Abstract
JC virus (JCV) is a human polyomavirus that infects the central nervous system (CNS) of immunocompromised patients. JCV granule cell neuronopathy (JCV-GCN) is caused by infection of cerebellar granule cells, causing ataxia. A 77-year-old man with iatrogenic lymphopenia presented with severe ataxia and was diagnosed with JCV-GCN. His ataxia and CSF improved with intravenous immunoglobulin, high-dose intravenous methylprednisolone, mirtazapine, and mefloquine. IL-7 therapy reconstituted his lymphocytes and reduced his CSF JCV load. One month after IL-7 therapy, he developed worsening ataxia and CSF inflammation, which raised suspicion for immune reconstitution inflammatory syndrome. Steroids were restarted and his ataxia stabilized.
Keywords: JC virus, granule cell neuronopathy, cerebellar ataxia, lymphopenia, IL-7
Introduction
JC virus (JCV) is a human polyomavirus with high seroprevalence that causes a quiescent infection in kidneys and urinary tract epithelium (Gheuens et al. 2013). JCV invades the brain of immunocompromised people, causing lytic infections of oligodendroglial cells and demyelinating white matter lesions, which is known as progressive multifocal leukoencephalopathy (PML) (Brew et al. 2010).
Rarely, JCV also infects neurons and glia in the grey matter. JCV granule cell neuronopathy (JCV-GCN) occurs when cerebellar granule cells are infected, causing subacute, progressive cerebellar ataxia. Mutations in the VP1 capsid protein of JCV confer the ability to infect granule cells (Dang and Koralnik 2006; Dang et al. 2012). JCV-GCN has been documented in immunocompromised patients with human immunodeficiency virus (HIV), CD40 ligand deficiency, sarcoidosis, and immunosuppression after natalizumab or rituximab treatment (Hecht et al. 2007; Granot et al. 2009; Agnihotri et al. 2014; Dang et al. 2014).
We report on JCV-GCN in a 77-year-old man with chronic lymphopenia, associated with prior chemotherapy. He presented with a progressive ataxia and an inflammatory cerebrospinal fluid (CSF) profile. We detail his clinical course including initial treatment with high-dose intravenous methylprednisolone (IVMP), intravenous immunoglobulin (IVIG), mirtazapine, and mefloquine, and later treatment with recombinant IL-7.
Patient and Methods
A 77-year-old man with a history of mantle cell lymphoma, status-post chemotherapy with rituximab and bendamustine (2010), presented with two months of progressive truncal and appendicular ataxia, rendering him non-ambulatory (Fig. 1). Prior to chemotherapy, his baseline absolute lymphocyte count (ALC) was 4000/μL, and immediately after chemotherapy, his ALC was 650/μL with a nadir of 300/μL. He was HIV negative, and he had a history of lung, bladder, and prostate cancers, all treated surgically. Additionally, he had chronic systolic heart failure and prior placement of a pacemaker/automatic implantable cardioverter/defibrillator (PPM/AICD) that was not magnetic resonance imaging (MRI) compatible.
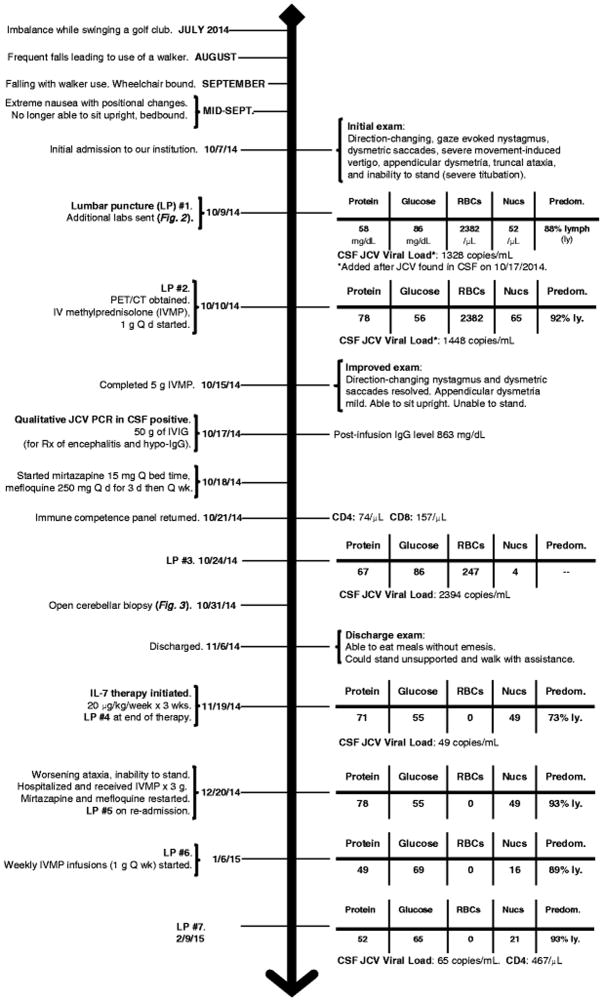
Fig. 1.
Case timeline. Important events and treatments are noted, in chronological order, on the left side of the timeline. Highlighted changes to the physical exam, cerebrospinal fluid (CSF) profile, JC virus (JCV) viral load, and CD4 and CD8 lymphocyte counts are noted on the right
LP = lumbar puncture; lymph/ly = lymphocytes; PET/CT = combined positron emission tomography, computed tomography; Q d = daily; Q wk = every week; IVMP = intravenous methylprednisolone; IVIG = intravenous immunoglobulin; Rx = treatment; IgG = immunoglobulin G
His exam showed direction-changing nystagmus, dysmetric saccades, and truncal and appendicular ataxia, rendering him unable to stand without support. Intractable nausea and vomiting, worsened by movement, resulted in additional morbidity. Brain computed tomography (CT) with contrast showed cerebellar atrophy. His ALC was 600/μL and CSF showed RBC count 2382/μL, WBC count 52/μL (88% lymphocytes), protein 56 mg/dL, and glucose 86 mg/dL. CSF cytology, flow cytometry, oligoclonal bands, and paraneoplastic antibodies were negative. A thorough screen for other etiologies of cerebellar ataxia was unrevealing (Fig. 2). Whole-body 18Fludeoxyglucose positron-emission tomography (PET)/CT scan showed cerebellar hypermetabolism and a right-upper lobe pulmonary nodule. The nodule could not be accessed by minimally-invasive biopsy procedures. Comparison to prior PET/CT scans revealed that the nodule had not changed in four years, rendering malignancy unlikely.
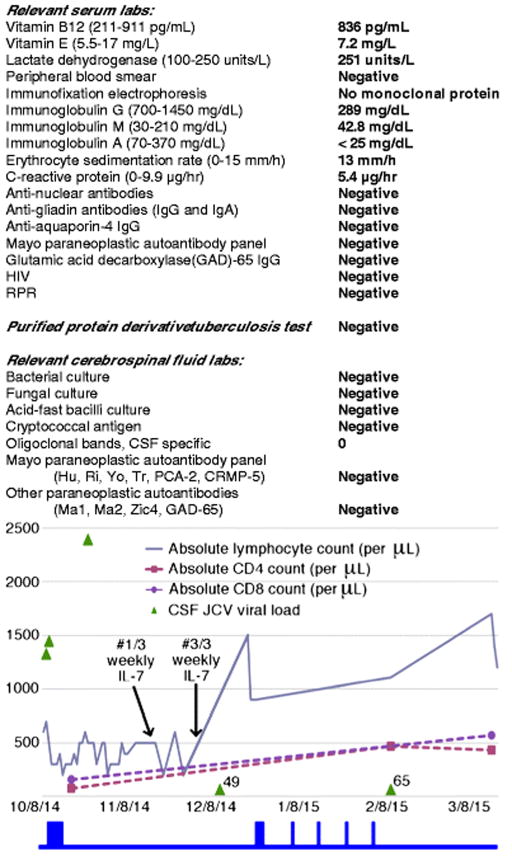
Fig 2.
Laboratory work-up and trends of absolute, CD4, and CD8 lymphocyte counts in serum alongside the trend of JC virus (JCV) viral load in cerebrospinal fluid (CSF). The start of the three-week course of weekly IL-7 therapy (#1/3) and the end of therapy (#3/3) are indicated by arrows. The blue boxes below the graph correspond with periods of time when IV methylprednisolone was administered (October 2014, 1g/day x 5 days; December 2014, 1g/day x 3 days; January – February 2015, 1g/week x 4 weeks). The JCV viral load in copies/mL is noted for the last two measurements
Given the possibility of an inflammatory or paraneoplastic etiology, the patient was treated with high-dose IVMP (five grams total, over five days) and plasma exchange. After completion of IVMP, his direction-changing nystagmus and dysmetric saccades resolved. He was able to sit upright, he could manipulate a spoon without spilling food, and his nausea, vomiting, and anorexia improved. JCV-GCN was added to the differential diagnosis because of his lymphopenia. A qualitative PCR detected JCV in CSF. Plasma exchange was stopped after two sessions. An immune competence panel showed ALC 300/μL, CD4 count 74/μL (normal range 365 – 1294/μL), and CD8 count 157/μL (normal range 187 – 781/μL) with a CD4:CD8 ratio of 0.47. The trends for ALC, CD4, and CD8 counts are highlighted in figure 2. Serum IgG was 289 mg/dL (normal range 700 – 1450 mg/dL). He was treated for hypogammaglobulinemia, in the context of an inflammatory CSF profile, with 50 g IVIG.
JCV DNA quantitative PCR (qPCR) was obtained from the first CSF sample, revealing 1328 copies/mL (Ryschkewitsch et al. 2013). The trend of the JCV qPCR is highlighted in figure 2. Repeat CSF studies after corticosteroid therapy showed RBC count 247/μL, WBC count 4/μL, glucose 86 mg/dL, protein 67 mg/dL, and JCV qPCR 2398 copies/mL. Treatment also included mirtazapine 15 mg daily and mefloquine 250 mg daily, for three days, followed by once-a-week (Brickelmaier et al. 2009; Marzocchetti et al. 2009; Wenning et al. 2009; Clifford et al. 2010).
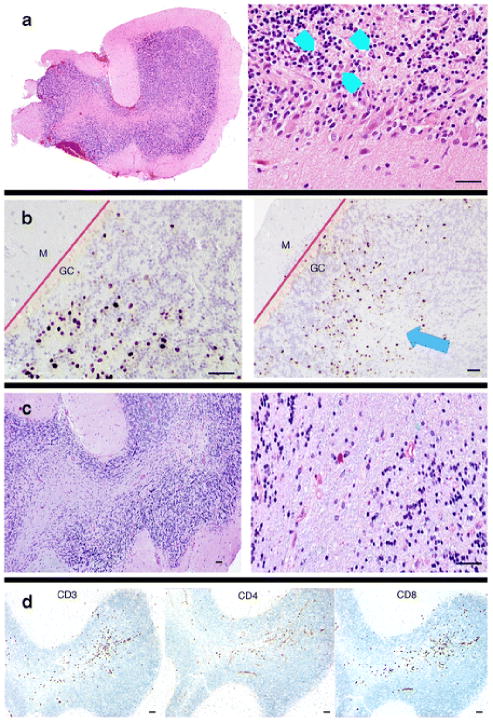
Because of the patient’s unanticipated, inflammatory CSF profile with detection of JCV, we sought to confirm the diagnosis of JCV-GCN. Biopsy of the left cerebellar hemisphere showed polyomavirus T antigen expressing cells and patchy areas of cell loss within the granule cell layer, without demyelinating lesions, findings consistent with JCV-GCN (Fig. 3). The granule cell layer had marked T cell infiltration (Fig. 3). JCV DNA was recovered from biopsy tissue and direct DNA sequencing was performed on the VP1 gene region where JCV-GCN associated mutations are found. Analysis of the associated amino acid sequence found a single change, lysine to arginine, at amino acid position 345 of the C-terminus of the VP1 capsid protein. This mutation lies in the area where JCV-GCN mutations are commonly found, but this mutation leads to a conservative amino acid substitution rather than a deletion or change in reading frame, as previously reported JCV-GCN cases (Dang et al. 2012). The mutation does not significantly alter the conformation of the VP1 capsid protein compared to wild-type JCV as modelled by Expert Protein Analysis System (EXPASY) (data not shown). The genotype was consistent with JCV type 2 (Agostini et al. 1997).
Fig. 3.
Pathology from open cerebellar biopsy. a, Hematoxylin and eosin staining shows a region of the granule cell layer with enlarged nuclei (blue arrowheads) next to normal-appearing granule cell neurons. b, Immunohistochemical staining of SV40 T antigen (ARUP Laboratories, Salt Lake City, Utah) was used to visualize JC virus-infected cells. Infected, positive staining cells are isolated to the granule cell (CG) layer. The molecular (M) layer is unaffected. The blue arrow highlights an area of focal granule cell loss. The red lines demarcate the boundary between the granule cell and molecular cell layers of the cerebellum. c, Luxol-fast blue/periodic acid Schiff (LFB-PAS) staining does not show demyelination, unlike PML. d, CD3, CD4, and CD8 immunohistochemical staining shows inflammatory T cell infiltrates affecting the granule cell layer. The pathology laboratory did not perform CD20 staining. The black scale bars at the bottom of each panel represent approximately 50 μM
His ataxia improved by the time of discharge. He could stand unsupported and walk with assistance. His ALC was 400/μL. Thirteen days after discharge, recombinant interleukin 7 (IL-7) therapy was administered as a 20 μg/kg subcutaneous injection once a week, for three weeks, in an attempt to promote immune recovery. The Washington University institutional review board approved the protocol and informed consent was obtained. There was additional clinical improvement (e.g. ambulating several steps independently). A lumbar puncture performed after completion of IL-7 therapy showed RBC count 0/μL, WBC count 49/μL, protein 71 mg/dL, glucose 55 mg/dL, and JCV qPCR 37 copies/mL.
One month after his last dose of IL-7, he presented with worsening ataxia. His ALC was 1500/μL and his CSF showed RBC count 0/μL, WBC count 49/μL, protein 78 mg/dL, and glucose 55 mg/dL. Given his clinical worsening despite an improved ALC there was suspicion for immune reconstitution inflammatory syndrome (IRIS). He was restarted on IVMP, mirtazapine, and mefloquine. He was treated empirically because MRI could not be done and CT is insensitive for detection of IRIS. His symptoms stabilized but his ataxia (e.g. difficulty sitting upright without support) and chronic nausea and vomiting persist. His latest lumbar puncture, performed in the setting of weekly 1 g IVMP infusions, showed RBC count 0/μL, WBC count 21/μL, protein 52 mg/dL, glucose 65 mg/dL, and JCV qPCR 65 copies/mL. His CD4 and CD8 counts were 433/μL and 570/μL, respectively.
Discussion
JCV-GCN should be considered in all lymphopenic patients with ataxia. CSF should be tested by JCV DNA PCR to establish the diagnosis. Genotyping is not necessary for diagnosis, but detection of known mutations in the C-terminus of the VP1 gene can be supportive. In this case, JCV present in the recovered tissue did not have the typical GCN mutations, which suggests that wild-type JCV also causes JCV-GCN. A growing body of literature shows that granule cells are frequently infected in patients with known PML lesions (Wijburg et al. 2015; Du Pasquier et al. 2003; Bustamante et al. 2009). There is ongoing debate whether this phenomenon is a consequence of a single, multifocal JCV infection or simultaneous infections from JCV variants with different tropisms. We would have liked to determine whether this patient had white matter lesions, but brain MRI could not be performed. Head CT with contrast did not show any suspicious lesions, but the sensitivity for detection of demyelinating lesions is poor compared to MRI. The cerebellar biopsy did not show demyelination.
Immunosuppressive therapies are a major risk factor for development of lymphopenia and resultant predisposition to JCV CNS infections. Our patient was previously treated with rituximab, a chimeric monoclonal antibody that depletes B cells. The relationship between rituximab and his JCV-GCN is unclear, for a couple of reasons. First, lymphopenia rarely lasts longer than 12 months after rituximab treatment (Lu et al. 2008). Second, the patient’s history of recurrent cancers suggests an underlying immune disorder that could have predisposed him to JCV infection of the CNS.
Currently there is no approved therapy for JCV infection in patients without a reversible, acquired lymphopenia. IL-7 is a rational therapy because it promotes rapid maturation and mobilization of available lymphocyte stores. After IL-7 therapy, our patient’s ALC, CD4, and CD8 counts normalized, and these improvements were inversely correlated with CSF JCV viral load. These results are consistent with other reports of IL-7 therapy (Alstadhaug et al. 2014; Gasnault et al. 2014). Unfortunately, at the time of publication, the drop in JCV viral load did not translate into additional clinical benefit for our patient.
The inflammatory nature of the patient’s JCV-GCN is unique. After completion of the first round of IVMP, the patient’s inflammatory CSF profile and ataxia markedly improved. Classically, PML and JCV-GCN are not associated with inflammation. If viral infection were the cause of inflammation, one would expect that reduction of the viral load by IL-7 therapy would reduce the inflammation, but this was not evident. The inflammation may be a consequence of his strengthened immune system attacking a persistent low-level JCV infection or it may be a consequence of the IL-7 therapy itself. He continues to receive regular low-dose oral steroid therapy, which has stabilized his symptoms.
Acknowledgments
We would like to acknowledge Arun Varadhachary, MD (Department of Neurology, Washington University, St. Louis, MO) and the Washington University neurology residents who were integral in providing care for this gentleman. Robyn Reese (Neuromuscular Laboratory, Washington University, St. Louis, MO) provided invaluable assistance in processing and managing the CSF and tissue samples. We would also like to acknowledge Michel Morre (Revimmune, Inc.) for his expertise on IL-7 treatment protocols and for helping expedite IL-7 therapy. This work was previously presented at the International Conference on Progressive Multifocal Leukoencephalopathy, August 25–26, 2015, Mölndal, Sweden; American Neurological Association Annual Meeting, September 27–29 2015, Chicago, IL, USA; Washington University Center for Neurology and Neuroinfectious Diseases Annual Symposium, October 28, 2015, St. Louis, MO, USA.
Footnotes
Authorship
D.N.S.-M.: acquisition and analysis of data, data interpretation, primary manuscript writer; K.E.S.: acquisition and analysis of pathological data, manuscript preparation; M.A.R.: manuscript preparation; C.R.: acquisition and analysis of JCV qPCR data; E.O.M.: acquisition and analysis of JCV qPCR data, manuscript revision; X.D.: acquisition and analysis of JCV DNA sequencing; I.J.K.: acquisition and analysis of JCV DNA sequencing, manuscript revision; R.E.S.: acquisition and analysis of pathological data; D.B.C. and F.M.K.: acquisition and analysis of data, data interpretation, manuscript revision; R.B.: acquisition and analysis of data, data interpretation, manuscript revision, supervision of case.
Conflicts of Interest
Dr. Koralnik reports grants from Biogen Idec., and personal fees from Medimmune, Antisense, Johnson & Johnson, Genzyme, Esai, and UpToDate, outside the submitted work. Drs. Major and Ryschkewitsch have patents pending for a multiplex qPCR assay for the ultrasensitive detection of JCV DNA. Dr. Clifford has received personal fees and support for consulting/advisory boards from Biogen Idec., Millenium/Takeda, Bristol Myers Squibb, Pfizer, Genzyme (Sanofi), Amgen, Genentech, GlaxoSmithKline, Merck/Serono, Inhibikinase, Quintiles, AstraZeneca, and DAC Beachcroft (Novartis), outside the submitted work. The other authors report no conflicts of interest.
References
- Agnihotri SP, Dang X, Carter JL, Fife TD, Bord E, Batson S, Koralnik IJ. JCV GCN in a natalizumab-treated MS patient is associated with mutations of the VP1 capsid gene. Neurology. 2014;83:727–732. doi: 10.1212/WNL.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini HT, Ryschkewitsch CF, Mory R, Singer EJ, Stoner GL. JC virus (JCV) genotypes in brain tissue from patients with progressive multifocal leukoencephalopathy (PML) and in urine from controls without PML: increased frequency of JCV type 2 in PML. J Infect Dis. 1997;176:1–8. doi: 10.1086/514010. [DOI] [PubMed] [Google Scholar]
- Alstadhaug KB, Croughs T, Henriksen S, Henriksen S, Leboeuf C, Sereti I, Hirsch HH, Rinaldo CH. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol. 2014;71:1030–1035. doi: 10.1001/jamaneurol.2014.825. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6:667–679. doi: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- Brickelmaier M, Lugovskoy A, Kartikeyan R, Reviriego-Mendoza MM, Allaire N, Simon K, Frisque RJ, Gorelik L. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53:1840–1849. doi: 10.1128/AAC.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante F, Luis Cartier R, Manuel Lavados M. Cerebellar atrophy by the JC virus in a patient with AIDS. Rev Chil Neuro-Psiquiatr. 2009;47:222–227. doi: 10.4067/S0717-92272009000300007. [DOI] [Google Scholar]
- Clifford DB, DeLuca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- Dang L, Dang X, Koralnik IJ, Todd PK. JC polyomavirus granule cell neuronopathy in a patient treated with rituximab. JAMA Neurol. 2014;71:487–489. doi: 10.1001/jamaneurol.2013.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang X, Koralnik IJ. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene. J Gen Virol. 2006;87:2533–2537. doi: 10.1099/vir.0.81945-0. [DOI] [PubMed] [Google Scholar]
- Dang X, Vidal JE, Penalva de Oliveira AC, Simpson DM, Morgello S, Hecht JH, Ngo LH, Koralnik IJ. JC virus granule cell neuronopathy is associated with VP1 C terminus mutants. J Gen Virol. 2012;93:175–183. doi: 10.1099/vir.0.037440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier RA, Corey S, Margolin DH, Williams K, Pfister LA, De Girolami U, Mac Key JJ, Wüthrich C, Joseph JT, Koralnik IJ. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61:775–782. doi: 10.1212/01.WNL.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- Gasnault J, de Goër de Herve M-G, Michot J-M, Hendel-Chavez H, Seta V, Mazet AA, Croughs T, Stankoff B, Bourhis JH, Lambotte O, Delfraissy JF, Taoufik Y. Efficacy of recombinant human interleukin 7 in a patient with severe lymphopenia-related progressive multifocal leukoencephalopathy. Open Forum Infect Dis. 2014;1:1–4. doi: 10.1093/ofid/ofu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheuens S, Wüthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. [DOI] [PubMed] [Google Scholar]
- Granot R, Lawrence R, Barnett M, Masters L, Rodriguez M, Theocharous C, Pamphlett R, Hersch M. What lies beneath the tent? JC-virus cerebellar granule cell neuronopathy complicating sarcoidosis. J Clin Neurosci. 2009;16:1091–1092. doi: 10.1016/j.jocn.2008.07.091. [DOI] [PubMed] [Google Scholar]
- Hecht JH, Glenn OA, Wara DW, Wu YW. JC virus granule cell neuronopathy in a child with CD40 ligand deficiency. J Pediatr Neurol. 2007;36:186–189. doi: 10.1016/j.pediatrneurol.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lu TY, Jonsdottir T, Vollenhoven RF, Issenberg DA. Prolonged B-cell depletion following rituximab therapy in systemic lupus erythematosus: a report of two cases. Ann Rheum Dis. 2008;67:1493–1494. doi: 10.1136/ard.2008.091124. [DOI] [PubMed] [Google Scholar]
- Marzocchetti A, Tompkins T, Clifford DB, Gandhi RT, Kesari S, Berger JR, Simpson DM, Prosperi M, De Luca A, Koralnik IJ. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73:1551–1558. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryschkewitsch CF, Jensen PN, Major EO. Multiplex qPCR assay for ultra sensitive detection of JCV DNA with simultaneous identification of genotypes that discriminates non-virulent from virulent variants. J Clin Virol. 2013;57:243–248. doi: 10.1016/j.jcv.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning W, Haghikia A, Laubenberger J, Clifford DB, Behrens PF, Chan A, Gold R. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N Engl J Med. 2009;361:1075–1080. doi: 10.1056/NEJMoa0810257. [DOI] [PubMed] [Google Scholar]
- Wijburg MT, van Oosten BW, Murk JL, Karimi O, Killestein J, Wattjes MP. Heterogeneous imaging characteristics of JC virus granule cell neuronopathy (GCN): a case series and review of the literature. J Neurol. 2015;262:65–73. doi: 10.1007/s00415-014-7530-5. [DOI] [PubMed] [Google Scholar]