Abstract
Background and Purpose
The differentiation of brain infarcts by region is important since their etiology and clinical implications may differ. Information on the incidence of these lesions and association with cognition and dementia from longitudinal population studies is scarce. We investigated the incidence of infarcts in cortical-, subcortical-, cerebellar- and overall brain regions and how prevalent and incident infarcts associate with cognitive change and incident dementia.
Methods
Participants (n=2,612, 41% men, mean age 74.6±4.8) underwent brain MRI for assessment of infarcts and cognitive testing at baseline and on average 5.2 years later. Incident dementia was assessed according to international guidelines.
Results
Twenty-one percent of the study participants developed new infarcts. The risk of incident infarcts in men was higher than the risk in women (1.8 (95%CI, 1.5–2.3)). Persons with both incident and prevalent infarcts showed steeper cognitive decline and had almost double relative-risk of incident dementia (1.7 (95%CI, 1.3–2.2)) compared to those without infarcts. Persons with new subcortical infarcts had highest risk of incident dementia compared to those without infarcts (2.6 (95%CI, 1.9–3.4)).
Conclusions
Men are at greater risk of developing incident brain infarcts than women. Persons with incident brain infarcts decline faster in cognition and have an increased risk of dementia compared to those free of infarcts. Incident subcortical infarcts contribute more than cortical and cerebellar infarcts to incident dementia which may indicate that infarcts of small vessel disease origin contribute more to the development of dementia than infarcts of embolic origin in larger vessels.
Keywords: MRI, Brain infarcts, Cognition, Dementia, Cohort study
Introduction
Brain infarcts are common findings on magnetic resonance (MR) images in older adults and have been associated with cognitive decline and dementia. Their prevalence has been well documented in several population-based studies.1–4 Imaging studies generally agree that the prevalence of brain infarcts increases steeply with increasing age and it is over 20% in the 70 to 79 age group and 35% in those older than 85.4, 5 Most studies with data on prevalence of brain infarcts show no sex disparity in infarct risk.5 Information on the incidence of brain infarcts from population based longitudinal studies is however scarce.
Previous studies suggest that cerebro-vascular abnormalities contribute to cognitive decline and the development of vascular dementia and Alzheimer’s disease (AD).6, 7 There is however limited information from imaging studies on whether this differs by brain region. The assessment of brain infarcts by region or type is important since the etiology and clinical implications of cerebellar-, cortical- and subcortical infarcts may differ. A distal branch middle cerebral artery occlusion resulting in a cortical stroke usually results from an embolus from either the heart, aortic arch or carotid artery, whereas a small infarct in the subcortical white matter is usually due to a blockage of small penetrating artery (lacunar infarct). The most common etiologies of cerebellar infarcts are however thought to be atherosclerosis, cardiac embolism and migraine.8, 9
At least three longitudinal population studies have shown an association of prevalent brain infarcts with increased risk of incident dementia.10–12 These studies had a relatively small number of incident dementia events and only one of them had MRI observations at two time points and none evaluated the association of infarcts with dementia by infarct location.
The objectives of this study were to investigate the incidence and risk of incident infarcts in cortical-, subcortical-, cerebellar- and overall brain regions by sex. Further, to investigate cognitive change and the risk of incident overall dementia in relation to prevalent and incident infarcts in those brain regions.
Materials and Methods
Study population
The longitudinal data in the present study are from a population-based cohort of men and women, who participated in the Age Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik Study). Briefly, from 2002 to 2006, 5,764 surviving participants of the Reykjavik Study were examined. From 2007 to 2011 a follow-up visit was conducted comprising of 3,316 surviving participants who agreed to participate. Reasons for not attending the follow-up examination included: death (n=1039), refusal (n=1198), and could not be contacted (n=211). The AGES-Reykjavik Study has been approved by the Icelandic National Bioethics Committee and by the Institutional Review Board for the Intramural Research Program of the National Institute on Aging, National Institutes of Health, USA. Written informed consent was obtained from all participants.
MRI acquisition
MR images were acquired on a single research-dedicated 1.5T Signa system (General Electric Medical Systems, Waukesha, WI). The image protocol used for the analysis of brain infarcts and described in detail elsewhere13 included a proton density (PD)/T2-w fast spin-echo (FSE) sequence, a fluid attenuated inversion recovery (FLAIR) sequence and a T2*-w gradient echo-planar-imaging (GRE-EPI) sequence. Imaging at both time-points used identical acquisition parameters.
MRI semi-quantitative rating
Brain infarcts from both time-points were rated semi-quantitatively by two trained radiographers who recorded the presence, number, and location of the lesions. An infarct was defined as a defect of the brain parenchyma with a signal intensity isointense to that of cerebrospinal fluid on all sequences used for the rating (FLAIR, PD/T2/T2*-w).14 All infarcts were included regardless of whether they were clinically apparent or not. Cortical infarcts were defined as defects involving or limited to the cortical ribbon and surrounded by an area of high signal intensity on FLAIR images (Figure 1A). Subcortical infarcts were defined as parenchymal defects not extending into the cortex, surrounded by an area of high signal intensity on FLAIR with a minimal size diameter of 4-mm (Figure 1B). This minimal size was used, because for smaller parenchymal defects it is harder to assess reliably whether they are based on perivascular spaces or lacunar infarcts. Defects surrounded by a rim of hemosiderin were excluded since it is not possible to distinguish parenchymal hematomas from hemorrhagic infarcts. The presence of hemosiderin was defined as an area of signal loss on T2*-w scans that was invisible or smaller on T2- and PD-w images. Cerebellar infarcts were defined as parenchymal defects in the cerebellum. They were not required to have a surrounding rim of high signal intensity on FLAIR or T2-w images since cerebellar infarcts often lack such rims (Figures 1C&D). There were no size criteria for cortical- nor cerebellar infarcts. Infarcts that spanned two different anatomical areas were assigned to the location with the largest diameter of the defect. Defects in the subcortical area without a rim or area of high signal intensity on FLAIR, with a minimal size diameter of 4-mm and without evidence of hemosiderin were regarded as enlarged perivascular-spaces and excluded.
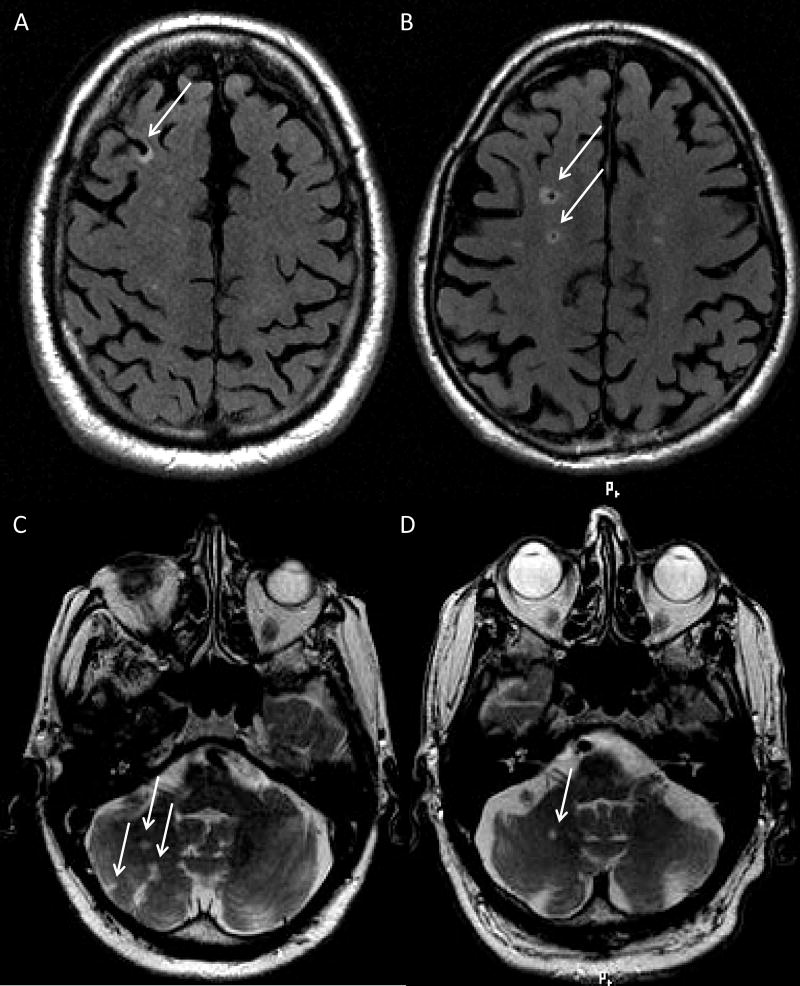
Figure 1. Example images of infarcts in sub-regions.
A) Cortical infarct in the right frontal lobe on a FLAIR image.
B) Subcortical infarcts on a FLAIR image in the right frontal lobe.
C)&D) Cerebellar infarcts on a T2-w image.
Intra- and inter-observer reliability was assessed for the two observers every 6 months and shown to be good. The intra-observer reliability (Kappa statistics) was 0.90 and 0.85 for cortical-; 0.85 and 0.87 for cerebellar- and 0.89 and 0.93 for subcortical infarcts. The inter-observer reliability for cortical-, cerebellar- and subcortical infarcts was 0.82, 0.70 and 0.76 respectively.
Cognition and dementia
The assessments of cognition and dementia have been described in detail elsewhere.15 In brief, a battery of cognitive tests was administered to all participants and 3 cognitive domain scores calculated: Memory, processing speed and executive function. We computed sex specific composite measures by averaging the standardized Z-scores across the tests in each domain. A change in cognition was calculated separately by domain by subtracting the follow-up from baseline scores.
Participants with dementia were identified in a 3-step procedure described previously.14 Briefly the Mini-Mental-State-Examination and the Digit-Symbol-Substitution-Test were administered to all participants. Screen-positives were administered a diagnostic battery of neuropsychological tests, and among them, screen positives were examined by a neurologist and a proxy interview was administered. A consensus diagnosis, according to international guidelines, was made by a panel of experts. In addition to case identification at the baseline and follow-up exams, all participants that attended the baseline exam were tracked for dementia diagnosis through vital statistics and hospital records, and the nursing and home based Resident Assessment Instrument (RAI)16, allowing for a more complete follow-up and less misclassification of cases as controls.
Analytic sample
Of the 3,316 participants who attended the follow-up study, 2,612 participants (1,070 men and 1,542 women) were included in the final sample. Compared to these participants, the 704 excluded persons were more often men, more likely to be older, to have hypertension, diabetes and atrial fibrillation. The reasons for exclusions were: MRI contraindications and claustrophobia (n=595), disability or refusals preventing a visit to the MRI facility (n=59). Additional 50 persons were excluded due to the diagnosis of dementia at baseline (n=31) or missing dementia assessment (n=19). Of the 5,764 participants in the baseline study, 4,766 had baseline MRI. Participants with MRI at baseline and not included in the final sample with follow-up MRI had higher prevalence of brain infarcts, more WMH volume and lower relative brain volume relative to intracranial volume.
Symptomatic infarcts
Prevalent strokes were obtained from medical records (69 of 2612 participants (3%)), 14% (11 of 69 participants) of which were adjudicated by a dementia neurologist, a stroke neurologist and a neuroradialogist. This same adjudication process was used to diagnose all incident strokes, which included strokes that occurred between the 1st and 2nd MRI (average 5.2 years between).
Statistical analysis
All analyses were performed with SAS/STAT®9.2 (SAS Institute Inc). For each infarct region (cortical-, cerebellar-, subcortical- and infarcts overall), subjects in the study sample were divided into four groups based on the absence/presence of infarcts: 1] No prevalent and no incident; 2] One or more prevalent and no incident; 3] No prevalent and one or more incident and 4] One or more prevalent and one or more incident. Baseline characteristics of the study sample between infarct groups were compared using a general linear model after adjusting for age.
Modelling the association between infarct groups with longitudinal change in the different cognitive domains was performed using a random effects model (PROC-MIXED) for men and women separately. The longitudinal change in cognition was presented in Z-scores with 95% confidence interval (95%CI) using time between MR scans as the time variable and adjusting for age at baseline. Multiplicative terms between time and infarct group, and time and baseline age was tested, to allow for the different estimates of change over time among the four brain infarct groups and to test if change between brain infarct groups was statistically significant or not.
The relative risks (risk-ratios, RRs) were estimated using a Poisson regression model with a robust variance estimator within PROC-GENMOD. The risk-ratios of incident infarcts in relation to sex were estimated after adjusting for age and the time interval between MR scans. The analysis of the effect of sex was repeated after additionally adjusting for brain volume and vascular risk-factors including smoking (current and former), total serum cholesterol, high-density lipoprotein cholesterol, use of cholesterol-lowering medication, hypertension (use of anti-hypertensive medication, systolic blood-pressure>140 mmHg and/or diastolic blood pressure>90 mmHg), C-reactive protein and coronary artery calcium-score.
Finally, the risk-ratios of incident dementia were estimated in relation to the four groups of infarcts after adjusting for age, sex and time interval between MR scans where the reference group was the group of persons without infarcts (model 1). These analyses were repeated after additionally adjusting for potential confounders: First by adding baseline vascular risk-factors and level of education (model 2), second, by additionally adding history of symptomatic brain infarcts (model 3) and third by additionally adding baseline presence of brain microbleeds and baseline white matter hyperintensity volume (model 4), both MRI markers of manifest cerebral small vessel disease17 Definitions of microbleeds and WMH are provided in the online-only Data Supplement).
Results
Overall, 803 of the 2612 participants (31%) had prevalent infarcts on MRI of whom 43 (5.4%) had clinical stroke events. Both men and women with one or more prevalent or incident infarct compared to men and women without infarcts were more likely to be older and to have higher coronary calcium (Table 1). For the same groups, men but not women were significantly more likely to have diabetes, atrial fibrillation, lower relative brain volumes and cognitive scores for all domains (age adjusted p-value for all <0.05). The average time between baseline and follow-up assessments was 5.2±0.2 (mean±SD) years.
Table 1.
Baseline characteristics of participants by infarcts overall and sex
| Characteristics | No prevalent & no incident |
One or more prevalent & no incident |
No prevalent & one or more incident |
One or more prevalent & one or more incident |
||||
|---|---|---|---|---|---|---|---|---|
| Men n=567 |
Women n=990 |
Men n=220 |
Women n=290 |
Men n=113 |
Women n=139 |
Men n=170 |
Women n=123 |
|
| Age, mean±SD | 74.1±4.4 | 74.0±4.7 | 74.8±4.6 | 75.3±5.0 | 75.0±4.4 | 75.5±5.4 | 76.4±4.9 | 76.1±4.7 |
| BMI, mean±SD | 26.8±3.6 | 27.6±4.6 | 27.2±3.5 | 27.3±4.2 | 26.6±3.3 | 27.5±4.3 | 26.8±3.5 | 27.7±4.3 |
| Hypertension % | 74.4 | 75.6 | 80.5 | 83.5 | 75.2 | 80.6 | 81.8 | 82.9 |
| Diabetes % | 9.7 | 6.7 | 16.9 | 8.3 | 9.7 | 8.6 | 15.9 | 8.9 |
| Smoking status % | ||||||||
| Never | 28.9 | 52.6 | 25.5 | 51.7 | 32.7 | 53.6 | 32.4 | 57.7 |
| Former | 60.9 | 32.5 | 61.8 | 38.3 | 59.3 | 32.6 | 58.2 | 32.5 |
| Current | 10.2 | 15.0 | 12.7 | 10.0 | 8.0 | 13.8 | 9.4 | 9.8 |
| Total cholesterol (mmol/L), mean±SD | 5.3±1.1 | 6.0±1.1 | 5.0±1.0 | 5.9±1.1 | 5.4±1.1 | 5.9±1.2 | 5.1±1.0 | 6.0±1.0 |
| Atrial Fibrillation % | 6.6 | 2.4 | 12.3 | 4.2 | 7.2 | 2.9 | 13.6 | 1.6 |
| Coronary calcium, mean±SD | 735±919 | 294±568 | 1061±1291 | 442±758 | 877±1112 | 416±607 | 1057±1168 | 521±743 |
| Education level % | ||||||||
| Primary | 14.1 | 25.0 | 12.8 | 23.8 | 10.8 | 23.2 | 11.2 | 29.5 |
| Secondary | 51.1 | 51.6 | 58.0 | 51.0 | 67.6 | 50.0 | 51.2 | 43.4 |
| College | 14.3 | 17.3 | 12.3 | 19.7 | 10.8 | 15.2 | 12.9 | 21.3 |
| University | 20.4 | 6.2 | 16.9 | 5.5 | 10.8 | 11.6 | 24.7 | 5.7 |
| Cognition Z-score, mean±SD | ||||||||
| Memory | −0.12±0.82 | 0.28±0.89 | −0.21±0.86 | 0.26±0.84 | −0.29±0.77 | 0.18±1.0 | −0.44±0.76 | 0.03±0.82 |
| Executive function | 0.08±0.80 | 0.09±0.75 | 0.01±0.75 | 0.05±0.78 | 0.06±0.75 | 0.03±0.70 | −0.25±0.78 | −0.04±0.79 |
| Processing Speed | 0.03±0.70 | 0.16±0.77 | −0.02±0.74 | 0.16±0.73 | −0.09±0.73 | 0.08±0.70 | −0.20±0.79 | −0.07±0.75 |
| Relative Brain Volume % | 72.2±3.4 | 74.3±3.6 | 71.1±3.2 | 73.3±3.4 | 71.7±3.3 | 73.8±3.5 | 70.9±3.6 | 73.1±3.2 |
| Relative WMH Volume % | 0.94±0.84 | 0.97±1.04 | 1.34±1.26 | 1.27±1.17 | 1.11±1.10 | 1.43±1.51 | 1.65±1.33 | 1.70±1.65 |
| Cerebral microbleeds % | 25.2 | 13.1 | 44.7 | 23.4 | 22.8 | 14.9 | 31.8 | 19.4 |
Abbreviations: SD=Standard deviation, ml=milliliters, BMI=Body Mass Index, BP=Blood pressure, mmHg=millimeter of Mercury, WMH=White Matter Hyperintensity.
Incidence and risk estimates of brain infarcts
Overall, 545 of 2612 individuals had 1240 new brain infarcts in total with an average size of 8.5±6.0 mm and n=87 (7%) being larger than 15 mm. The cumulative incidence of new MRI detected infarcts over a mean period of 5.2 years was 20.9% (26.4% in men vs. 17.0% in women). Of those with a new infarct on MRI, 37 (6.8%) had clinically recorded events. For cortical-, cerebellar- and subcortical infarcts detected with MRI, the incidence was 7.8%, 13.0% and 4.4% respectively. The incidence in all regions was higher in men compared to women and the age adjusted risk was significantly higher in men compared to women in all regions (p<0.05) with cortical infarcts having the strongest sex difference. After adjusting additionally for vascular risk-factors and brain volume, the increased risk in men compared to women remained significant for all infarct regions except cerebellar (Table 2).
Table 2.
Incidence and risk of brain infarcts by sex
| Overall (n=2612) | Men (n=1070) | Women (n=1542) | Men vs. Women | ||
|---|---|---|---|---|---|
|
|
|||||
| Infarct region | Incidence, n (%) | Incidence, n (%) | Incidence, n (%) | Model 1 Risk-ratio (95%CI) |
Model 2 Risk-ratio (95%CI) |
|
| |||||
| Overall | 545 (20.9) | 283 (26.4) | 262 (17.0) | 1.8 (1.5–2.3) | 1.5 (1.1–1.9) |
| Cortical | 203 (7.8) | 126 (11.8) | 77 (5.0) | 2.9 (2.1–4.0) | 2.4 (1.6–3.7) |
| Cerebellar | 340 (13.0) | 162 (15.1) | 178 (11.5) | 1.3 (1.0–1.7) | 1.0 (0.7–1.5) |
| Subcortical | 116 (4.4) | 68 (6.4) | 48 (3.1) | 2.3 (1.6–3.4) | 1.9 (1.0–3.4) |
Values in first three columns are number and percent of incident brain infarcts, overall and by sex. Values in last two columns are risk-ratios with 95% confidence intervals (95%CI). Model 1: Adjusted for age and time interval between MR scans. Model 2: Additionally adjusted for brain volume and vascular risk-factors.
Prevalence and incidence of brain infarcts and cognitive change
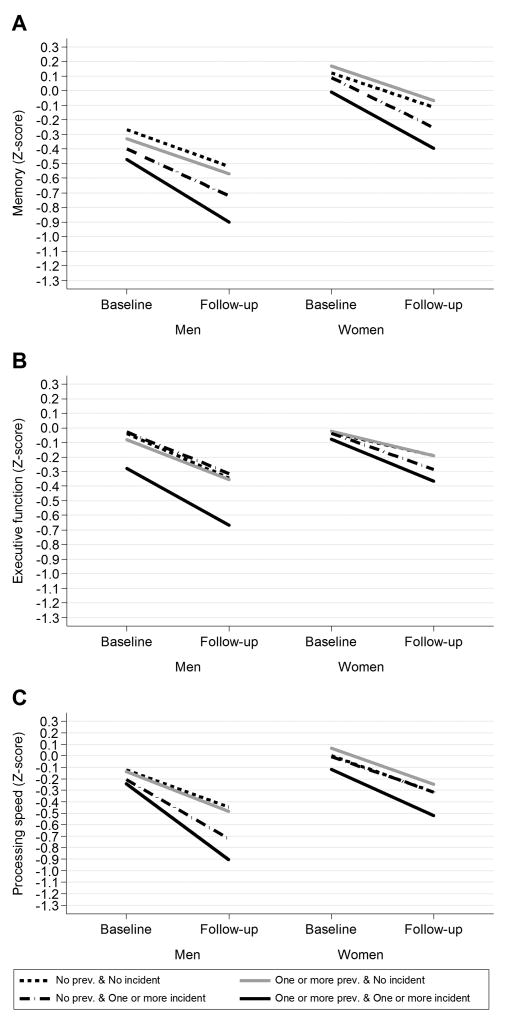
Individuals, especially men, with both prevalent and incident infarcts overall showed steeper cognitive decline in all domains compared to persons without infarcts (Figure 2). In men, this association was significant for all infarct regions and domains except overall- and cerebellar infarcts which were not significantly associated with performance in executive function (Tables I–IV and Figures I–III in the online-only Data Supplement). In women this association was significant for infarcts overall and performance in memory and executive function but not speed (Figure 1 and Table I in the online-only Data Supplement). Further, in women this association was only significant for cortical infarcts and performance in executive function (Tables II–IV and Figures I–III in the online-only Data Supplement).
Figure 2. Infarcts overall: Mean change in cognition by prevalence and incidence of infarcts and sex.
The graphs show mean change in A) memory, B) executive function and C) speed respectively for men and women by groups of prevalent- and incident infarcts overall after adjusting for age and time interval between MRI scans.
The risk of incident dementia
Dementia was diagnosed in 120 (4.6%) at the study consensus meeting (including 86 with AD, 21 with VaD, 9 with other subtype and 4 with mixed dementia) and 238 were identified later through vital statistics, hospital records and the RAI. Therefore, a total of 358 (13.7%) became demented during 9-year average follow-up.
Persons with new brain infarcts showed an increased risk of incident dementia compared to persons without infarcts or persons with prevalent infarcts. The risk of incident dementia after adjusting for age, sex and time interval between MRI scans, was almost double for persons with both prevalent and incident infarcts (Table 3, model 1). This relationship was independent of vascular risk-factors and level of education (model 2). This estimate remained statistically strong (p=0.009) after additionally adjusting for history of symptomatic infarcts (model 3), which attenuated risk estimate by 6% and by 18% after additionally adjusting for brain microbleeds and white matter hyperintensity volume (model 4). There was no significant interaction with sex in the relationship between overall infarcts and incident dementia (p=0.42).
Table 3.
Relationship between the presence of infarcts overall and incident dementia
| Overall infarcts | Infarcts vs. no infarcts (n) | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|---|
| Incident dementia (n=358) |
Without dementia (n=2254) |
Risk-ratio (95%CI) |
Risk-ratio (95%CI) |
Risk-ratio (95%CI) |
Risk-ratio (95%CI) |
|
| No prevalent & no incident | 169 | 1388 | Reference | Reference | Reference | Reference |
| One or more prevalent & no incident | 65 | 445 | 1.0 (0.8–1.3) | 1.1 (0.8–1.4) | 1.0 (0.8–1.4) | 1.0 (0.7–1.3) |
| One or more incident & no prevalent | 52 | 200 | 1.5 (1.2–2.00) | 1.6 (1.2–2.1) | 1.6 (1.2–2.1) | 1.6 (1.2–2.1) |
| One or more prevalent & one or more incident | 72 | 221 | 1.7 (1.3–2.2) | 1.7 (1.3–2.2) | 1.6 (1.2–2.1) | 1.4 (1.1–1.9) |
Values show number of persons with infarcts overall versus those without infarcts in groups of persons with and without incident dementia together with the risk-ratios of incident dementia by infarct group. Risk-ratios are with 95% confidence intervals (95%CI). Model 1: Adjusted for baseline age, sex, time interval between MRI scans. Model 2: Additionally adjusted for vascular risk-factors and education. Model 3: Additionally adjusted for symptomatic infarcts. Model 4: Additionally adjusted for brain microbleeds and white matter hyperintensity volume.
Compared to those without infarcts or with prevalent infarcts only, the risk of dementia was higher in persons with incident infarcts only or both prevalent and incident infarcts in all sub-regions (Table 4). Of the three sub-regions, the risk for persons with new cortical infarcts was similar to the risk of having new infarcts in any brain region; 1.7 (95%CI, 1.3 to 2.2). The risk of developing a new infarct was lowest for persons with cerebellar infarcts; 1.5 (95%CI, 1.1 to 2.1) and highest for persons with subcortical infarcts; 2.6 (95%CI, 1.9 to 3.4) (Table 4). The relationship of dementia with infarcts by sub-region was independent of vascular risk-factors and level of education, but similar to the results on all infarcts, estimates were attenuated by 5–15% after additionally adjusting for symptomatic infarcts, brain microbleeds and WMH volume. There were no significant interactions with sex in the relationship between infarcts in the sub-regions and dementia (data not shown).
Table 4.
Relationship between the presence of cortical, cerebellar, and subcortical infarcts and incident dementia
| Infarcts vs. no infarcts | |||
|---|---|---|---|
| Infarct region/infarct groups: | Incident dementia (n=358) | Without dementia (n=2254) | Risk-ratio (95%CI) |
| Cortical | |||
| No prevalent & no incident | 267 | 1928 | Reference |
| One or more prevalent & no incident | 39 | 175 | 1.3 (1.0–1.7) |
| No prevalent & one or more incident | 32 | 97 | 1.7 (1.3–2.2) |
| One or more prevalent & one or more incident | 20 | 54 | 1.5 (1.00–2.2) |
| Cerebellar | |||
| No prevalent & no incident | 234 | 1643 | Reference |
| One or more prevalent & no incident | 50 | 345 | 0.9 (0.7–1.2) |
| No prevalent & one or more incident | 39 | 155 | 1.2 (0.9–1.6) |
| One or more prevalent & one or more incident | 35 | 111 | 1.5 (1.1–2.1) |
| Subcortical | |||
| No prevalent & no incident | 290 | 2050 | Reference |
| One or more prevalent & no incident | 27 | 129 | 1.2 (0.8–1.6) |
| No prevalent & one or more incident | 32 | 48 | 2.6 (1.9–3.4) |
| One or more prevalent & one or more incident | 9 | 27 | 1.9 (1.1–3.3) |
Values show number of subjects with infarcts in the various regions versus those with no infarcts in corresponding regions in the groups of subjects with and without incident dementia together with the risk-ratios of incident dementia by infarct group. Risk-ratios are with 95% confidence intervals (95%CI) adjusted for baseline age, sex, time interval between MRI scans.
Discussion
We found the incidence of brain infarcts to be almost double in men compared to women. Persons, especially men with both incident and prevalent infarcts showed steeper cognitive decline than people without infarcts. The risk of incident dementia was 1.7-fold in those with both prevalent and incident infarcts compared to those without infarcts and was not significantly different in men compared to women. Of the brain sub-regions, incident subcortical infarcts contributed most to the development of dementia with 2.6-fold risk of incident dementia compared to persons without infarcts.
Three population based imaging studies determined brain infarct incidence.18–20 The overall incidence in these studies ranged from 9.5% to 18.5% in a 5-year follow-up period, which is consistent with the incidence in this study (20.9%) when considering age differences between these studies and an estimated 40% increase in relative-risk of new infarcts for every 5-years of increasing age.20
The age adjusted risk of incident infarcts overall in men compared to women was 1.8-fold higher and 2.9-fold higher in the cortical region. Conversely, two other studies of the older general population found no significant difference in brain infarct incidence between men and women19, 20 and most studies examining brain infarct prevalence do not support a sex disparity in infarct risk.5 In our study, men were at greater risk than women of developing new brain infarcts in all sub-regions. However, after adjusting additionally for vascular risk-factors and brain volume, the risk remained significant for cortical- and subcortical infarcts only, suggesting that the sex difference is driven by disease in both large and small vessels.
We found a trend of greater decline in cognitive function with increased overall infarct load. Persons with both prevalent and incident infarcts showed a steeper decline in cognition compared to persons without infarcts or persons with only prevalent- or only incident infarcts. This relationship was stronger and more often significant in men than in women for all infarct regions and cognitive domains. Men with prevalent and incident cortical- and subcortical infarcts showed significantly more cognitive decline than men without infarcts in all cognitive domains.
This study showed a significant increase in the risk of dementia in persons with new brain infarcts. Several clinical MRI studies have revealed increased odds of dementia in subjects with brain infarcts21–23 but others not.24 Four population based studies assessed this relationship, one using a cross-sectional design25 and three using a longitudinal design.10–12 Results from the Chicago Health and Aging Project25 showed no significant relationship between overall infarcts and dementia but did find that persons with cortical infarcts were fourfold more likely to have dementia. Our results are similar to the three longitudinal cohort studies where the presence of overall brain infarcts was shown to increase the risk of dementia. However, two of these studies did not have information on incident infarcts from a follow-up MRI10, 12 so there was no information on how those new infarcts may contribute to developing dementia. As we have shown, additional new infarcts are associated with more cognitive decline and an even higher risk for dementia than just having prevalent infarcts. Together our findings may well indicate a pattern of stepwise decline after first infarct and that new infarcts trigger steeper cognitive decline and increase the risk of dementia. There was also no assessment of the specific sub-regions we investigated10–12 which provides information about the location of the vascular damage, which has clinical implications.
Although evidence has accumulated that vascular abnormalities and brain infarcts contribute to the development of dementia, limited information exists on whether this contribution differs by brain regions. Alzheimer’s disease and small vessel disease share risk factors that lead to cognitive decline and dementia.26 In clinical pathological studies it has been demonstrated that individuals with subcortical infarcts are more likely to have dementia or require fewer plaques and tangles for a clinical diagnosis of AD.27, 28 Subcortical infarcts found by MRI are a measure of small vessel disease.17 Our results showed that persons with new subcortical infarcts on MRI were more than two-fold more likely to have incident dementia, even after adjusting for microbleeds and WMH volume that are other MRI markers of manifest small vessel disease. The association of cortical- and cerebellar infarcts with incident dementia was not as strong as for subcortical infarcts. This may indicate that infarcts of small vessel disease origin contribute more to the development of dementia than infarcts of embolic origin in larger vessels.
Strengths of this study include the very large well-characterized longitudinal cohort of older persons from the general population with a high number of incident dementia events. To the best of our knowledge this is the first imaging study of a population based cohort to report the incidence of infarcts and their associations with dementia in the subregions included in this study. However, persons with higher prevalence and incidence of brain infarcts may have been underrepresented as those not included in the sample were more often men, more likely to be older, to have hypertension, to have diabetes and to have atrial fibrillation.
Conclusions
The cumulated 5.2-year incidence of brain infarcts in the elderly general population is over 20%. Men are at greater risk of developing incident brain infarcts than women, particularly in the cortical region. Persons with incident brain infarcts decline faster in cognition and have an increased risk of dementia compared to those free of such lesions. The risk is higher in those with both prevalent and incident infarcts compared to those with either alone, suggesting a cascade where additional infarcts dispose people towards clinical dementia. Incident subcortical infarcts contribute more than cortical and cerebellar infarcts to incident dementia which may indicate that infarcts of small vessel disease origin contribute more to the development of dementia than infarcts of embolic origin in larger vessels.
Supplementary Material
Acknowledgments
The study is approved by the Icelandic National Bioethics Committee, VSN:00-063 and the IRB responsible for the National Institute on Aging (NIA) research. The researchers are indebted to the participants for their willingness to participate in the study.
Sources of Funding
This study has been funded by NIH-contract N01-AG-1-2100, the NIA Intramural-Research-Program, Hjartavernd and the Icelandic Parliament.
Footnotes
Disclosures
None.
References
- 1.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiology of Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Bryan RN, Cai J, Burke G, Hutchinson RG, Liao D, Toole JF, et al. Prevalence and anatomic characteristics of infarct-like lesions on mr images of middle-aged adults: The atherosclerosis risk in communities study. American Journal of Neuroradiology. 1999;20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 3.Baune BT, Schmidt WP, Roesler A, Berger K. Functional consequences of subcortical white matter lesions and mri-defined brain infarct in an elderly general population. Journal of Geriatric Psychiatry and Neurology. 2009;22:266–273. doi: 10.1177/0891988709342722. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MMB. Prevalence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 5.Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: A systematic review of population-based cohorts. BMC Medicine. 2014;12:119–119. doi: 10.1186/s12916-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chui HC, Ramirez-Gomez L. Clinical and imaging features of mixed alzheimer and vascular pathologies. Alzheimer's Research & Therapy. 2015;7:21. doi: 10.1186/s13195-015-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine. The population-based mri camera study. Brain. 2005;128:2068–2077. doi: 10.1093/brain/awh542. [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 12.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 13.Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Kjartansson O, Oskarsdottir B, et al. Brain tissue volumes in the general population of the elderly: The ages-reykjavik study. NeuroImage. 2012;59:3862–3870. doi: 10.1016/j.neuroimage.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saczynski JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Jonsson PV, Garcia ME, et al. Cerebral infarcts and cognitive performance: Importance of location and number of infarcts. Stroke. 2009;40:677–682. doi: 10.1161/STROKEAHA.108.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidal J-S, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Thorgeirsson G, Kjartansson O, et al. Coronary artery calcium, brain function and structure: The ages-reykjavik study. Stroke; a journal of cerebral circulation. 2010;41:891–897. doi: 10.1161/STROKEAHA.110.579581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JN, Hawes C, Fries BE, Phillips CD, Mor V, Katz S, et al. Designing the national resident assessment instrument for nursing homes. The Gerontologist. 1990;30:293–307. doi: 10.1093/geront/30.3.293. [DOI] [PubMed] [Google Scholar]
- 17.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: A prospective study. Brain. 2010;133:1987–1993. doi: 10.1093/brain/awq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longstreth WT, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, O’Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The cardiovascular health study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 20.Vermeer SE, den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MMB. Incidence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 21.Pohjasvaara T, Erkinjuntti T, Ylikoski R, Hietanen M, Vataja R, Kaste M. Clinical determinants of poststroke dementia. Stroke. 1998;29:75–81. doi: 10.1161/01.str.29.1.75. [DOI] [PubMed] [Google Scholar]
- 22.Pohjasvaara T, Mäntylä R, Salonen O, Aronen HJ, Ylikoski R, Hietanen M, et al. Mri correlates of dementia after first clinical ischemic stroke. Journal of the Neurological Sciences. 2000;181:111–117. doi: 10.1016/s0022-510x(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 23.Tatemichi TK, Paik M, Figueroa M, Gropen TI, Stern Y, et al. Clinical determinants of dementia related to stroke. Annals of Neurology. 1993;33:568–575. doi: 10.1002/ana.410330603. [DOI] [PubMed] [Google Scholar]
- 24.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JCL, Berman K, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: The sydney stroke study. Dementia and Geriatric Cognitive Disorders. 2006;21:275–283. doi: 10.1159/000091434. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal NT, Schneider JA, Wilson RS, Beck TL, Evans DA, De Carli C. Characteristics of mr infarcts associated with dementia and cognitive function in the elderly. Neuroepidemiology. 2012;38:41–47. doi: 10.1159/000334438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuropathology Group of the Medical Research Council Cognitive F, Ageing S. Pathological correlates of late-onset dementia in a multicentre, community-based population in england and wales. The Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 27.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of alzheimer disease: The nun study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 28.Jellinger KMD, Mitter-Ferstl EP. The impact of cerebrovascular lesions in alzheimer disease. Journal of Neurology. 2003;250:1050–1055. doi: 10.1007/s00415-003-0142-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.