.9 | −18.6; 17.0 | .918 | .001 |
| CES-D depression scale | 19.3 ± 10.3 | 16.6 ± 3.4 | 14.1 | −8.2; 13.6 | .585 | .034 |
| Fear of falling scale | 36.7 ± 11.1 | 35.6 ± 17.3 | 2.9 | −18.4; 20.5 | .904 | .002 |
| 0-10 Numeric Pain Intensity Scale | 2.8 ± 4.4 | 3.2 ± 4.6 | −12.9 | −6.5; 5.8 | .896 | .002 |
| SF12-PCS | 34.0 ± 10.9 | 36.4 ± 11.7 | −6.8 | −17.7; 13.1 | .741 | .013 |
| SF12-MCS | 47.8 ± 14.1 | 48.0 ± 11.6 | −0.4 | −18.0; 17.6 | .980 | .000 |
| Perceived stress scale | 16.7 ± 4.4 | 15.8 ± 4.9 | 5.2 | −5.5; 7.2 | .764 | .011 |
| SDNN, milliseconds | 30.4 ± 12.1 | 26.7 ± 14.2 | 14.2 | 12.4; 17.4 | .539 | .023 |
| RMSSD, milliseconds | 11.2 ± 5.8 | 13.7 ± 4.8 | −21.6 | 9.9; 17.4 | .334 | .055 |
| nLF, % | 70.4 ± 8.7 | 41.1 ± 17.9 | −41.7 | 16.0; 42.7 | .000* | .558 |
| nHF, % | 29.5 ± 8.7 | 58.7 ± 17.9 | 99.1 | −42.6; –15.8 | .000* | .555 |
| LF/HF, ratio | 2.8 ± 1.7 | 0.9 ± 0.8 | −68.5 | 0.6; 3.2 | .006* | .368 |
The results were adjusted by the wound size at the baseline.
P ≤ .05.
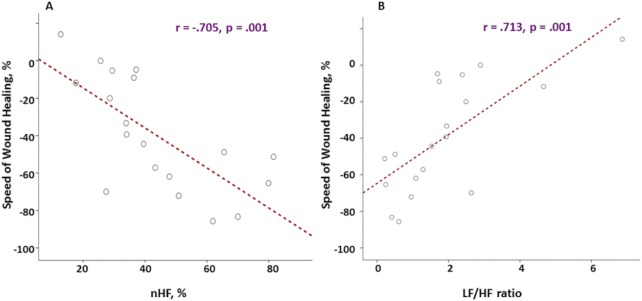
When the correlation between speed of wound healing and HRV drived parameters was examined irrespective of group assignment, a strong correlation was observed between wound healing speed and nHF (indication of vagal tone or relaxation, r = –.705, P = .001), LF/HF ratio (indication of stress, r = .713, P = .001), and nLF (indicator of stress, r = 706, P = .001) (Figure 1). A moderate but significant correlation (r = –.570, P = .014) was also observed between depression score and vagal tone as well as between CES-D depression scale and RMSSD (r = –.570, P = .014). None of other measured parameters including demographics, clinics, and questionnaires data were associated with the speed of wound healing (r = .017-.343, P > .050).
Figure 1.
The association between the speed of wound healing and heart rate variability (HRV). Column A: the nHF, which represents the vagal tone (relaxation) is higher in individuals with better healing pace. Column B: the LF/HF ratio, which represents the sympathetic (stress) is lower in the individuals with better healing speed. The y-axis represented the speed of wound healing in percentage ( = ).
Among the HRV parameters, nLF (indicator of physiological stress response) was significantly lower on average by 41.7% in the fast healers than the slow healers (P < .001, η2 = .558) (Figure 2A). Similarly, nHF (indicator of vagal tone or relaxation) was higher on average by 99.1% in the fast healers compared to the slow healers with a large effect size (P < .001, η2 = .555) (Figure 2A). When the balance between sympathetic and parasympathetic was assessed using LF/HF ratio, a lower balance toward stress by an average of 68.5% was observed in the fast healing group compared to the low healing group (P = .006, η2 = .368) (Figure 2B). The largest effect size to describe stress response in the fast healing group observed in the vagal tone response with an effect size of η2 = .555 indicating the higher ability of relaxation in the fast healers.
Figure 2.
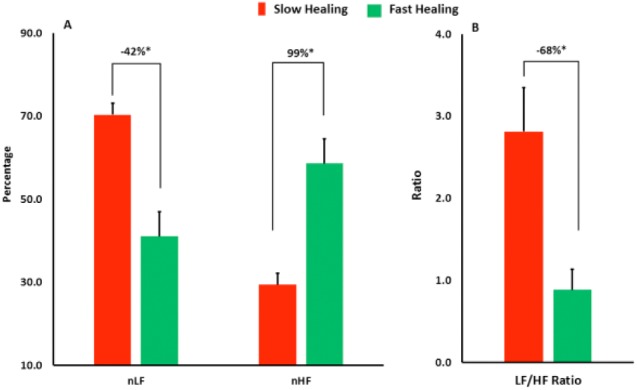
Comparing the speed of wound healing in the two groups: patients with fast healing speed and patients with slow healing speed. The univariate analysis shows that several heart rate variability parameters are independent predictors between the two groups such as normalized low frequency (nLF), normalized high frequency (nHF), and the ratio of low frequency to high frequency. *Difference is statistically significant (P value < .05). The analysis adjusted for the wound size at the baseline.
The predictive powers of demographic, clinical, and HRV parameters were assessed using discriminant analysis. Model 1 included demographics as well as patient’s level of HbA1c and years of diabetes, as potential independent predictors of wound healing rate, as suggested in the previous study43 (Figure 3). However, test of equality found both parameters to be nonsignificant (P > .400) contributors in our sample. The canonical correlation of coefficient for the model 1 was 0.231 indicating weak goodness of classification when demographic (age, BMI, etc) and clinical data (HbA1c and years of diabetes) were used as independent variables. The model yielded a cross-validation accuracy of 25% with sensitivity of 33.3% and specificity of 16.7% (Table 2).
Figure 3.
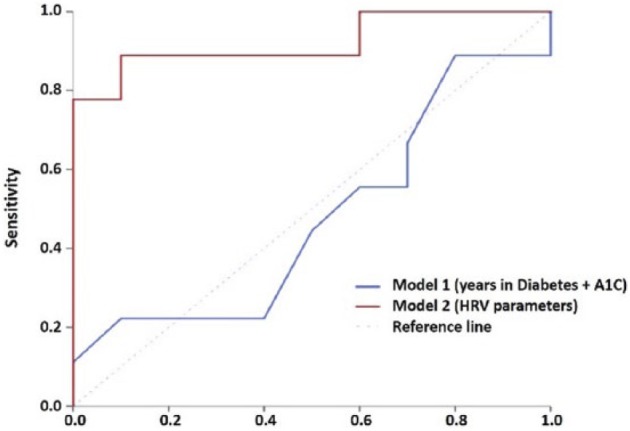
Receiver operating curve (ROC) of different models for predicting slow wound healing speed. Model 1. Demographic information of participants, ie, years in diabetes and HbA1c level. Model 2. The HRV parameters from the pre–wound dressing (preWD) interval.
Table 2.
Comparison of Logistic Models.
| Parameters | Sensitivity, % | Specificity, % | Accuracy, % | AUC |
|---|---|---|---|---|
| Model 1: Demographic and clinical parameters | 33.3 | 16.7 | 25.0 | 0.478 |
| Model 2: HRV parameters | 100 | 77.8 | 89.5 | 0.922 |
Demographic parameters, model using years in diabetes and A1c as input parameters; HRV parameters, model using HRV parameters.
Model 2 included nLF, nHF, and LF/HF. Test of equality found all three parameters to be significant (P < .05) contributors toward prediction of the fast healers (Figure 3). The canonical correlation of coefficient for HRV model was 0.748 indicating high goodness of classification when physiological stress derived parameters were used as independent variables. The model yielded a cross-validation accuracy of 89.5% with sensitivity of 100% and specificity of 77.8% (Table 2).
Discussion
The study assessed the association between physiological stress responses and wound healing outcomes among patients with diabetes who had plantar ulcers. While prior studies have demonstrated a negative association between psychological stress and acute wound healing processes44 or chronic wound healing process,45 little is known about the association between physiological stress response and chronic wound healing in patients with DFUs. This study proposed heart rate variability (HRV) measurement to objectively quantify physiological stress responses and examined its association with wound healing outcomes in diabetes patients with DFUs.
We did not observe an association between subjective stress assessment (eg, questionnaires) and wound healing speed. In contrast, objective stress assessment of physiological stress showed significant correlation with wound healing speed. Subjective tools like questionnaires assess patients’ emotional and psychological responses.20 Thus, questionnaires can sometimes cause patients to report high levels of stress to meet the expectations of the health care assistants.20 Similarly, patients may be accustomed to answering questions in a certain way, such as selecting the middle options on a rating scale rather than the extreme responses.20,46 Miller et al.47 examined more than 300 empirical articles describing a relationship between psychological stress and parameters of the immune system in human participants and revealed that while chronic stressors are associated with suppression of both cellular and humoral measures, subjective reports of stress generally did not associate with immune changes, highlighting limitation of subjective assessment of stress for chronic wound healing studies. A newly published study confirmed a lack of efficacy in subjective stress assessments to indicate the level of physiological stress in the participants.48 Thus, it has been reported that no correlation existed between subjective and objective metrics of stress; therefore, the self-stress evaluation questionnaires may not adequately capture the existence of physiological stress.
Several studies indicated that wound pain is a common cause of distress in patients with acute or chronic wounds.20,49 In patients with diabetes who had foot ulcers, wounds developed because of loss of protective sensation (diabetic peripheral neuropathy). Therefore, the previous conceptual model for the chronic wound which claimed wound pain as a primary source of discomfort may not be applicable in DFU patients. In this study, 67% of participants had mild pain (pain score ≤ 3.4) and no significant association between wound healing and pain observed. However, there is a need for further investment in DFU patients to build a better conceptual model which represents the underlying physiological process related to wound healing process and pain. One of the physiological factors is poor sleep, which has been shown to be underdiagnosed in the patient with chronic wounds.50 Poor sleep reduces the vagal tone/relaxation and increases the sympathetic/stress activation. Such a process may cause vasoconstriction which decreases perfusion in the wound area51 or may cause higher cortisol level in blood which increases blood sugar (HbA1c) and slows the wound healing process.51 In addition, sleep apnea, with its high prevalence in patients with diabetes,50 reduces the saturated oxygen level in the blood50 and speed of wound healing. Further study is needed to examine whether management of sleep disorders/stress may assist in reducing stress/sleep quality and improving wound healing outcomes.
A negative correlation (r = –.570, P = .014) was observed between depression and vagal tone (relaxation) suggesting the negative impact of depression on the ability to relax. Values of RMSSD revealed that patients with lower relaxation capability suffer from higher emotional depression status. These findings are in line with those in a previous study demonstrating the impact of depression on endocrine and immune function which triggers stress as a modulator of the healing process.52 In this study, the speed of wound healing and depression scale had no correlation. This finding is in contrast with a previous report on the chronic wound from Cole-King et al. in 2001.53 Cole-King et al. used (1) a hospital anxiety and depression Scale rather than CES-D, which has been reported to be a validated tool in the population of patients with diabetes,54 (2) a low cut point (9) while the generally recommended cutoff point is 11 for existence of depression,55 while all of our participants (except one) had a depression scale more than 10. Our results suggest that there may be a need for depression therapy along with stress management to reduce adverse outcomes associated with wound healing in patients with DFUs.
HRV parameters extracted form frequency domains (ie, nLF, nHl, and LF/HF) have achieved to significantly discriminate between slow or fast healers. While a trend was also observed for HRV parameters extracted from time-domain (i.e., SDDNN and RMSSD), these parameters didn’t achieve statistical significant level in our sample to discriminate between groups. This may suggest that HRV frequency derived parameters may be more sensitive to track stress/relaxation in people with diabetes and foot ulcers during short term monitoring (ie, pre–wound dressing interval). This finding is aligned with a previous study demonstrating that while there is an agreement between short-term (30 minutes) and long-term (24 hours) frequency domain HRV parameters, this agreement is diminished when time domain HRV parameters were examined.56 This may suggest that for an accurate information extracted from time domain HRV parameters, a longer recording (ie, minimum 2 hours57) may be needed.
This study suggests that frequency domain HRV parameters derived from a single chest worn sensor module yields a robust prediction model superior than a model based on demographic or clinical parameters (AUC > 0.9 for the model based on HRV vs AUC < 0.50 for a model based on demographic and clinical data; Figure 3). This finding is in line with a previous study43 demonstrating that nonmodifiable demographic (ie, sex, age, years in diabetes) or clinical (baseline wound size) factors are unable to predict the outcome of wound healing in a single visit.
Limitations
In this exploratory study, we faced a few limitations. The sample size in this study was small and may not have sufficient power to confirm significant statistical correlation with parameters of interest. The results should be confirmed in higher powered study. The time between the second visit and the first visit was not strictly controlled, which may affect the estimation of wound healing speed in our sample. We focused only on the pre–wound dressing period when the patient was in the waiting room and prior visiting his or her doctor, assuming that this time may better represent overall physiological stress in the subject. Another study is warranted to confirm whether the pre–wound dressing period could represent daily physiological stress response in diabetes patients with DFU. Despite these limitations, the results are encouraging and warrant future longitudinal studies with larger sample size to confirm the results.
Conclusions
The current study highlights the associations between physiological stress and speed of wound healing in patients with DFUs. The results also emphasize the potential of HRV and in particular the metrics representing the ability of relaxation to be a significant predictor of wound healing outcomes. The results of this exploratory study warrant further investigation in a large sample size. Our approach to measuring the association between physiological stress and speed of wound healing may open new avenues to better target slow healers and implement stress management strategies to speed up wound healing and prevent limb amputation in patients with diabetes.
Acknowledgments
We wish to thank Dr Saman Parvaneh, Ms Yuxi Xia, Mr Robert Menzies, and Mrs Samira Abdulla, who contributed in part to data analysis, data collection, or patient recruitment. The authors would like to thank Kimberly Macellaro, PhD, a member of the Baylor College of Medicine Michael E. DeBakey Department of Surgery Research Core Team, for her editorial assistance during the preparation of this manuscript.
Footnotes
Abbreviations: ANOVA, analysis of variance; AUC, area under the curve; BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression; CI, confidence interval; DFU, diabetic foot ulcer; ECG, electrocardiography; FES-I, Falls Self-Efficacy Scale; HRV, heart rate variability; IRB, institutional review board; LF/HF, ratio of low frequency components to high frequency components of normalized R-to-R waves; nHF, normalized high frequency components of normalized R-to-R waves; nLF, normalized low frequency components of normalized R-to-R waves; RMSSD, root mean squared of successive normalized R-to-R wave; ROC, receiver operating characteristic; SD, standard deviation; SDNN, standard deviation of normalized R-to-R wave; SF12, Short Health Survey; SF12-MCS, Mental Health Composite Scale score of SF12; SF12-PCS, Physical Health Composite Scale score of SF12.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported in part by Award Number NPRP 4-1026-3-277 from the Qatar National Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Qatar National Research Foundation.
References
- 1. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513-521. [DOI] [PubMed] [Google Scholar]
- 2. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217-228. [DOI] [PubMed] [Google Scholar]
- 3. Vileikyte L. Psychosocial and behavioral aspects of diabetic foot lesions. Current Diabetes Rep. 2008;8(2):119-125. [DOI] [PubMed] [Google Scholar]
- 4.Prevalence of diabetes and impaired fasting glucose in adults—United States, 1999-2000. MMWR Morb Mortal Wkly Rep. 2003;52(35):833-837. [PubMed] [Google Scholar]
- 5. World Diabetes Atlas. 4th ed. Brussels, Belgium: International Diabetes Federation; 2011. [Google Scholar]
- 6. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719-1724. [DOI] [PubMed] [Google Scholar]
- 7. Andersen H. Motor dysfunction in diabetes. Diabetes Metab Res Rev. 2012;28(suppl 1):89-92. [DOI] [PubMed] [Google Scholar]
- 8. Wallace C, Reiber GE, LeMaster J, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25(11):1983-1986. [DOI] [PubMed] [Google Scholar]
- 9. Barshes NR, Sigireddi M, Wrobel JS, et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabetic Foot Ankle. 2013;4:PMC3796020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994;2(3):165-170. [DOI] [PubMed] [Google Scholar]
- 11. Guo Sa, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736-1743. [DOI] [PubMed] [Google Scholar]
- 13. Ebrecht M, Hextall J, Kirtley L-G, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology. 2004;29(6):798-809. [DOI] [PubMed] [Google Scholar]
- 14. Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2007;13(5-6):337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Backe EM, Seidler A, Latza U, Rossnagel K, Schumann B. The role of psychosocial stress at work for the development of cardiovascular diseases: a systematic review. Int Arch Occup Environ Health. 2012;85(1):67-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361-370. [DOI] [PubMed] [Google Scholar]
- 17. Upton D, Solowiej K. Pain and stress as contributors to delayed wound healing. Wound Pract Res. 2010;18(3):114. [Google Scholar]
- 18. Spielberger CD. Manual for the State-Trait Anxiety Inventory STAI (Form Y)., Palo Alto, CA: Consulting Pschologists Press; 1983. [Google Scholar]
- 19. Solowiej K, Mason V, Upton D. Psychological stress and pain in wound care, part 2: a review of pain and stress assessment tools. J Wound Care. 2010;3:110-115. [DOI] [PubMed] [Google Scholar]
- 20. Solowiej K. Pain-induced stress in wound care, part 2: assessment and management. Brit J Healthcare Assistants. 2010;4(9). [Google Scholar]
- 21. Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S. eds. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 22. Cherrington CC, Moser DK, Lennie TA. The physiological response in patients with acute myocardial infarction to the administration of psychological instruments. Biol Res Nurs. 2002;4(2):85-91. [DOI] [PubMed] [Google Scholar]
- 23. Surwit RS, Van Tilburg MA, Zucker N, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25(1):30-34. [DOI] [PubMed] [Google Scholar]
- 24. Wise KD, Anderson DJ, Hetke JF, Kipke DR, Najafi K. Wireless implantable microsystems: High-density electronic interfaces to the nervous system. Proc IEEE. 2004;92(1):76-97. [Google Scholar]
- 25. Townsend KA, Haslett JW, Tsang TKK, El-Gamal MN, Iniewski K. Recent advances and future trends in low power wireless systems for medical applications. In: Proceedings of the Fifth International Workshop on System-on-Chip for Real-Time Applications. IEEE, 2005:476-481. [Google Scholar]
- 26. Hao Y, Foster R. Wireless body sensor networks for health-monitoring applications. Physiol Meas. 2008;29(11):R27-R56. [DOI] [PubMed] [Google Scholar]
- 27. Jovanov E, Lords AO, Raskovic D, Cox PG, Adhami R, Andrasik F. Stress monitoring using a distributed wireless intelligent sensor system. IEEE Eng Med Biol. 2003;22(3):49-55. [DOI] [PubMed] [Google Scholar]
- 28. Jovanov E, O’Donnel A, Morgan A, Priddy B, Hormigo R. Prolonged telemetric monitoring of heart rate variability using wireless intelligent sensors and a mobile gateway. P Ann Int IEEE Embs. 2002:1875-1876. [Google Scholar]
- 29. Parvaneh S, Grewal GS, Grewal E, et al. Stressing the dressing: assessing stress during wound care in real-time using wearable sensors. Wound Med. 2014;4:21-26. [Google Scholar]
- 30. Avlund K, Kreiner S, Schultz-Larsen K. Functional ability scales for the elderly. Eur J Public Health. 1996;6(3):35-42. [Google Scholar]
- 31. Wade D, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988;10(2):64-67. [DOI] [PubMed] [Google Scholar]
- 32. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385-401. [Google Scholar]
- 33. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:385-396. [PubMed] [Google Scholar]
- 34. Delbaere K, Close JC, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing. 2010;39(2):210-216. [DOI] [PubMed] [Google Scholar]
- 35. Ware JE, Keller SD, Kosinski M. SF-12: How to Score the SF-12 Physcial and Mental Health Summary Scales. 3rd ed. Boston, Mass: The Health Institute, New England Medical Center; 1998. [Google Scholar]
- 36. Zelman DC, Dukes E, Brandenburg N, Bostrom A, Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain. 2005;115(1):29-36. [DOI] [PubMed] [Google Scholar]
- 37. Tarvainen MP, Niskanen JP, Lipponen J, Ranta-Aho P, Karjalainen P. Kubios HRV—a software for advanced heart rate variability analysis. In: 4th European Conference of the International Federation for Medical and Biological Engineering Amsterdam, Netherlands: Springer; 2009:1022-1025. [Google Scholar]
- 38. Task Force of the European Society of Cardiology. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354-381. [PubMed] [Google Scholar]
- 39. Cohren J. Statistical Power Analysis for the Behavioural Sciences. New York, NY: Lawrence Erlbaum; 1988. [Google Scholar]
- 40. Pierce CA, Block RA, Aguinis H. Cautionary note on reporting eta-squared values from multifactor ANOVA designs. Educ Psychol Meas. 2004;64(6):916-924. [Google Scholar]
- 41. Tabachnick BG, Fidell LS, Osterlind SJ. Using Multivariate Statistics. In: Allyn, Bamberger Bacon, ed. 4th ed Needham Heights, Mass: Allyn and Bacon; 2001. [Google Scholar]
- 42. Thompson B. Canonical Correlation Analysis: Uses and Interpretation. Newbury Park, CA: Sage; 1984. [Google Scholar]
- 43. Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol. 2011;131(10):2121-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gouin JP, Kiecolt-Glaser JK. The impact of psychological stress on wound healing: methods and mechanisms. Immunol Allergy Clin North Am. 2011;31(1):81-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Upton D, Solowiej K, Hender C, Woo K. Stress and pain associated with dressing change in patients with chronic wounds. J Wound Care. 2012;21(2). [DOI] [PubMed] [Google Scholar]
- 46. Johnston M, Wright SC, Weinman J. Stress, Emotion and Life Events. Measures in health psychology: A user’s portofilo. Windsor: NFER-NELSON; 1995. [Google Scholar]
- 47. Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS, Treanor JJ. Psychological stress and antibody response to influenza vaccination: when is the critical period for stress, and how does it get inside the body? Psychosom Med. 2004;66(2):215-223. [DOI] [PubMed] [Google Scholar]
- 48. Joseph B, Parvaneh S, Swartz T, et al. Stress among surgical attendings and trainees: A quantitative assessment during trauma activation and emergency surgeries. J Trauma Acute Care Surg. 2016;81:723-728. [DOI] [PubMed] [Google Scholar]
- 49. Upton D, Solowiej K. The impact of atraumatic vs conventional dressings on pain and stress. J Wound Care. 2012;21(5):209-215. [DOI] [PubMed] [Google Scholar]
- 50. Patt BT, Jarjoura D, Lambert L, et al. Prevalence of obstructive sleep apnea in patients with chronic wounds. J Clin Sleep Med. 2010;6(6):541. [PMC free article] [PubMed] [Google Scholar]
- 51. Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008;4(3):261-272. [PMC free article] [PubMed] [Google Scholar]
- 52. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cole-King A, Harding KG. Psychological factors and delayed healing in chronic wounds. Psychosom Med. 2001;63(2):216-220. [DOI] [PubMed] [Google Scholar]
- 54. McHale M, Hendrikz J, Dann F, Kenardy J. Screening for depression in patients with diabetes mellitus. Psychosom Med. 2008;70(8):869-874. [DOI] [PubMed] [Google Scholar]
- 55. Stafford L, Judd F, Gibson P, Komiti A, Quinn M, Mann GB. Comparison of the Hospital Anxiety and Depression Scale and the Center for Epidemiological Studies Depression Scale for detecting depression in women with breast or gynecologic cancer. Gen Hosp Psychiatry. 2014;36(1):74-80. [DOI] [PubMed] [Google Scholar]
- 56. Voss A, Schroeder R, Vallverdú M, et al. Short-term vs. long-term heart rate variability in ischemic cardiomyopathy risk stratification. Frontiers Physiol. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Singh JP, Larson MG, O’Donnell CJ, et al. Association of hyperglycemia with reduced heart rate variability (the Framingham Heart Study). Am J Cardiol. 2000;86(3):309-312. [DOI] [PubMed] [Google Scholar]