Abstract
Introduction
A nonbehavioral method for monitoring ototoxicity in patients treated with cisplatin is needed because patients enduring chemotherapy may not be well or cooperative enough to undergo repeated hearing tests. Distortion-product otoacoustic emissions (DPOAEs) provide a nonbehavioral measure of auditory function that is sensitive to cisplatin exposure. However, interpreting DPOAE findings in the context of ototoxicity monitoring requires that their accuracy be determined in relation to a clinically accepted gold standard test.
Objectives
Among patients receiving cisplatin for the treatment of cancer, we sought to (1) identify the combination of DPOAE metrics and ototoxicity risk factors that best classified ears with and without ototoxic-induced hearing changes; and (2) evaluate the test performance achieved by the composite measure as well as by DPOAEs alone.
Design
Odds of experiencing hearing changes at a given patient visit were determined using data collected prospectively from 24 Veterans receiving cisplatin. Pure-tone thresholds were examined within an octave of each subject’s high-frequency hearing limit. DPOAE were collected as a set of four response growth (input/output) functions near the highest f2 frequency that yielded a robust response at L2 = L1 = 65 dB SPL. Logistic regression modeled the risk of hearing change using several DPOAE metrics, drug treatment factors, and other patient factors as independent variables. An optimal discriminant function was derived by reducing the model so that only statistically significant variables were included. Receiver operating characteristic curve analyses were used to evaluate test performance.
Results
At higher cisplatin doses, ears with better hearing at baseline were more likely to exhibit ototoxic hearing changes than those with poorer hearing. Measures of pre-exposure hearing, cumulative drug dose, and DPOAEs generated a highly accurate discriminant function with a cross-validated area under the receiver operating characteristic curve of 0.9. DPOAEs alone also provided an indication of ototoxic hearing change when measured at the highest DPOAE test frequency that yielded a robust response.
Conclusions
DPOAEs alone and especially in combination with pre-exposure hearing and cisplatin dose provide an indication of whether or not hearing has changed as a result of cisplatin administration. These promising results need to be validated in a separate sample.
INTRODUCTION
Anticancer drugs containing platinum are the basis for chemotherapy for a wide range of tumor types including ovarian, testicular, colorectal, head and neck, and lung cancer. The first-generation platinum drug, cisplatin, is widely used in both children and adults and is unrivaled in effectiveness against many cancers. However, it is also considered to be the most ototoxic compound in clinical use (Anniko & Sobin 1986; Hartmann & Lipp 2003). Schweitzer (1993) calculated that the incidence of cisplatin-induced hearing loss averaged across a large number of studies was 62%, indicating that cisplatin causes ototoxicity in a large percentage of patients treated with the drug.
Cisplatin causes hearing loss primarily by damaging the outer hair cells within the organ of Corti, and the stria vascularis, which provides the electrical drive to the outer hair cells. Initially, the first row of outer hair cells is affected, followed by the second and third rows of outer hair cells, inner hair cells, and finally supporting cells (Estrem et al. 1981; Nakai et al. 1982; Marco-Algarra et al. 1985; Tsukasaki et al. 2000). Direct damage to spiral ganglion cells can also occur concomitantly with organ of Corti damage (Hoistad et al. 1998; van Ruijven et al. 2005). The progression of damage is typically from the high-frequency coding cochlear base toward the apex (Brummett 1980; Komune et al. 1981; Nakai et al. 1982; Konishi et al. 1983; Schweitzer et al. 1984).
Hearing loss adversely impacts quality of life; psychosocial functioning (Dalton et al. 2003); and one’s ability to obtain, process, and understand basic health information (Amalraj et al. 2009) and so is arguably an important side effect to monitor for patients receiving ototoxic therapies. The impact of ototoxicity on patients having hearing impairment before cisplatin treatment may be especially important to consider because additional impairment can immediately impact communicative ability. However, these impacts can be mitigated through prospective ototoxicity monitoring. Early detection of ototoxicity provides physicians with the necessary information to prevent or minimize the progression of hearing loss, which helps to preserve frequencies critical for deciphering speech. Early detection also provides audiologists an opportunity to implement aural rehabilitation to lessen the impact of any unavoidable hearing loss.
Predicting which patients will experience ototoxic hearing loss is not possible without testing auditory function directly. Although the risk for developing hearing loss from ototoxic drugs is generally related to the dose, duration, frequency, and method of medication administration, there is marked individual variability in these relationships (Vermorken et al. 1983; Rademaker-Lakhai et al. 2006). It has also been shown that concomitant exposure to other toxins such as noise, chemicals, and other ototoxic medications can produce a synergistic effect leading to increased rates of ototoxicity (Komune & Snow 1981; Schweitzer et al. 1984; Boettcher et al. 1987; Gratton et al. 1990). Genetic factors (Peters et al. 2000; Oldenburg et al. 2008) and physiological factors, such as age and pre-exposure hearing ability, may further impact incidence rates (Blakley et al. 1994). Thus, accurately determining the cisplatin-ototoxicity relationship may require that a number of factors be considered.
Ototoxicity monitoring typically consists of the serial collection of behavioral pure-tone thresholds. The objective is to identify at a particular monitoring visit, whether hearing has changed in each ear. One monitors each ear, at each visit, so that the patient-ear visit is the object of monitoring. Test-retest comparisons of the hearing data are used to determine which patients exhibit a significant hearing change based on hearing change criteria. For incipient detection, change criteria developed by the American Speech-Language-Hearing Association (ASHA) and described in “Guidelines for the Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy” (ASHA 1994) are the most widely used (AAA 2009). For a list of these criteria, see the “Behavioral Pure-Tone Thresholds” section. Both ASHA guidelines and the more recent American Academy of Audiology “Position Statement and Clinical Practice Guidelines for Ototoxicity Monitoring” (AAA 2009) advocate the use of extended high-frequency testing when possible to improve test sensitivity. A major limitation to pure-tone threshold monitoring that includes both conventional and extended high frequencies at each visit is that it is time consuming, which may be a reason that it is not more commonly done.
Fausti et al. (1999, 2003) have suggested that a more effective behavioral monitoring method is one that targets frequencies near each patient’s high-frequency hearing limit, which may be within the conventional or extended high-frequency range, depending on the degree and configuration of one’s pre-exposure hearing impairment. This individualized test frequency range, called the sensitive range for ototoxicity (SRO), is relatively quick to monitor and when paired with ASHA’s threshold shift criteria erroneously identifies unexposed control subjects as having hearing shifts only about 5% of the time (Konrad-Martin et al. 2010).
However, we have found that significant numbers of patients administered ototoxic medications, roughly 33%, become sufficiently incapacitated during treatment that they are unable or unwilling to complete a behavioral hearing test (Fausti et al. 1992). A nonbehavioral, “objective” measure that is sensitive to changes in hearing is necessary for ototoxicity monitoring because it is not possible to know which patients will become incapacitated. Otoacoustic emission (OAE) testing has been proposed as an objective indicator of ototoxic damage because OAE generation depends on the physiological status of the outer hair cells (reviewed in Campbell & Durrant 1993; Whitehead et al. 1996; also see Hodges & Lonsbury-Martin 1999). Changes in the outer hair cell mechanism alter OAE responses and hearing thresholds.
Distortion-product otoacoustic emissions (DPOAEs) are generated using two stimulating tones, f1 and f2 (where f1 < f2). The response is initiated in the overlapping region of the basilar membrane’s response to the stimuli, somewhat nearer to the f2 tonotopic place. A second component arises near the basilar membrane place that codes the distortion-product (DP) frequency (2f1 − f2) (Kim 1980; Shera & Guinan 1999). Clinical DPOAEs are comprised of energy from these two sources combined within the ear canal (Konrad-Martin et al. 2001) and may include generator sources basal to the primary tones (Martin et al. 2009). The presence of DPOAEs are generally associated with normal hearing and are reduced in individuals with mild to moderate hearing losses up to approximately 50 to 60 dB HL. DPOAEs are rarely present in individuals with thresholds greater than about 60 dB HL (Gorga et al. 1996, 1997).
Changes in DPOAE responses have been associated with cisplatin exposure in both children and older adults (Katbamna et al. 1999; Ress et al. 1999; Stavroulaki et al. 2001, 2002; Knight et al. 2007). This is encouraging because it suggests that DPOAEs would be sensitive to ototoxic damage coincident with, or that might lead to, hearing changes. However, most prior studies used either statistical tests of group differences or clinical tests of differences derived from control populations. Although useful, these investigations add little to the literature with respect to the overall sensitivity and specificity of DPOAE measures for identifying ototoxicity in individuals.
An important next step in developing DPOAEs as a diagnostic test for ototoxicity is to determine the accuracy with which DPOAEs categorize patients exposed to cisplatin into two groups: those exhibiting hearing change and those who do not. This requires DPOAE monitoring results be compared with results of a “gold standard” test. Only once this is done will the clinical significance of DPOAE changes become clearer so that DPOAE monitoring results are actionable.
Previously, the level of agreement between DPOAE measurements and the audiogram has been used to determine the ability of DPOAEs to identify hearing loss (Gorga et al. 1993a, b, 1996, 1997; Kim et al. 1996; Stover et al. 1996; Dorn et al. 1999). Although no single DPOAE measurement (amplitude and signal-to-noise ratio [SNR]) has been capable of completely separating those with normal hearing from impaired hearing, multivariate DPOAE models have shown increased test accuracy (Dorn et al. 1999). Similarly, changes in DPOAE measurements can be compared with changes in hearing that meet gold standard criteria for ototoxic threshold shifts, such as those criteria recommended by ASHA (1994), and a multivariate approach will likely increase test accuracy.
This study sought to provide a DPOAE-based method of diagnosing cisplatin-induced hearing change to use when hearing cannot be directly measured. The approach was to compare for each ear and patient visit DPOAE level changes with ASHA-significant pure-tone thresholds changes in the SRO. Data evaluated for this study were obtained as part of a large, prospective study investigating methods of ototoxicity monitoring in Veterans. This report concerns only those results obtained in patients receiving cisplatin chemotherapy treatment. Objectives were to (1) identify combinations of DPOAE metrics and ototoxicity risk factors that best classified ears with and without cisplatin-induced hearing changes; and (2) evaluate the test performance achieved by the newly identified multivariate measure as well as by DPOAEs alone.
MATERIALS AND METHODS
Subjects
Subjects were recruited from the Portland Veteran Affairs Medical Center over the period from June 2004 through May 2006. A daily list was generated by the hospital pharmacy to identify patients prescribed cisplatin for the treatment of cancer. Patients’ electronic medical charts were reviewed, and the supervising nurse practitioner was consulted to verify pharmacy list information and to obtain treatment information. To be included as a study participant, patients could not be receiving any known ototoxins other than cisplatin. Subjects were screened during the initial baseline visit to ensure that these additional inclusion criteria were met: (a) ability to provide reliable pure-tone threshold responses (responses were considered unreliable if they varied by >5 dB during a threshold recheck administered during the baseline session); (b) measurable DPOAEs in at least one ear; and (c) no active or recent history of middle ear disorder, Meniere’s disease, or retrocochlear disorder. For a subject’s dataset to be included in this study, a complete baseline test and at least one complete post-treatment evaluation (behavioral audiometry and DPOAEs) were also required. All subjects were consented to participate in the study following the guidelines of the medical center’s Institutional Review Board and were compensated for their time. All subjects were counseled to reduce their noise exposure and protect their hearing when exposed to loud noise during and after cisplatin administration.
Subjects completed a battery of interviews and tests at baseline and during follow-up visits. Baseline was performed within the week before or within 24 hrs after initial treatment with the chemotherapeutic agent cisplatin. Subsequent monitoring visits were ideally conducted within 24 hrs after each treatment. However, monitoring visits sometimes occurred 2 to 4 days after treatment because of a host of factors, most significant being the subject’s ability to participate because of their overall health status. The chemotherapy regimen depended on the cancer staging, dose, presence of concomitant radiation, and overall health of the subject; therefore, the dosing schedule was variable across subjects. If a behavioral hearing change was noted, all conventional and extended high frequencies were retested, and the physician was notified. We attempted to conduct a test every week until thresholds stabilized. In addition, when possible, testing was performed immediately after treatment had been discontinued and at 1, 3, and 6 mos after treatment. Because the ultimate goal of this work is to provide a means of diagnosing hearing change in the two ears of a patient at a given follow-up visit, both ears on each subject were routinely tested. All testing at each visit was completed within 2 hrs.
Measurements
Behavioral Pure-Tone Thresholds
The gold standard for hearing change in this study was determined by serial audiometric SRO frequency monitoring. Behavioral pure-tone thresholds were obtained using the modified Hughson-Westlake technique (Carhart & Jerger 1959). Pure-tone thresholds were initially measured from 0.5 to 20 kHz using a Virtual Corporation, Model 320 (V320) audiometer. TDH-50P earphones in MX-41/AR cushions were used for testing 0.5 and 1 kHz thresholds. Koss Pro/4X Plus earphones, modified to improve SNR for high-frequency testing as described by Fausti et al. (1990), were used for testing frequencies from 2 to 20 kHz. Reliability, validity, and equipment limits (115 dB SPL) for frequencies 2 to 20 kHz for threshold responses using the Virtual V320 audiometer paired with modified Koss Pro/4X Plus earphones have been documented previously (Fausti et al. 1990).
Calibration of the Virtual V320 audiometer was conducted twice each month. TDH-50P earphones were calibrated according to ANSI S3.6-1989 and IEC 318 specifications. The earphone was coupled to a Bruel & Kjaer (B&K) 4153 artificial ear, and the acoustic output was measured by a B&K 4134 ½″ condenser microphone and read on a B&K 2231 sound level meter. KOSS Pro/4X Plus earphones were calibrated on a 6cc flat-plate coupler with a B&K 4134 ½″ condenser microphone in the center of the cavity (as described in Fausti et al. 1979).
A behavioral SRO was identified for each ear from the baseline pure-tone thresholds (0.5 to 20 kHz). The upper bound of the SRO was defined as the highest frequency at which the subject responded to a pure-tone signal of 100 dB SPL or less. This frequency is denoted FB. The pure-tone thresholds of the six lower adjacent frequencies in 1/6-octave steps were then obtained and are similarly denoted; FB − 1 is 1/6-octave below FB, FB − 2 is 1/6-octave below FB − 1, and so on. These seven frequencies constituted the behavioral SRO, which was the target range monitored at all visits. Thus, for subsequent test sessions, pure-tone thresholds were obtained only within the subject’s SRO, as defined at baseline. If a hearing change was noted within the SRO, then full frequency testing resumed.
Presence or absence of behavioral hearing change was based on published clinical guidelines (ASHA 1994) and includes: (a) ≥20 dB change at any one test frequency; (b) ≥10 dB change at any two consecutive test frequencies; or (c) loss of response at three consecutive test frequencies at which responses were previously obtained. Using these criteria, a binary indicator for presence or absence of hearing change was constructed for each postbaseline patient-ear visit in the sample. The ultimate goal of this analysis was to accurately predict this binary indicator (behavioral hearing change in an ear: yes/no) among patients treated with cisplatin.
Distortion Product Otoacoustic Emissions
DPOAEs were collected using custom software (the Otoacoustic Emission Averager, EMAV from Boys Town National Research Hospital; Neely & Liu 1993) run on a computer. The software utilized a Card Deluxe digital signal processing board (Digital Audio Labs) to generate stimuli and record responses. The two DPOAE stimulus frequencies (f1 and f2, where f1 < f2) were separately digitized, converted to analog voltages, passed through custom headphone buffers to two Etymotic Research (ER-2) tubephones, and delivered to the sealed ear canal through separate ports in the probe assembly. The probe also contained an ER-10B+ microphone to record the DPOAE responses. The signal recorded by the microphone was amplified 20 dB by the ER-10B+ preamplifier, digitized in 64-msec time windows, and stored in two interleaved buffers, which were averaged in the time domain. The DPOAE level at 2f1 − f2 was estimated from a Fast Fourier transform of the grand average of the two response buffers ([A + B]/2). The noise level was estimated at the DPOAE frequency from the A to B spectrum. Measurement-based stopping rules were used such that at any test frequency, sampling stopped when the noise floor was < −20 dB SPL or after 32 secs of artifact-free averaging, whichever occurred first.
Both DPOAEs and stimulus levels were measured at the plane of the microphone near the entrance to the ear canal. In the ear calibration was used to adjust voltage applied to the tubephones to set the SPL of f1 and f2 to desired values. In the ear calibration procedures using SPL measurements at the plane of the microphone are known to produce calibration errors, particularly at higher frequencies secondary to interactions between incident and reflected waves producing pressure nodes within the ear canal (Siegel & Hirohata 1994; Siegel 2007). These pressure nodes, in addition to leading to inaccurate estimations of the SPL at the surface of the tympanic membrane, may lead to an increase in the driving-point voltage required to achieve the desired SPL at the plane of the microphone. A concern with increased driving-point voltages is increased system distortion located at the 2f1 − f2 place, which can be incorrectly interpreted as a DPOAE. This was mitigated to some extent by the use of ER-2 tubephones and custom headphone buffers, which were capable of providing essential amplification while maintaining low system distortion and noise. Calibration procedures that measure sound intensity level or the forward pressure level of the stimulus rather than sound pressure level may reduce the effects of standing waves (Neely & Gorga 1998; Scheperle et al. 2008) and thus decrease the overall variability in DPOAE measurements. Calibration strategies in small cavities should be thoughtfully considered when measuring DPOAEs and certainly warrant further investigation.
System distortion was estimated in an occluded ear simulator (B&K 4153 Coupler) weekly. Estimates of system distortion were less than −20 dB SPL for the frequencies and intensity levels used in this study. The system was also electrically calibrated annually according to the EMAV manual. Ear-canal transfer functions obtained during in the ear calibration for baseline recordings were employed as target calibration spectra to ensure consistent probe placement across follow-up visits and thus improve test-retest reliability.
DPOAE responses were considered valid and present if they met all of the following criteria: (1) DPOAE amplitude was greater than −20 dB SPL, a conservative estimate of the system distortion; (2) DPOAE amplitude was at least 6 dB or greater than the measured noise floor (biologic and system noise); and (3) primary levels L1 and L2 measured with the ER-10B+ probe microphone were within 3 dB of the targeted stimulus level. DPOAE responses were considered valid and absent if (1) DPOAE amplitude was less than −20 dB regardless of the SNR ratio; (2) the measured noise floor was sufficiently low (−20 dB SPL); and (3) primary levels were within 3 dB of the targeted stimulus level.
DPOAE stimuli were presented at a fixed primary frequency ratio f2/f1 = 1.22. DPOAE responses were obtained using a primary frequency sweep (DP-gram) from 1 to 10 kHz in 1/6-octave increments at stimulus frequency levels of L1 = L2 = 65 dB SPL to identify the highest frequency that produced a valid present response, which constituted the upper bound of the DPOAE range. The highest f2 able to generate a DPOAE was marked, and response growth (input/output) functions were obtained for that frequency and the three lower adjacent frequencies using 1/3-octave frequency steps. DPOAE stimulus input levels were optimized based on a covaried paradigm (L1 = 0.4 L2 + 39) following Kummer et al. (1998) to obtain input/output frequency responses at six intensity levels: L1/L2 in dB SPL = 63/60, 61/55, 59/50, 57/45, 55/40, and 53/35.
DPOAEs were recorded in dB SPL and converted to pressure (Pa) post-hoc for summary metric calculations and analyses. For each function, six input levels (L2) ranging from 35 to 60 dB SPL (0.00112 to 0.02 Pa) could result in six output amplitudes with a minimum emission of −20 dB SPL (0.000002 Pa). A lack of response not attributable to a high noise floor masking the DPOAE was arbitrarily set to 0 Pa. Similar to the behavioral SRO, DPOAE frequencies were normalized to each subject’s highest frequency with an emission and termed FD, which was defined during the baseline test. The FD − 1 is 1/3-octave below FD, FD − 2 is 1/3-octave below FD − 1, and FD − 3 is 1/3-octave below FD − 2.
Using separate measurements for each DPOAE frequency and level combination would have generated a large number of variables (4 frequencies × 6 levels = 24 potential variables for analysis) for the limited amount of data collected, making the model prone to overfitting. Overfitting data occurs when a model is highly customized to the dataset, making the model less likely to be generalizable to other datasets. Therefore, to minimize the risks of overfitting the dataset and concomitant loss of generalizability, we restricted our analysis to three simple summary DPOAE metrics calculated from each of the four input/ output functions. This reduced the number of variables for analysis to 12. These were (1) the sum of the stimulus level input (I) defined as the sum of the L2 values (in Pa) that were associated with a valid DPOAE response; (2) the sum of the emission output (O) defined as the sum of the valid DPOAE amplitudes (in Pa) generated within an input/output function; and (3) the sum of the SNR ratio defined as the SPL of the noise floor subtracted from the SPL of the DPOAE and the difference was converted to Pascal and summed over the range of valid responses within the input/output function. Thus, there were 12 DPOAE metric-frequency combinations per ear. The difference between DPOAE metrics measured at baseline and DPOAE metrics measured during subsequent follow-up visits were calculated.
Treatment Measures
Disease, dose regimen, concomitant radiation therapy, and single-dose volume information was obtained from the patient’s physicians or from medical records. Cumulative dose was computed from patient records and is defined as the sum of the single-dose volumes of cisplatin (mg) received up to the date that hearing measures were taken.
Other Patient Characteristics
Gender, age, pre-exposure hearing loss, and other hearing measures were obtained during audiological exam of the patient or by questionnaire. At each session, the presence or absence of tinnitus was recorded. If the patient reported tinnitus, a verbal questionnaire was administered to characterize the tinnitus. In addition, the patient was asked about noise exposure during treatment including type of noise and duration and whether hearing protection devices were used. All patient interviews were conducted based on a standard questionnaire. Otoscopy and acoustic immittance testing were performed at every visit. Average pre-exposure pure-tone threshold in the SRO region was computed from the baseline audiogram. The reciprocal of the SRO average threshold was used for the purposes of the regression analyses (see below) such that larger values would reflect better hearing. To correct for scaling problems in the model-fitting effort, the reciprocal of the SRO average threshold variable was standardized to a mean of 0 and a SD of 1.
Statistical Analysis
The goal of this study was to develop an approach to detect ototoxic hearing change using DPOAE-based, objective techniques that do not rely on the patient being behaviorally responsive during chemotherapy. A first step involved evaluating the accuracy of different DPOAE metrics individually for identifying ototoxic hearing change. However, the predictions may be more accurate by simultaneously considering other patient or treatment features. Following Dorn et al. (1999), our approach was to use a multivariate statistical approach to find a single, composite measurement, called a “discriminant function,” that best distinguished ears with an ototoxic hearing change from those without such a change. The discriminant function was derived by fitting a logistic regression model to the risk of hearing change using DPOAE metrics, cancer treatment factors, and other patient factors as independent variables.
The goal of identifying ototoxic hearing change departs from that of Dorn et al. (1999) who were concerned with identifying hearing impairment. Specifically, current ototoxicity monitoring requires clinicians to determine whether or not hearing thresholds have shifted substantially at each patient visit for each ear separately. Our sample was therefore composed of the ears of cisplatin-treated patients evaluated repeatedly during the course of chemotherapy, and the unit of analysis was termed the patient-ear visit. Repeated measurements under this protocol induce correlations among outcomes both between ears and within ears over time. Accordingly, we used the generalized estimating equations (GEEs) approach (Fitzmaurice et al. 2004) to estimate regression coefficients and their SEs. This estimation algorithm allows one to account for correlation among the repeated measurements on each ear and each patient by stipulating a “working” covariance structure along with other model components. The result was a discriminant function that predicted the risk of hearing change in an ear at a particular visit without regard to whether changes were noted previously or subsequently.
GEE logistic regression models were first fit using each risk factor and DPOAE metric difference individually. Parameters with p < 0.1 were considered for further analysis. The final multivariate model was developed in two stages. First, a risk factor model relating the risk of hearing change to treatment and other patient features was established. All potential two-way interactions were also assessed. This model was reduced by backward elimination and assessed for lack of fit using cumulative residuals (Lin et al. 2002). The second stage involved adding DPOAE metrics and further reducing by backward elimination and again assessing using cumulative residuals. The final model was comprised only of significant variables at the 0.05 level and significant interactions at the 0.15 level.
These modeling techniques were used to select the most important predictors of hearing change but in no way guarantee that the optimal discriminant function thus determined is accurate. Small p values for the independent variables in the best-fitting model do not necessarily indicate an accurate model.
The accuracy of the model was assessed using receiver operating characteristic (ROC) curve analysis. A ROC curve is a plot of test sensitivity against 1 − specificity and has been used to assess the ability of DPOAEs to differentiate normal hearing from impaired hearing based on audiometric results (Gorga et al. 1993a, b, 1996, 1997; Kim et al. 1996; Stover et al. 1996). The area under the ROC curve (AUC) summarizes the average sensitivity across all false-positive rates yielding an estimate of the overall test accuracy, and was estimated using an AUC estimator that is analogous to the Wilcoxon statistic (Hanley & McNeil 1982).
It is well known that evaluating a discriminant function on the data that were also used to develop the model will lead to overly optimistic estimates of classification accuracy. A suitable alternative is to use leave-one-out cross-validation to estimate the accuracy. Briefly, this procedure entails generating multiple training datasets using a leave-one-out partitioning of the complete dataset. For this study, individual patients, as opposed to patient-ear visits, constituted the units “left-out” in the leave-one-out procedure. A GEE logistic regression model using the variables selected according to the procedure described above was fit to each training dataset. The fitted model was then used to predict hearing change in each of the omitted patient’s ears at each visit. This procedure was iterated until all patient-ear visits of all patients were diagnosed. Summary measures of cross-validated classification accuracy, including the ROC curve and the AUC, were computed from this set of predictions. Details of this and other approaches to classification are described elsewhere (Radmacher et al. 2002; Simon 2005).
RESULTS
Forty subjects receiving the anticancer drug cisplatin consented to participate in the study and underwent baseline testing. Of the 40 subjects, three withdrew after the baseline test, six had incomplete data at baseline, and seven others did not meet the inclusion criteria (3 = poor thresholds precluded DPOAE measurements; 2 = active middle ear pathologies; 2 = unreliable). Of the remaining 24 subjects, 12 contributed one ear and 12 contributed two ears to the analysis. Of the 12 ears excluded, three had incomplete data at baseline, four had incomplete follow-up data, and five ears did not meet the inclusion criteria (2 = poor thresholds precluded DPOAE measurements; 3 = active middle ear pathologies). The final sample comprised 36 ears from 24 subjects.
The majority of subjects in the analysis were Caucasian males with a mean age of 58.5 yrs (Table 1). On average, each subject had 3.4 follow-up visits. Of the 24 subjects receiving cisplatin chemotherapy, half met the criteria for a hearing change according to the ASHA definition of ototoxicity in at least one ear during at least one follow-up visit. On average, patients received approximately 350 mg of cisplatin over an average of 42 days in treatment during which hearing was measured.
TABLE 1.
Characteristics of cisplatin subjects (n = 24)
| Gender | |
| Male | 22 (91.7%) |
| Female | 2 (8.3%) |
| Ethnicity | |
| Non-Hispanic white | 15 (62.5%) |
| American Indian/Alaskan | 1 (4.2%) |
| Hispanic | 0 (0.0%) |
| African American | 1 (4.2%) |
| Other | 7 (29.2%) |
| Age (mean, range) | 58.5 (28–75) |
| Number of follow-up tests (mean, range) | 3.4 (1–9) |
| Final cumulative drug dose, mg (mean, range) | 347.5 (150–600) |
| Total days exposed (mean, range) | 41.7 (1–160) |
| Total number of doses (mean, range) | 3.3 (1–14) |
| Number of subjects with no hearing change | 12 |
| Number of subjects with unilateral hearing change | 8 |
| Number of subjects with bilateral hearing change | 4 |
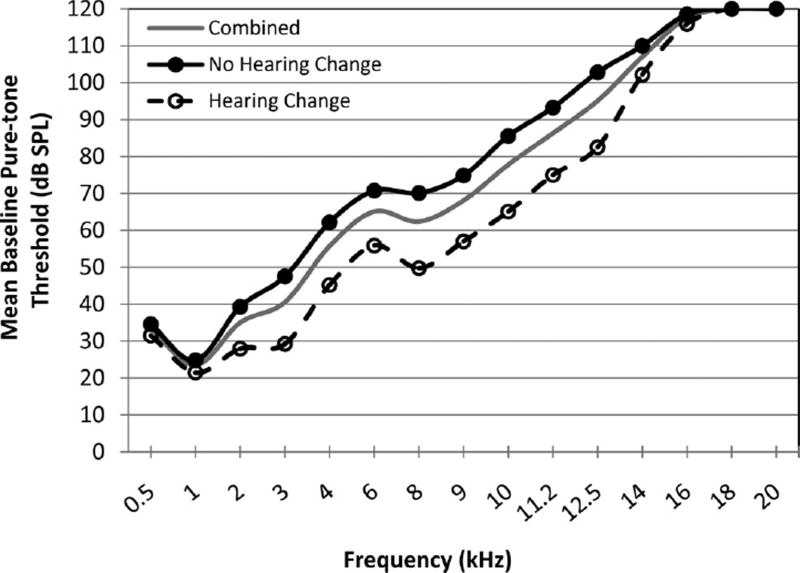
Baseline mean behavioral hearing thresholds for the entire sample (solid gray line) are plotted in Figure 1 as a function of audiometric test frequency from 0.5 to 20 kHz. Pure-tone threshold responses that could not be obtained at equipment limits (115 dB SPL) were arbitrarily set to 120 dB SPL for inclusion into the average. Ninety-seven percent (35 of 36) of ears in this sample had measurable thresholds above 8 kHz at baseline. The percentage of ears with pure-tone thresholds that could be measured within the intensity limits of the audiometric equipment declined as frequency increased. At baseline, approximately 83% of ears (30 of 36) had measurable hearing thresholds at ≥12.5 kHz; this rapidly declined to 28% (10 of 36) at 16 kHz and 0% (0 of 36) at 20 kHz. In Figure 1, mean thresholds at frequencies beginning around 16 kHz were near 120 dB SPL, indicating that many subjects had no responses at these higher frequencies.
Fig. 1.
Mean baseline pure-tone thresholds by audiometric frequency. Mean thresholds in dB SPL are given for all ears at baseline (gray solid line), ears that eventually experienced subsequent hearing changes (black dashed line, open circles), and ears with no hearing changes (black solid line, filled circles).
In addition to baseline hearing thresholds for the entire group, the baseline hearing thresholds (dB SPL) for the no hearing change group (filled circles) and the hearing change group (open circles) are plotted separately in Figure 1. Both groups follow a similar audiometric pattern with normal to near-normal hearing in the low frequencies followed by a sloping hearing loss. However, the ears that went on to experience hearing change had better pre-exposure hearing across the majority of frequencies compared with the ears that did not have hearing change.
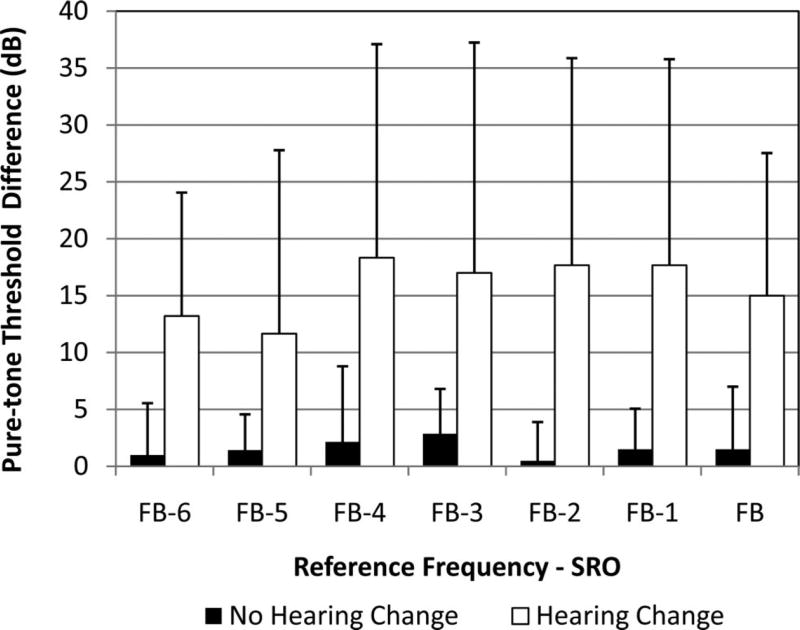
Postexposure hearing changes for normalized behavioral SRO frequencies are plotted in Figure 2. The plot includes magnitude of hearing change (dB) at the final test by the seven SRO frequencies in rank order of the lowest frequency, FB − 6, to highest frequency, FB. The median upper bound of the SRO (FB) at baseline was 12.5 kHz (range: 5 to 16 kHz) and baseline SRO pure-tone thresholds averaged 74.3 dB SPL (range: 48.6 to 97.5 dB SPL). Average hearing shift among ears with hearing change (white bars) was at least 10 dB in each frequency. There was an average of at least 15 dB threshold shifts within the five highest frequencies (FB − 4 through FB). Note that these are within frequency averages only and do not represent an average hearing change over the SRO range.
Fig. 2.
Postexposure pure-tone threshold shifts by audiometric frequencies from baseline to final test. Amount of hearing change (in dB; y axis) at the seven SRO normalized frequencies (x axis). Error bars represent 1SD.
Patient and drug regimen factors that, based on previous studies, could be risk factors for ototoxic hearing change were analyzed for their predictive utility through separate logistic regression analyses. A repeated measures statistical approach incorporating a correlation matrix was used to adjust for correlations between the two ears of a subject and between repeated measures on each ear. Tables 2 and 3 show statistics for continuous and categorical variables, respectively, and include patient and drug regimen factors that may be risk factors for ototoxicity by hearing change group. Table 2 includes age, high-frequency pure-tone average (PTA) at 2, 4, and 6 kHz at baseline, SRO average pure-tone threshold at baseline, upper frequency bound of the SRO at baseline, cumulative cisplatin dose, total number of days exposed to cisplatin, and the total number of cisplatin doses by hearing change group. The degree of hearing loss reported by high-frequency PTA at 2, 4, and 6 kHz is by definition restricted to the conventional frequencies, whereas the SRO average threshold reflects the degree of hearing loss in the individualized frequency range most sensitive to ototoxicity. Therefore, although high-frequency PTA and SRO average threshold measures are highly correlated (r = 0.78), they most often represented hearing in different frequency ranges. Similarly, upper frequency bound of the SRO is correlated with both SRO average threshold (r = −0.62) and high-frequency PTA (r = −0.66). However, it was of interest to determine which measure of baseline hearing was the best predictor of hearing change, so all three measures were evaluated individually as well as together.
TABLE 2.
Continuous variables by hearing change group across all visits
| No Hearing Change | Hearing Change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Variable | N† | Mean | SD | Min | Max | N† | Mean | SD | Min | Max | p* |
| Age | 79 | 59.8 | 6.4 | 28 | 75 | 47 | 56.4 | 7.0 | 28 | 68 | 0.03 |
| Baseline SRO average threshold (dB SPL) | 79 | 76.1 | 13.2 | 48.6 | 97.5 | 47 | 62.3 | 13.6 | 48.6 | 93.6 | <0.01 |
| Baseline high-frequency PTA (2, 4, and 6 kHz) | 79 | 57.3 | 16.2 | 25.0 | 83.3 | 47 | 43.3 | 13.0 | 25.0 | 71.7 | <0.01 |
| Baseline SRO upper frequency bound (kHz) | 79 | 11.2 | 2.3 | 5.0 | 14.0 | 47 | 12.6 | 1.8 | 8.0 | 16.0 | <0.01 |
| Cumulative dose (mg) | 79 | 252.4 | 98.6 | 55.0 | 540.0 | 47 | 425.3 | 121.1 | 150.0 | 600.0 | <0.01 |
| Total number of days exposed | 79 | 57.7 | 43.7 | 1 | 160 | 47 | 54.2 | 33.7 | 1 | 160 | 0.95 |
| Total number of doses | 79 | 3.2 | 1.7 | 1 | 14 | 47 | 3.4 | 2.4 | 1 | 14 | 0.55 |
p value from GEE logistic regression model coefficients.
N is patient-ear visits.
TABLE 3.
Categorical variables by hearing change group across all visits
| Variable | No Hearing Change | Hearing Change | Total | p* |
|---|---|---|---|---|
| Gender | 0.27 | |||
| Male | 70 (65%) | 38 (35%) | 108 (100%) | |
| Female | 9 (50%) | 9 (50%) | 18 (100%) | |
| Total | 79 | 47 | 126 | |
| Tinnitus at baseline | 0.21 | |||
| No | 28 (84.9%) | 5 (15.1%) | 33 (100%) | |
| Yes | 51 (54.8%) | 42 (45.2%) | 93 (100%) | |
| Total | 79 | 47 | 126 | |
| Concurrent radiation therapy | 0.96 | |||
| No | 20 (69.0%) | 9 (31.0%) | 29 (100%) | |
| Yes | 59 (60.8%) | 38 (39.2%) | 97 (100%) | |
| Total | 79 | 47 | 126 |
p value from GEE logistic regression model coefficients.
Each baseline hearing measure (high-frequency PTA, SRO average threshold, and upper frequency bound of the SRO) reflected significant differences at baseline between the ears that went on to experience hearing change and no hearing change. Ears with subsequent hearing changes had significantly better hearing at baseline, roughly 14 dB better as determined by both high-frequency PTA and SRO average threshold (both p < 0.01), and they could hear more extended high frequencies (upper frequency bound of the SRO, p < 0.01).
Furthermore, ears with hearing changes also received a significantly greater total cumulative drug dose on average, 425 mg, compared with those without hearing changes who received on average 252 mg (p = <0.01). However, other cisplatin exposure descriptors such as total number of days exposed and total number of doses were not different between groups.
Among the patient factors, age (Table 2) was significantly different among groups, with the hearing change group being slightly younger than the no hearing change group, 56 years versus 60 years, respectively (p = 0.03). However, gender, tinnitus at baseline, and presence of concurrent radiation (Table 3) did not differ between groups after accounting for the correlation among the repeated measurements.
DPOAE Accuracy for Ototoxicity Monitoring
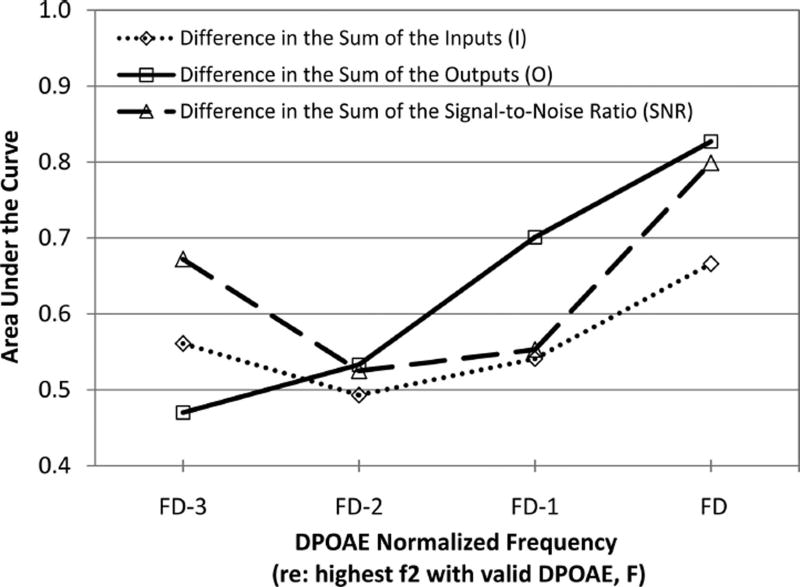
Table 4 shows the average difference in the DPOAE metrics by normalized test frequency between ears without hearing change and ears with hearing change. The median high frequency with a valid DPOAE (FD) was 4 kHz and ranged from 1.5 to 8 kHz. The difference in DPOAE metrics was calculated by subtracting the follow-up visit measurement from the baseline visit measurement. Positive differences indicate a decrease in the DPOAE metric from baseline to follow-up and a negative difference indicates an increase in the DPOAE metric from baseline to follow-up. The largest positive differences (i.e., an indication of decreasing emissions) occurred at FD corresponding to the highest frequencies measured. Statistically significant differences were observed for the I:FD, O:FD, and SNR:FD (all p < 0.05). The estimated AUC of each DPOAE metric is plotted against normalized frequency in Figure 3. The AUC for each of the DPOAE metrics is high at FD, indicating good test performance but declines as test frequency decreased to FD − 2.
TABLE 4.
DPOAE variables according to hearing change across all visits
| No Hearing Change | Hearing Change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| N† | Mean | SD | Min | Max | N† | Mean | SD | Min | Max | p* | |
| Difference in the sum of the inputs | |||||||||||
| FD | 56 | 13.6 | 126.5 | −270.1 | 343.7 | 42 | 110.3 | 170.2 | −176.4 | 438.5 | 0.04 |
| FD − 1 | 72 | 19.7 | 110.7 | −295.1 | 335.2 | 44 | 30.6 | 101.2 | −355.2 | 302.0 | 0.38 |
| FD − 2 | 69 | 29.8 | 121.3 | −289.1 | 301.9 | 39 | 27.6 | 86.8 | −139.3 | 317.2 | 0.67 |
| FD − 3 | 66 | 25.5 | 144.6 | −346.8 | 376.1 | 32 | 14.7 | 97.4 | −100.6 | 335.6 | 0.40 |
| Difference in the sum of the outputs | |||||||||||
| FD | 56 | −0.02 | 0.15 | −0.68 | 0.23 | 42 | 0.15 | 0.13 | −0.19 | 0.39 | <0.01 |
| FD − 1 | 72 | 0.003 | 0.17 | −0.37 | 0.49 | 44 | 0.09 | 0.29 | −1.63 | 0.43 | 0.24 |
| FD − 2 | 69 | 0.09 | 0.28 | −0.68 | 1.19 | 39 | 0.09 | 0.43 | −0.89 | 1.63 | 0.76 |
| FD − 3 | 66 | 0.13 | 0.44 | −0.95 | 1.31 | 32 | 0.13 | 0.82 | −0.58 | 3.25 | 0.60 |
| Difference in the sum of the SNRs | |||||||||||
| FD | 56 | −1.81 | 11.37 | −57.87 | 10.74 | 42 | 6.19 | 5.26 | −3.69 | 14.30 | 0.03 |
| FD − 1 | 72 | 0.02 | 8.56 | −43.53 | 22.69 | 44 | 0.00 | 6.34 | −19.19 | 13.14 | 0.25 |
| FD − 2 | 69 | 0.43 | 6.93 | −15.42 | 17.03 | 39 | −0.63 | 9.51 | −23.01 | 22.26 | 0.35 |
| FD − 3 | 66 | 2.37 | 13.86 | −64.50 | 37.56 | 32 | −2.83 | 17.26 | −30.60 | 43.69 | 0.28 |
p value from GEE logistic regression model coefficients.
N is patient-ear visits.
Fig. 3.
Accuracy of OAE metrics as predictors of ototoxicity by DPOAE frequency. The area under the ROC curve (AUC; y axis) is plotted for each DPOAE metric as a function of normalized frequency (x axis).
Risk Factor Model
According to other published reports, multivariate solutions may be more accurate than univariate DPOAE methods for identifying hearing loss (Dorn et al. 1999). Herein, we develop a risk factor model to use as a base model. In the next section, an “optimal” multivariate discriminant function is developed by combining this risk factor model with DPOAE variables described above.
The risk factor model was established by including predictors observed in Tables 2 and 3 having p values for the regression coefficients <0.1. This model was reduced by backward elimination and is shown in Table 5. This risk factor model includes the transformed pre-exposure SRO average pure-tone threshold, cumulative dose of cisplatin, and an interaction between these two terms.
TABLE 5.
Regression coefficients for the multivariate discriminant function
| Variable | β | SE | p* |
|---|---|---|---|
| SRO average threshold† | −3.352 | 1.174 | 0.01 |
| −3.504 | 1.136 | 0.01 | |
| Cumulative dose | 0.017 | 0.004 | <0.01 |
| 0.018 | 0.004 | <0.01 | |
| SRO average threshold† × dose | 0.013 | 0.004 | 0.03 |
| 0.014 | 0.003 | 0.01 | |
| I:FD | 0.006 | 0.002 | <0.01 |
| 0.007 | 0.002 | <0.01 | |
| O:FD | −0.606 | 4.143 | 0.861 |
| SNR:FD | 0.078 | 0.104 | 0.422 |
Bold, italicized numbers indicate results for the optimal multivariate discriminant function that was selected by backward elimination.
p value from GEE logistic regression model coefficients.
Reciprocal of “SRO Average Threshold” and standardized to a mean of 0 and SD of 1.
Indicates order in which DPOAE metrics were removed from model.
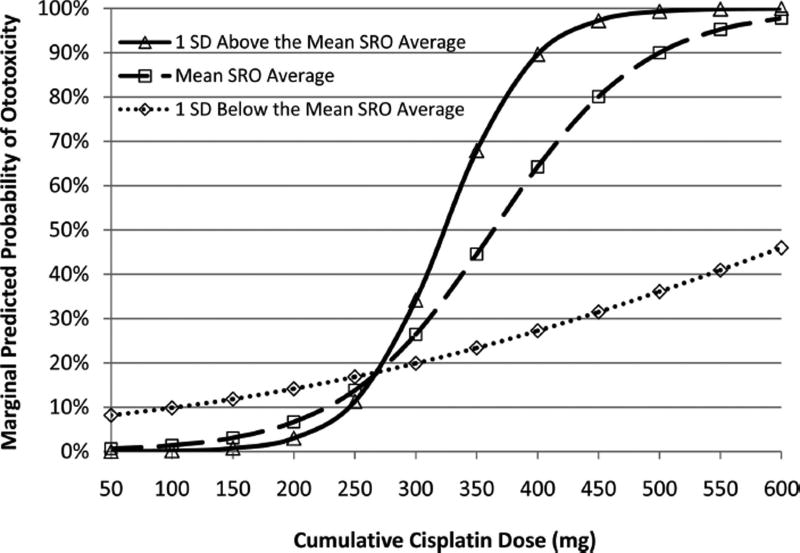
The marginal probability of hearing loss predicted by the risk factor model is plotted in Figure 4 for cumulative drug doses ranging from 100 to 600 mg by pre-exposure hearing status. At cumulative drug doses of 300 mg or lower, the predicted probability of ototoxicity differs very little by pre-exposure hearing status. Those with better hearing have a higher probability of experiencing ototoxicity at cumulative cisplatin doses of 400 mg and greater. At 400 mg cumulative drug dose, the predicted probability of ototoxicity increases from 28% to 64% to 90% with increasingly better pre-exposure hearing status. At a cumulative cisplatin dose of 600 mg, a dose typically associated with ototoxicity, the predicted probability of ototoxicity is nearly 100% for ears with better hearing. However, for ears with poorer hearing (1SD below the mean), the predicted probability of ototoxicity does not reach 50%. At high cisplatin doses, those with better hearing are twice as likely to experience ototoxicity.
Fig. 4.
Marginal predicted probability of ototoxicity by cumulative drug dose and pre-exposure hearing. Plot of predicted probability of ototoxicity (y axis) by cumulative drug dose (x axis), ranging from 100 to 600 mg, which is stratified by pre-exposure hearing for the mean (dashed line with open squares), 1SD below the mean (poorer hearing indicated by the dotted line with open diamonds), and 1SD above the mean (better hearing indicated by solid line with open triangles).
Multivariate Discriminant Function
To develop an “optimal” multivariate discriminant function for separating ears with and without ASHA-significant ototoxic hearing change, the three significant DPOAE metrics shown in Table 4 (difference in I:FD, difference in O:FD, and the difference in SNR:FD) were introduced to the risk factor model shown in Table 5. The model was again reduced in a backward fashion. Only the difference in I:FD remained in the final model. No two-way interactions between the SRO average threshold or cumulative dose and I:FD were significant at the 0.05 test level. Analysis of the cumulative residuals indicated no systematic lack of fit to the data.
The final model included SRO average threshold at baseline, cumulative cisplatin dose, difference in I:FD, and the interaction between SRO average threshold at baseline and cumulative dose (Table 5). The model indicates that, for every 100 Pa drop from baseline in I:FD, there is a 2.3-fold increase (95% CI: 1.53 to 3.36) in the predicted risk of hearing change after controlling for baseline hearing and cumulative cisplatin dose.
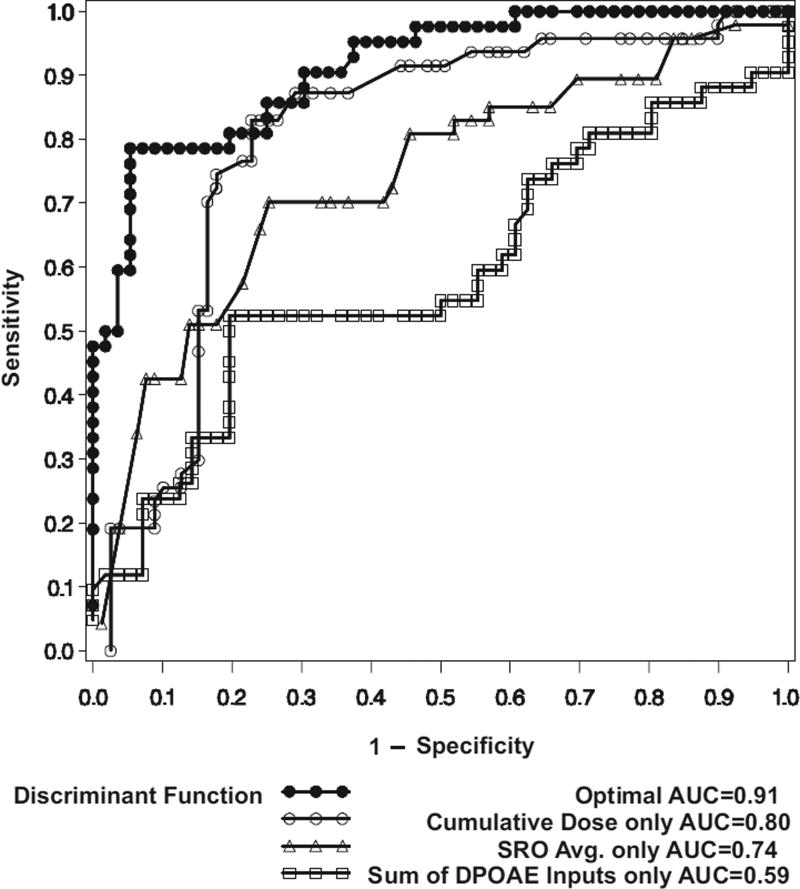
Multivariate Discriminant Function Accuracy for Ototoxicity Monitoring
A ROC curve and its corresponding AUC was estimated using the leave-one-out cross-validation methodology. These are measures of the accuracy with which the multivariate discriminant function can differentiate ears with an ASHA-significant hearing change from those without. Results are shown in Figure 5, along with ROC curves and AUCs for discriminant functions relying only on the cumulative dose, SRO average threshold, or the difference in the I:FD alone. In ROC curve analysis, the overall best discriminator is the one that is concentrated toward the upper left region of the graph. The ROC curve for the multivariate discriminant function, which is a combination of information from the three sources, had the highest AUC (0.91). The ROC curves show that the multivariate discriminant function has the highest sensitivity across the spectrum of false-positive rates. The optimal multivariate discriminant function clearly predicts hearing change better than any single variable.
Fig. 5.
ROC curves. Sensitivity (y axis) is plotted by 1 − specificity (x axis) for the independent variables in the discriminant function and for the optimal discriminant function.
DISCUSSION
As long as the best evidence-based practice for the treatment of certain cancers includes treatment with cisplatin, some patients will experience ototoxic hearing loss. The investigation of DPOAE changes as an indicator of hearing changes attributed to ototoxic medication administration is directed toward disability control. If patients receiving cisplatin can be effectively identified as having incipient ASHA-significant hearing threshold changes by DPOAEs, then disability control measures can be implemented, such as early intervention to minimize hearing loss when possible and more timely and appropriate aural rehabilitation.
These results indicate that when measured near a patient’s DPOAE high-frequency limit, DPOAEs are a reliable indicator whether or not an ASHA-significant ototoxic hearing change has occurred. In addition, the results show that an optimal multivariate discriminant function constructed of the patient’s pre-exposure hearing together with cumulative cisplatin dose and DPOAE changes at follow-up predicts the probability of hearing change at that visit with greater accuracy than do DPOAEs alone. If validated on an independent sample, these results provide clinicians a means of entering the relevant patient, treatment, and DPOAE data into the multivariate model to detect clinically significant ototoxic hearing shifts when hearing cannot be directly measured. Because our results are based on comparisons of DPOAE changes to hearing changes as the “gold standard” measure of ototoxicity, they represent an important step toward a DPOAE-based method by which clinical decisions of ototoxicity can be made.
Although this study considered only hearing and DPOAE changes at a particular visit computed relative to results obtained at baseline, we envision that this multivariate solution may be implemented to monitor for signs of progressive hearing change over multiple visits by shifting the baseline DPOAE amplitudes and/or frequencies once a significant change has been confirmed on repeat testing. The shifted baseline values then serve as the basis for subsequent comparisons. Continuously shifting the baseline following confirmed changes allows this method to be applied over time to monitor for progressive hearing changes until DPOAEs are no longer recordable.
DPOAE Test Performance
Highest Recordable DPOAEs Change First
Twelve DPOAE metric-frequency combinations were evaluated for predictive accuracy. The most predictive frequency based on estimates of the AUC was the highest valid DPOAE able to be recorded, FD. Predictive accuracy decreased as frequency relative to FD decreased. These results suggest there is a DPOAE-sensitive region to ototoxic insult, similar to the behavioral SRO. This is not surprising because animal and human studies indicate that damage from cisplatin progresses in a base to apex manner. Although previous reports in adults have also noted that the higher DPOAE frequencies are those most vulnerable to ototoxicity (Ress et al. 1999; Reavis et al. 2008), this study seems to be the first to demonstrate that higher DPOAE frequencies are statistically more sensitive to incipient ototoxicity than lower DPOAE frequencies.
DPOAEs Detect Hearing Loss at Higher Behavioral Frequencies
The DPOAEs in these analyses detected concurrent pure-tone threshold changes with high accuracy. Initial baseline recordings were made through 10 kHz for DPOAEs. However, the highest frequency with a valid DPOAE was always <10 kHz, and the median DPOAE upper frequency bound at baseline was 4 kHz. In contrast, the median upper frequency bound of the behavioral SRO was 12.5 kHz. Consequently, the DPOAEs that could be monitored for changes were most often at lower frequencies compared with the behavioral SRO. This pattern of change could potentially be reflecting DPOAE sensitivity to damage at regions of the cochlea basal to the DPOAE primary frequencies, preclinical degradation of auditory function within the tonotopic region coding the primary and DP frequencies, or a combination of these influences.
We have shown previously that DPOAE sensitivity to ototoxic hearing changes declined significantly when DPOAEs able to be monitored were separated from the behavioral SRO by more than one and one-half octaves (Reavis et al. 2008). Other studies have demonstrated that DPOAE level measures correlate best with pure-tone thresholds obtained at the same test frequency, but that hearing at higher frequencies also impacts DPOAE levels (Dorn et al. 1999). It has also been suggested that extended high-frequency hearing may be associated with DPOAEs measured at ≤8 kHz (Arnold et al. 1999; Driesbach et al. 2008). Given the correlation between DPOAEs and higher frequency behavioral threshold sensitivity, it is unclear how to interpret evidence that DPOAEs change in the absence of pure-tone threshold changes at corresponding frequencies in patients receiving ototoxic medications (Mulheran & Degg 1997; Katbamna et al. 1999; Stavroulaki et al. 2001, 2002).
One interpretation is that DPOAE generator components are spread over a greater region of the cochlea than previously thought, because DPOAEs can be affected by an acoustic interfering tone (IT) set much higher in frequency than the primaries (Martin et al. 1987, 2009). Most recently, Martin et al. (2010) recorded DPOAEs in normal-hearing and noise-damaged rabbit ears over a wide range of stimulus frequencies and levels, both with and without a high-level IT placed slightly below 2f1 − f2 or far above f2. Both the ITs influenced DPOAE level and phase responses. The high-frequency IT could have potentially affected the DPOAE energy generated near f2; however, phase behavior of the component removed by the high-frequency IT bore the signature of a reflection emission (vertical bands in emission phase-frequency plots) and so is inconsistent with that explanation. A DPOAE component with horizontal phase banding was associated with the f2 region in the absence of the high-frequency IT, presumably indicative of a wave-fixed distortion emission.
On the other hand, there is also evidence from animal models that DPOAEs are sensitive to preclinical damage. Such studies have provided much needed insight into the relationship between DPOAE changes and the pathophysiology of cisplatin. Alam et al. (2000) found that among cisplatin-treated gerbils, DPOAEs were diminished from 0.5 to 16 kHz compared with controls, with the greatest level decreases observed at the highest frequencies. Through molecular methods, they confirmed that a variety of cochlear tissues undergo apoptosis in response to cisplatin exposure including outer and inner hair cells, supporting cells, spiral ganglion cells, and the stria vascularis. In addition to the majority of outer hair cell death observed toward the basal turn of the cochlea, they also found that the stria vascularis undergoes apoptotic changes in all three turns. The authors suggested that observed DPOAE level changes in frequency regions where outer hair cell apoptotic changes were minimal might suggest that the DPOAEs were reflecting endocochlear potential decreases associated with the strial damage and as such might be reflecting a preclinical pathological state. It is beyond the scope of this study to address the complex generator sources of DPOAEs or the pathophysiology of ototoxicity; however, regardless of the mechanism, it is clear that DPOAEs have utility for detecting behavioral hearing threshold shifts occurring at higher frequencies.
DPOAE Test Performance
The three DPOAE metrics examined were simple quantitative summary measures of input-output growth functions related to the input levels (I), DPOAE amplitudes or output levels (O), and SNRs that were associated with valid DPOAE responses. In normal-hearing subjects (defined as thresholds ≤20 dB HL), Gorga et al. (1997) found that DPOAE test performance increased with increasing test frequency, reporting AUCs ranging from 0.76 to 0.95 for frequencies 750 to 6000 Hz, respectively. In our study, DPOAE test frequencies were normalized to each subject’s high-frequency DPOAE limit, yet we found that each of the DPOAE metrics performed well on their own. The best performer was change in O with an AUC of 0.8. Our results in Veterans suggest that with no other information regarding cisplatin dosage or patient factors, DPOAEs perform as well categorizing ears as having stable or changed hearing as categorizing normal ears as having normal or impaired hearing (Gorga et al. 1997).
DPOAE Test Performance Improves by Adding Other Factors
We expected and found that known ototoxicity risk factors could be statistically adjusted in a prediction model to improve the ability of DPOAEs to correctly classify ears as having stable or changed hearing. The final model incorporated DPOAEs, cumulative cisplatin dose, and pre-exposure hearing to give an AUC of 0.9. This can be interpreted as, on average, a patient experiencing hearing changes will have a more abnormal test result than 90% of the patients with stable, unchanged hearing. Most adults receiving cisplatin are capable of taking a behavioral hearing test before the start of their chemotherapy, and information about cumulative drug dose can be readily obtained from the medical chart. Therefore, it is clinically feasible to include these important risk factors along with DPOAEs test results in a multivariable DPOAE model to determine whether or not hearing is likely to have changed after cisplatin administration.
Risk Factors for Ototoxicity
The data presented here were from a homogenous group of older, adult, male Veterans who received only the ototoxin, cisplatin. Furthermore, no subject reported noise exposure during treatment without the use of hearing protection devices. Therefore, concurrent exposure to other ototoxins such as noise and other medications were controlled by the study design and not evaluated statistically. Other patient variables that were not controlled by the study design and were investigated included age; gender; pre-exposure hearing status; pre-exposure tinnitus status as a proxy for cochlear degradation; concurrent radiation; and the number, dose, and duration of cisplatin exposure. Ultimately, cumulative dose of cisplatin and pre-exposure hearing proved to be the most important risk factors for hearing change.
Pre-Exposure Hearing Status
The behavioral threshold monitoring strategy used in this study tests the entire range of human hearing (frequencies from 0.25 to 20 kHz) to determine the highest audible frequencies, considered the most vulnerable frequency range, to monitor each patient. Using this SRO monitoring strategy yielded results that suggested individuals with better than average hearing calculated at baseline are at greater risk for experiencing pure-tone threshold shifts compared with individuals with poorer than average hearing at baseline. Even so, the functional consequence of hearing changes may be even greater in individuals with poor pre-exposure hearing than for patients with good pre-exposure hearing because additional loss can immediately affect their communication ability. Furthermore, there is no indication that individuals with poorer hearing at baseline who experience hearing changes experience a smaller magnitude (decibel) of hearing change. Reavis et al. (2008) found no differences in the magnitude of hearing change between a group of cisplatin-exposed Veterans with good hearing (high-frequency PTA = 43 dB SPL) and exposed Veterans with poor hearing (high-frequency PTA = 70 dB SPL) at baseline.
In contrast, there are several clinical studies that have suggested that patients with pre-exposure sensorineural hearing loss may develop greater hearing loss from cisplatin than those with normal hearing (Aguilar-Markulis et al. 1981; Fleming et al. 1985; van der Hulst et al. 1988; Bokemeyer et al. 1998). Other investigations in both animal and human studies found no association between pre-exposure hearing and ototoxicity (Boheim & Bichler 1985; Laurell & Borg 1986, 1988; Laurell & Jungnelius 1990). It is noteworthy that in most previous reports examining the association between pre-exposure hearing and ototoxicity, hearing threshold shifts were monitored only for frequencies up to 8 kHz. Because ototoxic hearing shifts seem to progress from the higher frequencies to the lower frequencies, it can be argued that a monitoring strategy consisting only of test frequencies within the conventional audiometric range (i.e., 250 to 8000 Hz) would not be equally as sensitive for all pre-exposure audiometric configurations; in fact, it would initially be less sensitive in ears with reasonably good high-frequency hearing than in ears with substantial hearing loss. Differences in the behavioral frequency ranges assessed and subjects’ hearing characteristics may explain the lack of uniformity across previous studies.
Pre-Exposure Hearing and Cisplatin Dose Interaction
In this report, the mean cumulative cisplatin dosage at the final test date among ears with hearing change was 425 mg, consistent with previous findings that ototoxicity is typically associated with cumulative doses of 400 mg or greater (Schaefer et al. 1985). However, we noted that pre-exposure hearing was an effect modifier of the cisplatin dose-hearing change relationship. A logistic regression model predicts ototoxicity twice as frequently in ears with better than average pre-exposure hearing (average pre-exposure hearing in this report was a mild sloping to a moderate sensorineural hearing loss) compared with ears with poorer than average pre-exposure hearing. The predicted probability of ototoxicity in ears with average to better-than-average pre-exposure hearing markedly rises as cumulative cisplatin dose exceeds 300 mg and reaches nearly 100% at 600 mg. The predicted probability of ototoxicity at 600 mg in ears with poorer than average pre-exposure hearing is <50%. It is apparent that higher drug doses were required among individuals with poorer hearing to achieve a rate of ototoxicity equal to that seen in subjects with better pre-exposure hearing.
Given that cisplatin causes hearing loss initially by damaging the outer hair cells near the cochlear base, and because outer hair cell damage is associated with hearing losses only up to about 60 dB SPL, these findings might suggest that ototoxic damage occurs at lower cisplatin doses for subjects with some preserved outer hair cell function within the SRO at baseline. Higher drug doses may be needed to damage the inner hair cells/auditory nerve fibers within the SRO region. Of course this is speculative, because direct measurements of hair cell and auditory nerve activity are beyond the scope of the current project.
Other Nonsignificant Risk Factors
No effects were found between the variables age, gender, tinnitus at baseline, and concurrent radiation and the outcome variable, behavioral hearing change. After controlling for pre-exposure hearing, age was no longer significantly associated with hearing change. Tinnitus at baseline, a proxy in this study for cochlear degeneration resulting from noise-induced hearing loss, yielded no association with hearing change. In addition, concurrent radiation was found not to be associated with hearing change. However, these findings should be interpreted with caution because concomitant toxins such as noise (Boettcher et al. 1987; Gratton et al. 1990) and radiation therapy to the head and neck (Chen et al. 2006; Pearson et al. 2006) have previously been shown to produce a synergistic effect leading to increased rates of ototoxicity. Neither of these two variables was explored in any detail, for example, radiation location was not taken into account.
Limitations and Future Directions
There were several limitations to this study and data analysis. First, the study was conducted using a small sample (36 ears), as are most ototoxicity studies and many other biological datasets. Analysis of diagnostic test performance in small samples might be impacted by the idiosyncratic nature of the sample and there is always the risk of overfitting the data making the results less generalizable. This makes model validation an important step in accurately estimating diagnostic test performance. However, with small datasets, setting aside data for both parameter estimation and validation of the discriminant function is not possible. To overcome the small dataset and its inherent limitations, we used the popular leave-one-out cross-validation approach. The consequence of modeling data and estimating test performance on the same data used to fit the model is that test accuracy will always be overly optimistic. We advocate careful validation studies, because test performance may be poorer in a different study with independent samples (Simon 2005). Next, the discriminant function established in this study can only be applied to a sample collected with the same stimulus parameters and recording conditions used in this study. Separate studies would need to be conducted to test the performance of other DPOAE protocols, which also would need to be validated in a series of independent samples.
In addition, the Veteran population is different from non- Veterans in their overall health status in that Veterans traditionally present to the hospital with more advanced stages of cancer and multiple, more significant comorbidities (Agha et al. 2000), which may limit the generalizability of this multivariate solution to non-Veteran populations. Future DPOAE ototoxicity monitoring investigations and validation studies would benefit from being conducted among other populations including children to determine whether the observed associations and solutions are confounded by age, disease status, or a cultural trait, either social and/or genetic.
A potential problem with comparing DPOAE changes to hearing changes in patients treated with cisplatin is that any observed disagreement between the two tests will be interpreted as a diagnostic error involving the DPOAE test. DPOAE test performance in this context might be driven down if DPOAEs change when hearing does not, because this would be interpreted as a false-positive response. In reality, an unknown portion of these “false positives” could be preclinical ototoxic changes (changes that do not yet produce audiometric hearing changes). Potentially as new gold standard methods emerge for monitoring ototoxicity, estimates of DPOAE test accuracy may increase.
Another limitation is that clinically feasible gold standards such as the modified Hughson-Westlake pure-tone threshold approach are often imperfect. There is marked variability associated with audiometric threshold testing, which sacrifices threshold accuracy for reduced test time. Audiometric testing following this approach is done using 5-dB steps, resulting in threshold estimates that are more variable compared with more time-consuming classical psychophysical procedures, and this variability could result in misclassification of the outcome, for example, pure-tone threshold measurements would suggest a hearing change but in truth, no hearing change existed. However, when the modified Hughson-Westlake approach is paired with ASHA-significant change criteria applied to the SRO region, false-positive rates were about 5% (Konrad-Martin et al. 2010). This suggest that the gold standard used in this study for comparison was sufficient to judge DPOAE performance.
Finally, ROC curve analyses limit the outcome variable to a dichotomous measure. Quantitative relationships between the magnitude of ototoxic changes in behavioral hearing measures and DPOAEs remain to be studied and warrant further investigation. A DPOAE model predicting magnitude of hearing change would afford the prescribing clinician more detailed information for determining continued treatment.
CONCLUSIONS
Consistent with previous reports, these data indicate that the risk for hearing threshold shifts from ototoxic medications is generally related to the drug dose in patients receiving cisplatin. However, we find that especially at high cisplatin dose levels, the better the hearing at baseline, the more important an indicator dosage becomes. Furthermore, in contrast to previous reports, we find that having better pre-exposure hearing (a mild sloping to moderate hearing loss among the Veterans we tested) is an important indicator of threshold sensitivity to cisplatin exposure, with differences across studies attributable to testing methodology, specifically different test frequency ranges. Finally, DPOAEs alone are strong predictors of ASHA-significant hearing change but perform better when accompanied by other patient information including pre-exposure hearing ability and drug dose. Once validated, this multivariate solution proposed (DPOAE, cisplatin dose, and pre-exposure hearing ability) can be a useful tool for clinicians monitoring ototoxicity through repeated measurements of DPOAE.
Acknowledgments
The authors thank Steve Neely for providing the EMAV program and Peter Jacobs for his help in computer programming to automate data processing.
This work was supported by the National Center for Rehabilitative Auditory Research and the Office of Rehabilitation Research and Development Service, Department of Veterans Affairs (Grants C3213R and C4447K).
Footnotes
Presented in part at the 2009 American Auditory Society Meeting, Scottsdale, AZ.
References
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- Aguilar-Markulis NV, Beckley S, Priore R, et al. Auditory toxicity effects of long-term cis-dichloro-diammineplatinum II therapy in genitourinary cancer patients. J Surg Oncol. 1981;16:111–123. doi: 10.1002/jso.2930160203. [DOI] [PubMed] [Google Scholar]
- Alam SA, Ikeda K, Oshima T, et al. Cisplatin-inducted apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000;141:28–38. doi: 10.1016/s0378-5955(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Amalraj S, Starkweather C, Nguyen C, et al. Health literacy, communication, and treatment decision-making in older cancer patients. Oncology (Williston Park) 2009;23:369–375. [PubMed] [Google Scholar]
- American Academy of Audiology (AAA) American Academy of Audiology Position Statement and Clinical Practice Guidelines: Ototoxicity Monitoring. Reston, VA: American Academy of Audiology; 2009. pp. 1–25. [Google Scholar]
- American National Standards Institute. American National Standard Specification for Audiometers. New York: ANSI; 1989. ANSI S3.6-1989. [Google Scholar]
- American Speech-Language-Hearing Association (ASHA) Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. ASHA. 1994;36:11–19. [Google Scholar]
- Anniko N, Sobin A. Cisplatin: Evaluation of its ototoxic potential. Am J Otolaryngol. 1986;7:276–293. doi: 10.1016/s0196-0709(86)80050-3. [DOI] [PubMed] [Google Scholar]
- Arnold DJ, Lonsbury-Martin BL, Martin GK. High frequency hearing influences lower-frequency distortion-product otoacoustic emissions. Arch Otolaryngol Head Neck Surg. 1999;125:215–222. doi: 10.1001/archotol.125.2.215. [DOI] [PubMed] [Google Scholar]
- Blakley BW, Gupta AK, Myers SF, et al. Risk factors for ototoxicity due to cisplatin. Arch Otolaryngol Head Neck Surg. 1994;120:541–546. doi: 10.1001/archotol.1994.01880290051009. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Henderson D, Gratton MA, et al. Synergistic interactions of noise and other ototraumatic agents. Ear Hear. 1987;8:192–212. doi: 10.1097/00003446-198708000-00003. [DOI] [PubMed] [Google Scholar]
- Boheim K, Bichler E. Cisplatin-induced ototoxicity: Audiometric findings and experimental cochlear pathology. Arch Otorhinolaryngol. 1985;242:1–6. doi: 10.1007/BF00464398. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett RE. Drug-induced ototoxicity. Drugs. 1980;19:412–428. doi: 10.2165/00003495-198019060-00002. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Durrant J. Audiologic monitoring for ototoxicity. Otolaryngol Clin North Am. 1993;26:903–914. [PubMed] [Google Scholar]
- Carhart R, Jerger J. Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Disord. 1959;24:330–345. [Google Scholar]
- Chen WC, Jackson A, Budnick AS, et al. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer. 2006;106:820–829. doi: 10.1002/cncr.21683. [DOI] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BEK, et al. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- Dreisbach LE, Torre P, III, Kramer SJ, et al. Influence of ultrahigh-frequency hearing thresholds on distortion-product otoacoustic emission levels at conventional frequencies. J Am Acad Audiol. 2008;19:325–336. doi: 10.3766/jaaa.19.4.5. [DOI] [PubMed] [Google Scholar]
- Dorn PA, Piskorski P, Gorga MP, et al. Predicting audiometric status from distortion product otoacoustic emissions using multivariate analyses. Ear Hear. 1999;20:149–163. doi: 10.1097/00003446-199904000-00006. [DOI] [PubMed] [Google Scholar]
- Estrem SA, Babin RW, Ryu JH, et al. Cis-diamminedichloroplatinum (II) ototoxicity in the guinea pig. Otolaryngol Head Neck Surg. 1981;89:638–645. doi: 10.1177/019459988108900424. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Frey RH, Erickson DA, et al. A system for evaluating auditory function from 8000–20 000 Hz. J Acoust Soc Am. 1979;66:1713–1718. doi: 10.1121/1.383643. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Frey RH, Henry JA, et al. Reliability and validity of high frequency (8–20 kHz) thresholds obtained on a computer-based audiometer as compared to a documented laboratory system. J Am Acad Audiol. 1990;1:162–170. [PubMed] [Google Scholar]
- Fausti SA, Frey RH, Henry JA, et al. Portable stimulus generator for obtaining high-frequency (8–14 kHz) auditory brainstem responses. J Am Acad Audiol. 1992;3:166–175. [PubMed] [Google Scholar]
- Fausti SA, Henry JA, Helt WJ, et al. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20:497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Helt WJ, Phillips DS, et al. Early detection of ototoxicity using 1/6th-octave steps. J Am Acad Audiol. 2003;14:444–450. [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, Inc; 2004. [Google Scholar]
- Fleming S, Peppard S, Ratanatharathon V, et al. Ototoxicity from cis-platinum in patients with stages III and IV previously untreated squamous cell cancer of the head and neck. Am J Clin Oncol. 1985;8:302–306. doi: 10.1097/00000421-198508000-00005. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman B, et al. Otoacoustic emissions from normal-hearing and hearing-impaired subjects: Distortion product responses. J Acoust Soc Am. 1993a;93(4 Pt 1):2050–2060. doi: 10.1121/1.406691. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman BM, et al. A comparison of transient-evoked and distortion product ototacoustic emissions in normal-hearing and hearing-impaired subjects. J Acoust Soc Am. 1993b;94:2639–2648. doi: 10.1121/1.407348. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B, et al. From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 1997;18:440–455. doi: 10.1097/00003446-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Stover L, Neely ST, et al. The use of cumulative distributions to determine critical values and levels of confidence for clinical distortion product ototacoustic emission measurements. J Acoust Soc Am. 1996;100(2 Pt 1):968–977. doi: 10.1121/1.416208. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Salvi RJ, Kamen BA, et al. Interaction of cisplatin and noise on the peripheral auditory system. Hear Res. 1990;50:211–224. doi: 10.1016/0378-5955(90)90046-r. [DOI] [PubMed] [Google Scholar]
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Hartmann JT, Lipp HT. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- Hodges AV, Lonsbury-Martin BL. Hearing management. In: Sullivan PA, Guilford AM, editors. Swallowing Intervention in Oncology. San Diego, CA: Singular Publishing Group; 1999. pp. 269–290. [Google Scholar]
- Hoistad DL, Ondrey FG, Mutlu C, et al. Histopathology of human temporal bone after cis-platinum, radiation, or both. Otolaryngol Head Neck Surg. 1998;118:825–832. doi: 10.1016/S0194-5998(98)70276-1. [DOI] [PubMed] [Google Scholar]
- Katbamna B, Homnick DN, Marks JH. Effects of chronic tobramycin treatment on distortion product otoacoustic emissions. Ear Hear. 1999;20:393–402. doi: 10.1097/00003446-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Kim DO. Cochlear mechanics: Implications of electrophysiological and acoustical observations. Hear Res. 1980;2:297–317. doi: 10.1016/0378-5955(80)90064-7. [DOI] [PubMed] [Google Scholar]
- Kim DO, Paparello J, Jung MD, et al. Distortion product otoacoustic emission test of sensorineural hearing loss: Performance regarding sensitivity, specificity and receiver operating characteristics. Acta Otolaryngol. 1996;116:3–11. doi: 10.3109/00016489609137705. [DOI] [PubMed] [Google Scholar]
- Knight KR, Kraemer DF, Winter C, et al. Early changes in auditory function as a result of platinum chemotherapy: Use of extended high frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- Komune S, Asakuma S, Snow JB., Jr Pathophysiology of the ototoxicity of cis-diamminedichloroplatinum. Otolaryngol Head Neck Surg. 1981;89:275–282. doi: 10.1177/019459988108900226. [DOI] [PubMed] [Google Scholar]
- Komune S, Snow JB., Jr Potentiating effects of cisplatin and ethacrynic acid in ototoxicity. Arch Otolaryngol Head Neck Surg. 1981;107:594–597. doi: 10.1001/archotol.1981.00790460006003. [DOI] [PubMed] [Google Scholar]
- Konishi T, Gupta BN, Prazma J. Ototoxicity of cis-dichlorodiammine platinum (II) in guinea pigs. Am J Otolaryngol. 1983;4:18–26. doi: 10.1016/s0196-0709(83)80003-9. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, James KE, Gordon JS, et al. Evaluation of audiometric threshold shift criteria for ototoxicity monitoring. J Am Acad Audiol. 2010;21:301–314. doi: 10.3766/jaaa.21.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad-Martin D, Neely ST, Keefe DH, et al. Sources of distortion product otoacoustic emissions revealed by suppression experiments and inverse fast Fourier transforms in normal ears. J Acoust Soc Am. 2001;109:2862–2879. doi: 10.1121/1.1370356. [DOI] [PubMed] [Google Scholar]
- Kummer P, Janssen T, Arnold W. The level and growth behavior of the 2 f1–f2 distortion product otoacoustic emission and its relationship to auditory sensitivity in normal hearing and cochlear hearing loss. J Acoust Soc Am. 1998;103:3431–3444. doi: 10.1121/1.423054. [DOI] [PubMed] [Google Scholar]
- Laurell G, Borg E. Cis-platin ototoxicity in previously noise-exposed guinea pigs. Acta Otolaryngol. 1986;101:66–74. doi: 10.3109/00016488609108609. [DOI] [PubMed] [Google Scholar]
- Laurell G, Borg E. Ototoxicity of cisplatin in gynecological cancer patients. Scand Audiol. 1988;17:241–247. doi: 10.3109/01050398809070712. [DOI] [PubMed] [Google Scholar]
- Laurell G, Jungnelius H. High-dose cisplatin treatment: Hearing loss and plasma concentrations. Laryngoscope. 1990;100:724–734. doi: 10.1288/00005537-199007000-00008. [DOI] [PubMed] [Google Scholar]
- Lin DY, Wei LJ, Ying Z. Model-checking techniques based on cumulative residuals. Biometrics. 2002;58:1–12. doi: 10.1111/j.0006-341x.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- Marco-Algarra J, Basterra J, Marco J. Cis-diaminedichloro platinum ototoxicity. An experimental study. Acta Otolarynogol. 1985;99:343–347. doi: 10.3109/00016488509108921. [DOI] [PubMed] [Google Scholar]
- Martin GK, Lonsbury-Martin BL, Probst R, et al. Acoustic distortion products in rabbit ear canal. II. Sites of origin revealed by suppression contours and pure-tone exposures. Hear Res. 1987;28:191–208. doi: 10.1016/0378-5955(87)90049-9. [DOI] [PubMed] [Google Scholar]
- Martin GK, Stagner BB, Fahey PF, et al. Steep and shallow phase gradient distortion product otoacoustic emissions arising basal to the primary tones. J Acoust Soc Am. 2009;125:85–92. doi: 10.1121/1.3073734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GK, Stagner BB, Lonsbury-Martin BL. Evidence for basal distortion-product otoacoustic emission components. J Acoust Soc Am. 2010;127:2955–2972. doi: 10.1121/1.3353121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulheran M, Degg C. Comparison of distortion product OAE generation between a patient group requiring frequent gentamicin therapy and control subjects. Br J Audiol. 1997;31:5–9. doi: 10.3109/03005364000000004. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Konishi K, Chang KC, et al. Ototoxicity of the anticancer drug cisplatin. An experimental study. Acta Otolaryngol. 1982;93:227–232. doi: 10.3109/00016488209130876. [DOI] [PubMed] [Google Scholar]
- Neely ST, Gorga MP. Comparison between intensity and pressure as measures of sound level in the ear canal. J Acoust Soc Am. 1998;104:2925–2934. doi: 10.1121/1.423876. [DOI] [PubMed] [Google Scholar]
- Neely ST, Liu Z. Tech Memo No. 17. Omaha, NE: Boys Town National Research Hospital; 1993. EMAV: Otoacoustic emission averager. [Google Scholar]
- Oldenburg J, Fossa SD, Ikdahl T. Genetic variants associated with cisplatin-induced ototoxicity. Pharmacogenomics. 2008;9:1521–1530. doi: 10.2217/14622416.9.10.1521. [DOI] [PubMed] [Google Scholar]
- Pearson SE, Meyer AC, Adams GL, et al. Decreased hearing after combined modality therapy for head and neck cancer. Am J Otolaryngol Head Neck Med Surg. 2006;27:76–80. doi: 10.1016/j.amjoto.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Peters U, Preisler-Adams S, Hebeisen A, et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs. 2000;11:639–643. doi: 10.1097/00001813-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Rademaker-Lakhai J, Crul M, Zuur L, et al. Relationship between cisplatin administration and the development of ototoxicity. J Clin Oncol. 2006;24:918–924. doi: 10.1200/JCO.2006.10.077. [DOI] [PubMed] [Google Scholar]
- Radmacher MD, McShane LM, Simon RJ. A paradigm for class prediction using gene expression profiles. Comput Biol. 2002;9:505– 511. doi: 10.1089/106652702760138592. [DOI] [PubMed] [Google Scholar]
- Reavis KM, Phillips DS, Fausti SF, et al. Factors affecting sensitivity of distortion-product otoacoustic emissions to ototoxic hearing loss. Ear Hear. 2008;29:875–893. doi: 10.1097/AUD.0b013e318181ad99. [DOI] [PubMed] [Google Scholar]
- Ress BD, Sridhar KS, Balkany TJ, et al. Effects of cis-platinum chemotherapy on otoacoustic emissions: The development of an objective screening protocol. Otolaryngol Head Neck Surg. 1999;121:693–701. doi: 10.1053/hn.1999.v121.a101567. [DOI] [PubMed] [Google Scholar]
- Schaefer SD, Post JD, Close LG, et al. Ototoxicity of low- and moderate-dose cisplatin. Cancer. 1985;56:1934–1939. doi: 10.1002/1097-0142(19851015)56:8<1934::aid-cncr2820560807>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Scheperle RA, Neely ST, Kopun JG, et al. Influence of in situ, sound-level calibration on distortion-product otoacoustic emission variability. J Acoust Soc Am. 2008;124:288–300. doi: 10.1121/1.2931953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer VG. Ototoxicity of chemotherapeutic agents. Otolaryngol Clin North Am. 1993;26:759–789. [PubMed] [Google Scholar]
- Schweitzer VG, Hawkins JE, Lilly DJ, et al. Ototoxic and nephrotoxic effects of combined treatment with cis-diamminedichloroplatinum and kanamycin in the guinea pig. Otolaryngol Head Neck Surg. 1984;92:38–49. doi: 10.1177/019459988409200109. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Siegel JH. Calibrating otoacoustic emission probes. In: Robinette MS, Glattke TJ, editors. Otoacoustic Emissions: Clinical Applications Third Edition. New York: Thieme Medical Publishers, Inc; 2007. pp. 403–427. [Google Scholar]
- Siegel JH, Hirohata ET. Sound calibration and distortion-product otoacoustic emissions at high frequencies. Hear Res. 1994;80:146–152. doi: 10.1016/0378-5955(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Simon R. Roadmap for developing and validating therapeutically relevant genomic classifiers. J Clin Oncol. 2005;23:7332–7341. doi: 10.1200/JCO.2005.02.8712. [DOI] [PubMed] [Google Scholar]
- Stavroulaki P, Apostolopoulos N, Segas J, et al. Evoked otoacoustic emissions—An approach for monitoring cisplatin induced ototoxicity in children. Int J Pediatr Otorhinolaryngol. 2001;59:47–57. doi: 10.1016/s0165-5876(01)00455-4. [DOI] [PubMed] [Google Scholar]
- Stavroulaki P, Vossinakis IC, Dinopoulou D, et al. Otoacoustic emissions for monitoring aminoglycoside-induced ototoxicity in children with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 2002;128:150–155. doi: 10.1001/archotol.128.2.150. [DOI] [PubMed] [Google Scholar]
- Stover L, Gorga MP, Neely ST, et al. Toward optimizing the clinical utility of distortion product otoacoustic emission measurements. J Acoust Soc Am. 1996;100(2 Pt 1):956–967. doi: 10.1121/1.416207. [DOI] [PubMed] [Google Scholar]
- Tsukasaki N, Whitworth CA, Rybak LP. Acute changes in cochlear potentials due to cisplatin. Hear Res. 2000;149:189–198. doi: 10.1016/s0378-5955(00)00182-9. [DOI] [PubMed] [Google Scholar]
- van der Hulst RJ, Dreschler WA, Urbanus NA. High frequency audiometry in prospective clinical research of ototoxicity due to platinum derivatives. Ann Otol Rhinol Laryngol. 1988;977(2 Pt 1):133–137. doi: 10.1177/000348948809700208. [DOI] [PubMed] [Google Scholar]
- van Ruijven MW, de Groot JC, Klis SF, et al. The cochlear targets of cisplatin: An electrophysiological and morphological time-sequence study. Hear Res. 2005;205:241–248. doi: 10.1016/j.heares.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Vermorken JB, Kapteijn TS, Hart AA, et al. Ototoxicity of cis-diamminedichloroplatinum (II): Influence of dose, schedule and mode of administration. Eur J Cancer Clin Oncol. 1983;19:53–58. doi: 10.1016/0277-5379(83)90398-x. [DOI] [PubMed] [Google Scholar]
- Whitehead ML, Lonsbury-Martin BL, Martin GK, et al. Otoacoustic emissions: Animal models and clinical observations. In: Van De Water TR, Popper AN, Fay RR, editors. Clinical Aspects of Hearing. New York, NY: Springer-Verlag; 1996. pp. 199–257. [Google Scholar]