Abstract
Mild traumatic brain injury (mTBI) results in variable clinical trajectories and outcomes. The source of variability remains unclear, but may involve genetic variations, such as single nucleotide polymorphisms (SNPs). A SNP in catechol-o-methyltransferase (COMT) is suggested to influence development of post-traumatic stress disorder (PTSD), but its role in TBI remains unclear. Here, we utilize the Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot) study to investigate whether the COMT Val158Met polymorphism is associated with PTSD and global functional outcome as measured by the PTSD Checklist – Civilian Version and Glasgow Outcome Scale Extended (GOSE), respectively. Results in 93 predominately Caucasian subjects with mTBI show that the COMT Met158 allele is associated with lower incidence of PTSD (univariate odds ratio (OR) of 0.25, 95% CI [0.09–0.69]) and higher GOSE scores (univariate OR 2.87, 95% CI [1.20–6.86]) 6-months following injury. The COMT Val158Met genotype and PTSD association persists after controlling for race (multivariable OR of 0.29, 95% CI [0.10–0.83]) and pre-existing psychiatric disorders/substance abuse (multivariable OR of 0.32, 95% CI [0.11–0.97]). PTSD emerged as a strong predictor of poorer outcome on GOSE (multivariable OR 0.09, 95% CI [0.03–0.26]), which persists after controlling for age, GCS, and race. When accounting for PTSD in multivariable analysis, the association of COMT genotype and GOSE did not remain significant (multivariable OR 1.73, 95% CI [0.69–4.35]). Whether COMT genotype indirectly influences global functional outcome through PTSD remains to be determined and larger studies in more diverse populations are needed to confirm these findings.
Keywords: Traumatic brain injury, Genetic factors, PTSD, Outcome measures, Human studies
1. Introduction
Traumatic brain injury (TBI) is a common and often debilitating injury in modern societies. In the United States alone, at least 2.5 million people suffer TBIs annually which accounts for 52,000 deaths, 275,000 inpatient hospitalizations, and 1,365,000 Emergency Room visits [1]. Approximately 70–90% of all TBI is characterized as ‘mild TBI’ (mTBI) defined by a Glasgow Coma Scale score of 13–15. The vast majority of patients make a complete recovery following mTBI in the ensuing weeks to months [2,3]. However, a small but significant number of patients suffer from persistent neurologic, cognitive and/or neuropsychiatric sequelae – including headache, dizziness, fatigue, memory impairment, slowed processing speed, depression and post-traumatic stress disorder (PTSD), among others [4]. In many instances, individuals enduring similar mechanisms and magnitude of brain injury follow different clinical trajectories, and there are limited metrics to identify and/or sub-stratify those at greatest risk for persistent post-traumatic impairment [5].
The Human Genome Project has allowed the identification of single nucleotide polymorphisms (SNPs) – single nucleotide substitutions which alter amino acid sequence and protein function and/or levels of protein expression. Several SNPs are good candidates for allelic association studies aimed at explaining the divergence in outcome or in the prevalence of cognitive, behavioral and neuropsychiatric symptoms following TBI [6–8]. Catechol-O-methyltransferase (COMT; encoded by the gene COMT on chromosome 22q11.2) represents one such candidate gene. COMT enzymatically inactivates the catecholamine neurotransmitters, i.e., norepinephrine and dopamine, through 3-O-methylation of the benzene ring [9]. A common coding SNP, Val158Met (rs4680), results in a substitution of valine (G; Val158) for methionine (A; Met158) at codon 158. The Met158 substitution reduces COMT enzymatic activity [10,11]. In areas with limited reuptake transporters, i.e., the prefrontal cortex, COMT-mediated inactivation is the principal mechanism of inactivation of dopaminergic signal transmission, and the Met158 variant is associated with higher catecholamine bioavailability [12–14].
Numerous studies have investigated the effects of the COMT Val158Met polymorphism on human behavior and/or brain function [9,15,16]. In general, the Met158 allele confers a performance advantage in cognitive tasks – including measures of memory and attention – attributed to the higher catecholamine bioavailability in the prefrontal cortex [9,15–17]. The Val158 allele, on the other hand, may confer advantage in aversive stimulus processing [18]. Consistent with this principle, the Met158 allele has been associated with a number of anxiety disorders – including generalized anxiety disorder, panic disorder, obsessive compulsive disorder, and PTSD in some, but not all study populations [19–23]. In TBI, prior studies have shown that Val158/Val158 homozygosity may be associated with greater impairment in certain cognitive domains, e.g., perseveration, but not others [24–27]. However, whether the COMT Val158Met polymorphism influences psychiatric health following brain injury – such as TBI – has yet to be studied.
PTSD is a relatively common and often debilitating neuropsychiatric sequela of TBI with published rates ranging from of 17% to 33% of patients [28–32]. The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) classifies PTSD as an anxiety disorder presenting with three concurrent symptom clusters after exposure to a traumatic event: persistent re-experiencing of the traumatic event, persistent avoidance of stimuli associated with the traumatic event, and persistent symptoms of increased arousal. The event can involve actual or perceived serious injury, a threat to one’s physical integrity or the integrity of another individual, or an unexpected death or serious harm to a close family member or friend. The symptoms must be present for more than one month and cause clinically significant distress or impairment in social, occupational, or other important areas of functioning [33]. Although PTSD may occur with any severity of TBI, the highest incidence occurs in individuals with mTBI [31,34–36]. Furthermore, symptoms of PTSD in the context of a history of mTBI is often associated with poorer outcome [37].
In this study we utilize the Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot) dataset, a comprehensive database of demographic history, biomarkers, neuroimaging, and neuropsychiatric and neurocognitive outcomes [38], to investigate whether the COMT Val158Met polymorphism is associated with the development of symptoms meeting DSM-IV criteria of PTSD and global functional outcome following isolated and uncomplicated mild closed head injury. On the basis of the literature of anxiety disorders, we hypothesize that subjects with the Met158 allele will more frequently develop symptoms associated with PTSD and have poorer 6-month global functional outcome following mTBI.
2. Materials and methods
2.1. Study design
The TRACK-TBI Pilot Study is a multicenter prospective observational study conducted at three Level 1 Trauma Centers in the United States – San Francisco General Hospital, University of Pittsburgh Medical Center and University Medical Center Brackenridge (UMCB) in Austin, Texas [38]. Institutional review board approval was obtained at all participating sites. Informed consent was obtained for all subjects prior to enrollment in the study. For patients unable to provide consent due to their injury, consent was obtained from their legally authorized representative (LAR). Patients were then re-consented, if cognitively able at later inpatient and/or outpatient follow-up assessments for continued participation in the study.
2.2. Patient selection
Inclusion criteria for the pilot study were patients presenting to a Level I trauma center with external force trauma to the head and clinically indicated head computed tomography (CT) scan within 24 h of injury. TRACK-TBI Pilot patients age ⩾16 completed outcome measures. Exclusion criteria were pregnancy, comorbid life-threatening disease, incarceration, serious psychiatric and neurologic disorders that would interfere with outcome assessment, and non-English speakers due to limitations in participation with outcome assessment. For the present study, our goal was to study the effects of COMT Val158Met in isolated and uncomplicated mTBI. Therefore, our analysis was restricted to a subset of patients with a GCS ⩾13, loss of consciousness (LOC) <30 min, post-traumatic amnesia <24 h, no skull fracture or acute intracranial pathology – defined as the absence of intraparenchymal contusions or hemorrhage, axonal injury, ventricular hemorrhage, epidural hematoma, acute subdural hematoma or traumatic subarachnoid hemorrhage – on non-contrasted head CT, and no polytrauma as defined by an Abbreviated Injury Scale (AIS) score >1 in any extracranial body region. To avoid potential confounding with measures of PTSD, patients who reported pre-injury PTSD or schizophrenia – variables known to associate with COMT – were excluded from the present study [39,40]. Patients with previous cerebrovascular accidents, brain tumor, and baseline developmental delay were also excluded.
2.3. Biospecimen collection and genotyping
Specimen acquisition was performed as previously described [38]. In brief, blood samples for DNA genotyping analysis were collected via peripheral venipuncture or existing peripheral venous indwelling catheters within 24 h of injury. Samples were collected in BD Vacutainer K2-EDTA vacutainer tubes, and subsequently aliquoted and frozen in cryotubes at −80 °C within 1 h of collection in accordance with recommendations from the NIH-CDE Biomarkers Working Group [41]. DNA was extracted from isolated leukocytes using the Wizard® Genomic DNA Purification Kit as described by the manufacturer (Promega, Madison, WI). COMT Val158Met polymorphism (rs4680) was genotyped utilizing TaqMan®SNP Genotyping Assay as described by the manufacturer (Applied Biosystems, Carlsbad, CA). For the purposes of evaluating a potential protective benefit of the Met158 allele, Met158/Met158 and Met158/Val158 were combined as a single group as previously described for COMT [42–45], and other genetic polymorphisms in TBI [46–48]. Therefore, for data recording and all figures this group is referred to as Met158.
2.4. Neuropsychiatric assessment and outcome parameters
All participants underwent a neuropsychiatric and outcome assessment at 6 months following TBI, including the PTSD Checklist – Civilian Version (PCL-C) and the Glasgow Outcome Scale Extended (GOSE). To evaluate for the presence of PTSD, the PCL-C was utilized as previously described [49–51]. The PCL-C is a standardized self-report rating scale of 17 PTSD symptoms that can be mapped to the three required criteria for PTSD, as outlined in the DSM-IV. Respondents are asked to rate on a 5-point scale (1- “not at all” to 5-“extremely”) how much they have been bothered by each symptom in the past month. Subjects endorsing a score of ⩾3 in one or more symptoms in “Re-experiencing”, three or more symptoms in “Avoidance”, and two or more symptoms in the “Hypervigilance” categories on the PCL-C were coded as “Yes PTSD” during analysis in accordance to the DSM-IV clinical screening criteria for PTSD.
The GOSE was utilized to assess global functional outcome following TBI as previously described [52]. The GOSE provides an overall measure of disability based on information obtained through a structured interview focused on cognition, independence, employability, and social/community participation. Individuals are described by one of the eight ordinal outcome categories: 1-Dead, 2-Vegetative State, 3-Lower Severe Disability, 4-Upper Severe Disability, 5-Lower Moderate Disability, 6-Upper Moderate Disability, 7-Lower Good Recovery, and 8-Upper Good Recovery.
2.5. Statistical analysis
Group differences in baseline descriptors across COMT Met158 carriers versus Val158/Val158 homozygotes were assessed by Pearson’s chi-squared test (χ2) for categorical variables, and analysis of variance (ANOVA) for continuous variables. Fisher’s Exact Test was used to assess differences in categorical variables with cell counts ≤5. Predictors examined in addition to COMT genotype were selected on the basis of prior published reports, and limited to variables that were previously associated with the response variable and relevant within our study population of isolated, uncomplicated mTBI to help control for potential confounding in multivariable analyses. Given the constraints of our exclusion criteria, remaining variables known to associate with PTSD include the presence of a self-reported pre-existing psychiatric disorder (defined by the major categories of diagnosed depression, anxiety, sleep disorder, and bipolar disorder) and history of substance abuse [2,53–55]. For GOSE, age (per-year increase) and GCS (15 vs. 13–14) were selected as consistent predictors cited in literature [56]. Binary logistic regression was performed with PTSD as the response, and COMT genotype, presence of pre-existing psychiatric disorder, and illicit drug use history as predictors. Ordinal logistic regression was performed with GOSE as the response, and COMT genotype, age, and GCS as predictors. All multivariable regression models conformed to tests for goodness-of-fit. The Nagelkerke pseudo-R-square (NR2) used to estimate the variance explained by the model. Race effects were independently assessed in the presence of COMT genotype for each response. Significance was assessed at α = 0.05. All analyses were performed using Statistical Package for the Social Sciences (SPSS) v.21 (IBM Corporation, Chicago, IL).
3. Results
3.1. Demographic and clinical descriptors
In total, the present study included 93 subjects (Table 1). Overall, the mean age of included subjects was 40 years old, and the majority of subjects were male (60%). Subjects were predominately Caucasian (70%). Subjects also self-identified as African American (14%), Asian (7%), mixed race (7%), American Indian/Native Alaskan (2%) or Hawaiian/Pacific Islander (2%). With respect to psychiatric health, 39% of subjects had self-reported presence of one or more psychiatric conditions – including depression, anxiety, sleep disorder, or bipolar disorder – and 24% percent of subjects reported a history of substance abuse. Subjects had a multitude of different mechanisms of injury including motor vehicle or motorcycle collision, bicycle accident, pedestrian versus automobile, assault and struck by or against an object. COMT genotype distribution was 29% Met158/Met158 (n = 27), 46% Met158/Val158 (n = 43) and 25% Val158/Val158 (n = 23). COMT allelic frequencies (A = 0.53, G = 0.47) were not found to deviate significantly from Hardy–Weinberg equilibrium (X2 = 0.5, p = 0.778). A higher prevalence of African-Americans (p = 0.032) and preexisting psychiatric disorder (p = 0.043) were noted in the Val158/Val158 homozygotes. No other significant differences were observed in the distribution of each demographic and clinical descriptor across COMT Met158 and Val158/Val158 genotypes (Table 1).
Table 1.
Demographic and clinical information of included subjects with mild traumatic brain injury.
| Variable |
Met158 (N = 70) |
Val158/Val158 (N = 23) |
Significance (p) |
|---|---|---|---|
| Age (y) | |||
| Mean ± SD | 40 ± 17 | 42 ± 14 | 0.682 |
| Gender | |||
| Male | 42 (60%) | 14 (61%) | 0.941 |
| Female | 28 (40%) | 9 (39%) | |
| Race | |||
| Caucasian | 52 (80%) | 13 (20%) | 0.032 |
| African-American/African | 6 (46%) | 7 (54%) | |
| Other races | 12 (80%) | 3 (20%) | |
| Pre-existing psychiatric disorder | |||
| No | 47 (67%) | 10 (44%) | 0.043 |
| Yes | 23 (33%) | 13 (56%) | |
| Substance abuse | |||
| No | 56 (80%) | 15 (65%) | 0.148 |
| Yes | 14 (20%) | 8 (35%) | |
| Mechanism of injury | |||
| Motor vehicle crash | 22 (31%) | 2 (9%) | 0.140 |
| Cyclist/pedestrian hit | 15 (21%) | 6 (26%) | |
| Fall | 21 (30%) | 8 (35%) | |
| Assault | 8 (11%) | 6 (26%) | |
| Struck by/against object | 4 (6%) | 1 (4%) | |
| ED arrival GCS | |||
| 13 | 1 (1%) | 0 (0%) | 0.817 |
| 14 | 12 (17%) | 5 (22%) | |
| 15 | 57 (81%) | 18 (78%) |
Race distributions are reported as row percentages. All other distributions reported as column percentages. The race subgroup “Other races” was combined due to individual small sample sizes of Asian (N = 6; Met158 = 5, Val158/Val158 = 1), American Indian/Alaskan Native (N = 1; Met158 = 1), Hawaiian/Pacific Islander (N = 2; Met158 = 1, Val158/Val158 = 1), and more than one race (N = 6; Met158 = 5, Val158/Val158 = 1).
3.2. COMT is associated with PTSD independent of pre-existing psychiatric disease, substance abuse and race
We first tested our hypothesis that COMT Met158 is associated with higher incidence of PTSD following mTBI. In total, 28 of 96 subjects (29%) qualified for PTSD on screening criteria. Sixteen of 73 (22%) of Met158 carriers and 12 of 23 (52%) of Val158/Val158 homozygotes met qualifying screening criteria for PTSD, respectively (X2 = 7.75, p = 0.005) (Fig. 1). COMT Met158 had a univariate odds ratio (OR) of 0.25 (95% CI [0.09–0.69], NR2 11.0%) for the presence of PTSD (Table 2). Therefore, contrary to our initial hypothesis, COMT Met158 was associated with lower incidence of PTSD.
Fig. 1.
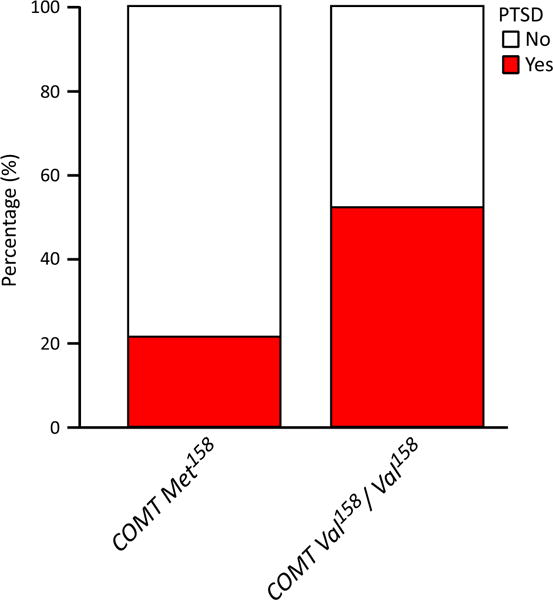
The COMT Val158Met polymorphism is associated with lower prevalence of qualifying for screening criteria for post-traumatic stress disorder (PTSD) at 6-months following mild traumatic brain injury. White, did not meet PTSD qualification on screening criteria; red, met PTSD qualification on screening criteria. COMT = Catechol-O-Methyltransferase. red, met PTSD qualification on screening criteria.
Table 2.
Univariate and multivariable statistical analysis of the association between the COMT Val158Met polymorphism and a history of preexisting psychiatric disease or substance abuse with post-traumatic stress disorder (PTSD) at 6-months post-injury.
| Predictor | Odds ratio (OR) [95% CI] | Predictor sig. (p) | Nagelkerke pseudo-R2 | Model sig. (p) |
|---|---|---|---|---|
| Univariate analysis | ||||
| COMT Met158 | 0.25 [0.09–0.69] | 0.006 | 11.0% | – |
| Preexisting psychiatric disorder | 6.85 [2.54–18.49] | 6.3 × 10−5 | 22.6% | – |
| Substance abuse | 3.44 [1.26–9.38] | 0.016 | 8.6% | – |
| Multivariable analysis | ||||
| COMT Met158 | 0.32 [0.11–0.97] | 0.044 | 29.5% | 8.1 × 10−5 |
| Preexisting psychiatric disorder | 5.17 [1.80–14.89] | 0.002 | – | – |
| Substance abuse | 1.88 [0.60–5.88] | 0.281 |
OR >1 represents greater odds of having six-month PTSD. CI = Confidence Interval; COMT = Catechol-O-Methyltransferase; OR = Odds Ratio; PTSD = post-traumatic stress disorder.
Given that COMT genotype unevenly distributed across racial subgroups, we next utilized multivariable regression for PTSD to control for the potential confounding influence of race. In the studied population, univariate analysis failed to show a statistically significant relationship between race and PTSD following mTBI (p = 0.092). When only the two largest racial groups were compared populations were compared (Caucasian and African American), African American race emerged as a predictor for PTSD with a univariable OR of 3.89 (95% CI [1.13–13.35]). However, only COMT genotype, but not African American racial background, remained a statistically significant predictor of PTSD when included in a multivariable model as evidenced by a multivariable OR of 0.29 (95% CI [0.10–0.83]) and 2.76 (95% CI [0.75–10.22]) for the COMT Met158 allele and African American race, respectively. Collectively, these data preliminarily suggest that the association of COMT and PTSD is not influenced by race, but larger future studies in more diverse populations are needed to confirm and/or refute these findings.
We next sought to determine whether controlling for established risk factors for PTSD, e.g., pre-existing psychiatric disease and substance abuse [2,53–55], would influence the observed association between COMT genotype and PTSD. Univariate analysis confirmed that pre-existing psychiatric disorder (OR 6.85, 95% CI [2.54–18.49], NR2 22.6%) and substance abuse (OR 3.44, 95% CI [1.26–9.38], NR2 8.6%) was associated with greater univariate odds of PTSD (Table 2). We also confirmed that there was no effect of interaction between COMT and preexisting psychiatric disorder on PTSD (p = 0.195). We then performed multivariable regression with PTSD as the response and COMT, preexisting psychiatric disorder, and substance abuse as predictors. In the multivariable model COMT Met158 remained a significant predictor of decreased odds of PTSD (OR 0.32, 95% CI [0.11–0.97]) after adjusting for pre-existing psychiatric disorder and illicit drug use. In the model, pre-existing psychiatric disorder associated with greater odds of PTSD (OR 5.17, 95% CI [1.80–14.89]) while drug use did not significantly associate with PTSD (Table 2). This model was statistically significant (p = 8.1 × 10−5) and explained 29.5% of the variability in PTSD – values higher than COMT Met158 or any pre-existing risk factor alone.
3.3. Functional outcome is associated with COMT and inversely related to PTSD
We next investigated whether an association exists between COMT Met158 carriers or Val158/Val158 homozygotes and functional outcome following mTBI. For the 70 COMT Met158 carriers, 6%, 17%, 37%, and 40% were found to have GOSE scores of 5, 6, 7, 8, respectively. In comparison, 35%, 9%, 30% and 26% of the 23 COMT Val158/Val158 homozygotes were found to have GOSE scores of 5, 6, 7, 8, respectively, which differed from Met158 carriers (p = 0.008) (Fig. 2). Univariate ordinal logistic regression showed that the presence of COMT Met158 allele was associated with an OR of 2.87 for higher GOSE scores (95% CI [1.20–6.86]) and explained 5.9% of the variance. Race was not a significant univariate predictor of GOSE (p = 0.158). After correcting for age (per-year increase: univariate OR 0.99, 95% CI [0.97–1.02]; multivariable OR 0.99, 95% CI [0.97–1.02]), and no GCS deficit (GCS 15: univariate OR 2.55 95% CI [1.00–6.57]; multivariable OR 2.68, 95% CI [1.03–6.94]), COMT Met158 remained a significant predictor of higher functional outcome on GOSE (OR 2.96, 95% CI [1.23–7.13], NR2 6.2%).
Fig. 2.

The COMT Val158Met polymorphism is associated with greater global functional outcome as measured by the Glasgow Outcome Scale Extended (GOSE) at 6-months following mild traumatic brain injury. White, GOSE score of 8; light gray, GOSE score of 7; dark gray, GOSE score of 5; red, GOSE score of 5. COMT = Catechol-O-Methyltransferase.
Given the lower incidence of PTSD with COMT Met158, we sought to determine whether the observed association between GOSE and COMT may in part be explained by the differences in the incidence of post-TBI PTSD between COMT Met158 carriers or Val158/Val158 homozygotes. Overall, low GOSE scores were associated with a higher incidence of PTSD (Fig. 3A–C) Among all subjects and Met158 carriers a statistically significant increase in PTSD was observed with decreasing GOSE groups as reflected by p-values of 5.32 × 10−7 and 2.27 × 10−5, respectively (Fig. 3A and B). Overall a similar trend was observed in Val158/Val158 homozygotes (Fig. 3C), but this failed to reach statistical significance (p = 0.087). Collectively, this suggests that PTSD may influence functional outcome as previously described for other outcome metrics [57].
Fig. 3.

Global functional outcome is negatively associated with the presence of concomitant post-traumatic stress disorder at 6-months post-injury. (A–C) Graphs depicting proportion of individuals not meeting (white) or meeting (red) post-traumatic stress disorder (PTSD) screening criteria in all subjects (A), subjects with the COMT Met158 allele (B), and subjects with COMT Val158/Val158 homozygosity (C). COMT = Catechol-O-Methyltransferase.
To offer further support to this hypothesized relationship, we verified that PTSD is a univariate predictor of lower GOSE by ordinal logistic regression (OR 0.08, 95% CI [0.03–0.21]), and explains 30.6% of the variance in GOSE. We next performed a multivariable ordinal logistic regression with COMT Met158 and PTSD as predictors, and GOSE as the dependent variable, adjusting for age. The model was statistically significant (p = 9.06 × 10−7) and explained 32.8% of the variability in GOSE. Analysis confirmed that PTSD was associated with greater odds of lower GOSE score as evidenced by multivariable OR of 0.09 (95% CI [0.03–0.26]), but the association of COMT with global functional outcome was no longer statistically significant (multivariable OR 1.73, 95% CI [0.69–4.35]) (Table 3). These analyses suggest that PTSD is inversely associated with GOSE and may indirectly contribute to the association of COMT and GOSE following mTBI. However, the directionality of this relationship could not be conferred by the present analysis and future studies are needed to more clearly delineate the influences of PTSD on functional outcome.
Table 3.
Univariate and multivariable statistical analysis of the association between the COMT Val158Met polymorphism and post-traumatic stress disorder (PTSD) with global functional outcome (GOSE) at six months post-injury.
| Predictor | Odds ratio (OR) [95% CI] | Predictor sig.(p) | Nagelkerke pseudo-R2 | Model sig. (p) |
|---|---|---|---|---|
| Univariate analysis | ||||
| COMT Met158 | 2.87 (1.20–6.86) | 0.018 | 5.9% | – |
| PTSD | 0.08 [0.03–0.21] | 3.62 × 10−7 | 30.6% | – |
| Age (y) | 0.99 [0.97–1.02] | 0.499 | 0.5% | – |
| No GCS deficit | 2.55 [1.00–6.57] | 0.051 | 3.9% | – |
| Multivariable analysis | ||||
| COMT Met158 | 1.73 [0.69–4.35] | 0.243 | 32.8% | 9.06 × 10−7 |
| PTSD | 0.09 [0.03–0.26] | 5.0 × 10−6 | – | – |
| Age (y) | 1.00 [0.98–1.03] | 0.723 | ||
| No GCS deficit | 1.86 [0.69–5.01] | 0.218 |
OR >1 represents greater odds of higher six-month functional outcome score on GOSE. COMT = Catechol-O-Methyltransferase; CI = Confidence Interval; GOSE = Glasgow Outcome Scale Extended; OR = Odds Ratio; PTSD = post-traumatic stress disorder.
4. Discussion
In the present study, we sought to investigate whether the COMT Val158Met polymorphism is associated with PTSD and functional outcome following mild closed head injury in a predominately Caucasian population. We found that subjects with the COMT Met158 allele have lower rates of PTSD and better functional outcomes when compared to Val158/Val158 homozygotes at 6-months following injury. Much of the effect on functional outcome may be related to differences in PTSD between COMT genotypes. How PTSD relates to outcome measures, such as GOSE, remains unclear and future studies addressing this issue are needed.
Prior reports examining the potential influence of the COMT Val158Met polymorphism on long-term outcomes following TBI have been predominately restricted to measures of cognition in patients with predominantly moderate and severe TBI, with varying results [24–27]. Consistent with a potential deleterious role of COMT Val158/Val158 in TBI, homozygotes have been shown to have a greater number of perseverative errors following TBI [24,25]. More recently, no relationship between the COMT Met158-Val genotype and cognitive performance was found; however, this study did not include measures of perseveration. The authors also failed to find an association between COMT Val158Met polymorphism and functional outcome as measured by the GOSE at 12 and 24 months post-injury [27]. The source of this discrepancy with the present report is unclear. The incidence of PTSD is greatest following mTBI [31,34–36], and greater impairment of cognition or prolonged amnesia with more severe TBI may protect against development of subsequent PTSD [58]. Therefore, subjects with more severe injury as studied by Willmott et al., 2014, may not show similar outcome associations in the absence of PTSD. Whether this reflects differences in severity of injury in its entirety and/or trial design – namely interval of follow-up (6-months vs. 12- and 24-months) – or a combination thereof remains to be determined.
To the best of our knowledge, no prior reports have been published investigating the relationship between the COMT Val158Met polymorphism and development of PTSD following head trauma. The rate of PTSD of 26.5% in this study is within the published range for mTBI [28–32]. The role of COMT in the development of PTSD following other forms of emotional and/or physical trauma remains unclear [21–23,59]. For example, in survivors of the Rwandan genocide, COMT Met158/Met158 homozygotes demonstrated higher risk for PTSD independent of their traumatic load [21]. The COMT Met158 allele has also been associated with PTSD following urban violence [22]. However, these studies were conducted in an African and Brazilian population, respectively. It has recently been demonstrated that COMT Val158Met polymorphism exerts differential effects on risk of PTSD in children or different ethnic groups [20]; this suggests that different ethnic backgrounds and presumably heterogeneity of genetic modifiers therein modulates the effects of the COMT Val158Met polymorphism on genetic propensity for PTSD. Consistent with this principle, a study of predominately Caucasian veterans following deployment to Iraq failed to find an association between Met158/Met158 and more prevalent PTSD, but did demonstrate that Met158/Val158 heterozygotes developed fewer PTSD symptoms than either homozygous group [59]. Gene functions and associated phenotypic manifestations are modified through a complex interplay with environmental stimuli [6]. Therefore, whether the predominately Caucasian population, the nature and severity of traumatic event and/or combination thereof contribute to the potentially protective effect of the COMT Met158 allele following mTBI remains to be seen. We show that the association between COMT and PTSD following mTBI persists despite controlling for race. However, stratification of our population into racial groups showed a trend towards lower PTSD with the presence of the COMT Met158 allele in all racial groups, but failed to reach statistical significance which was in part likely the result of the small sample size of each racial group. Larger studies are therefore needed to fully delineate the potential modifying influence of ethnicity on behavioral and psychiatric associations with COMT Val158Met in the setting of head trauma.
The mechanism(s) through which COMT influences propensity to develop PTSD also remain unclear. In support of a potentially protective role of the COMT Met158 allele, a recent study has shown that COMT Val158/Val158 homozygotes are associated with heightened reacquisition of fear from presumed alterations in reconsolidation of fearful memories [42]. Conversely, the COMT Met158 allele has been also been associated with impaired fear extinction in some, but not all studies [60,61], and may therefore increase propensity for PTSD development in other contexts. However, a detailed review of the hypothesized mechanisms is beyond the scope of the present study.
Although we designed our study with restrictive inclusion criteria, it is not without limitations. Our data was obtained for a relatively small sample size (n = 93) in a predominately Caucasian male population and did not conform to known HapMap Phase III subpopulations; therefore, the need for studies in larger and more diverse study populations cannot be overstated. We also included patients only with isolated mTBI in the absence of intracranial findings on CT and a limited period of diminished consciousness and/or post-traumatic amnesia; thus, the generalizability of our results is limited. We pursued analyses designed to investigate a hypothesized relationship between the COMT Val158Met polymorphism and PTSD and did not explore the structure–function implications of COMT with specific brain pathology or variables important to the trajectory of recovery such as treatment and support. There is also a need to examine gene-gene interaction with other susceptibility loci in the context of mTBI to better elucidate complex interactions and mechanisms through which the COMT molecular pathway may influence response and recovery to TBI.
4.1. Conclusions
The COMT Val158Met polymorphism (rs4680) is associated with incidence of PTSD and functional outcome following isolated, uncomplicated mTBI. The COMT Met158 allele is associated with lower incidence of PTSD and improved functional outcome, and may exert a protective effect. However, larger studies in more diverse populations are needed to confirm the role of COMT Met158Val in psychological health following mTBI. Whether COMT Val158/Val158 homozygotes would benefit from heightened clinical surveillance and/or pharmacologic and behavior therapy targeted towards symptomatic manifestations of PTSD remain to be determined and should be the subject of future studies.
Acknowledgments
The authors would like to thank the following contributors to the development of the TRACK-TBI database and repositories by organization and alphabetical order by last name:
QuesGen Systems, Inc.: Vibeke Brinck, MS, and Michael Jarrett, MBA.
One Mind for Research: General Peter Chiarelli, US Army (Ret.), and Magali Haas, MD, PhD.
Thomson Reuters: Tatiana Khasanova, PhD, and Sirimon O’Charoen, PhD.
Sources of support
This work was supported by the following grants: NIH RC2 NS069409, NIH RC2 NS069409-02S1, NIH U01 NS086090-01, DOD USAMRAA W81XWH-13-1-0441.
Appendix
TRACK-TBI Investigators
Wayne A. Gordon, PhD (Department of Rehabilitation Medicine, Mount Sinai School of Medicine, New York, NY), Allison J. Hricik (Department of Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, PA), Andrew I. R. Maas, MD, PhD (Department of Neurosurgery, Antwerp University Hospital, Edegem, Belgium), David K. Menon, MD, PhD (Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, United Kingdom), Ava M. Puccio, RN, PhD (Department of Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, PA), David M. Schnyer, PhD (Department of Psychology, University of Texas at Austin, Austin, TX), Alex B. Valadka (Seton Brain and Spine Institute, Austin, TX), and Mary J. Vassar, RN, MS (Department of Neurosurgery, University of California, San Francisco, San Francisco, CA).
Footnotes
Conflict of interest
No competing financial interests exist.
Disclosure statement
The authors have no competing interests to disclose.
References
- 1.Faul M, Xu L, Wald MM, et al. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths, 2002–2006. Centers for Disease Control and Prevention, National Center for Injury; 2010. [Google Scholar]
- 2.Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004:84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 3.McCrea M, Iverson GL, McAllister TW, et al. An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin Neuropsychol. 2009;23:1368–90. doi: 10.1080/13854040903074652. [DOI] [PubMed] [Google Scholar]
- 4.Arciniegas DB, Anderson CA, Topkoff J, et al. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat. 2005;1:311–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Ponsford J, Draper K, Schonberger M. Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J Int Neuropsychol Soc. 2008;14:233–42. doi: 10.1017/S1355617708080272. [DOI] [PubMed] [Google Scholar]
- 6.Dardiotis E, Fountas KN, Dardioti M, et al. Genetic association studies in patients with traumatic brain injury. Neurosurg Focus. 2010;28:E9. doi: 10.3171/2009.10.FOCUS09215. [DOI] [PubMed] [Google Scholar]
- 7.Davidson J, Cusimano MD, Bendena WG. Post-traumatic brain injury: genetic susceptibility to outcome. Neuroscientist. 2015;21(4):424–41. doi: 10.1177/1073858414543150. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Arrastia R, Baxter VK. Genetic factors in outcome after traumatic brain injury: what the human genome project can teach us about brain trauma. J Head Trauma Rehabil. 2006;21:361–74. doi: 10.1097/00001199-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Witte AV, Floel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res Bull. 2012;88:418–28. doi: 10.1016/j.brainresbull.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–10. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 12.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci. 2006;17:359–67. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- 14.Klucken T, Kruse O, Wehrum-Osinsky S, et al. Impact of COMT Val158Metpolymorphism on appetitive conditioning and amygdala/prefrontal effective connectivity. Hum Brain Mapp. 2015;36(3):1093–101. doi: 10.1002/hbm.22688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickinson D, Elvevag B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–27. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 17.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–51. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectr. 2006;11:745–8. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- 19.Gatt JM, Burton KL, Williams LM, et al. Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. J Psychiatr Res. 2015;60:1–13. doi: 10.1016/j.jpsychires.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys KL, Scheeringa MS, Drury SS. Race moderates the association of catechol-O-methyltransferase genotype and posttraumatic stress disorder in preschool children. J Child Adolesc Psychopharmacol. 2014;24:454–7. doi: 10.1089/cap.2014.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolassa IT, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val (158)Met polymorphism. Biol Psychiatry. 2010;67:304–8. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Valente NL, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci. 2011;43:516–23. doi: 10.1007/s12031-010-9474-2. [DOI] [PubMed] [Google Scholar]
- 23.Wilker S, Elbert T, Kolassa IT. The downside of strong emotional memories: how human memory-related genes influence the risk for posttraumatic stress disorder–a selective review. Neurobiol Learn Mem. 2014;112:75–86. doi: 10.1016/j.nlm.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Flashman LA, Saykin AJ, Rhodes CH, et al. Effect of COMT Val/Met genotype on frontal lobe functioning in traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2004;16:238–9. [Google Scholar]
- 25.Lipsky RH, Sparling MB, Ryan LM, et al. Association of COMT Val158Met genotype with executive functioning following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17:465–71. doi: 10.1176/jnp.17.4.465. [DOI] [PubMed] [Google Scholar]
- 26.Willmott C, Ponsford J, McAllister TW, et al. Effect of COMT Val158Met genotype on attention and response to methylphenidate following traumatic brain injury. Brain Inj. 2013;27:1281–6. doi: 10.3109/02699052.2013.809553. [DOI] [PubMed] [Google Scholar]
- 27.Willmott C, Withiel T, Ponsford J, et al. COMT Val158Met and cognitive and functional outcomes after traumatic brain injury. J Neurotrauma. 2014;31:1507–14. doi: 10.1089/neu.2013.3308. [DOI] [PubMed] [Google Scholar]
- 28.Bryant RA, Harvey AG. Relationship between acute stress disorder and posttraumatic stress disorder following mild traumatic brain injury. Am J Psychiatry. 1998;155:625–9. doi: 10.1176/ajp.155.5.625. [DOI] [PubMed] [Google Scholar]
- 29.Harvey AG, Brewin CR, Jones C, et al. Coexistence of posttraumatic stress disorder and traumatic brain injury: towards a resolution of the paradox. J Int Neuropsychol Soc. 2003;9:663–76. doi: 10.1017/S1355617703940069. [DOI] [PubMed] [Google Scholar]
- 30.Harvey AG, Bryant RA. Two-year prospective evaluation of the relationship between acute stress disorder and posttraumatic stress disorder following mild traumatic brain injury. Am J Psychiatry. 2000;157:626–8. doi: 10.1176/appi.ajp.157.4.626. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy JE, Jaffee MS, Leskin GA, et al. Posttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injury. J Rehabil Res Dev. 2007;44:895–920. doi: 10.1682/jrrd.2006.12.0166. [DOI] [PubMed] [Google Scholar]
- 32.McCauley SR, Boake C, Levin HS, et al. Postconcussional disorder following mild to moderate traumatic brain injury: anxiety, depression, and social support as risk factors and comorbidities. J Clin Exp Neuropsychol. 2001;23:792–808. doi: 10.1076/jcen.23.6.792.1016. [DOI] [PubMed] [Google Scholar]
- 33.Association AP. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: 1994. [Google Scholar]
- 34.Carlson KF, Kehle SM, Meis LA, et al. Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. J Head Trauma Rehabil. 2011;26:103–15. doi: 10.1097/HTR.0b013e3181e50ef1. [DOI] [PubMed] [Google Scholar]
- 35.Luethcke CA, Bryan CJ, Morrow CE, et al. Comparison of concussive symptoms, cognitive performance, and psychological symptoms between acute blast-versus nonblast-induced mild traumatic brain injury. J Int Neuropsychol Soc. 2011;17:36–45. doi: 10.1017/S1355617710001207. [DOI] [PubMed] [Google Scholar]
- 36.McCauley SR, Wilde EA, Miller ER, et al. Preinjury resilience and mood as predictors of early outcome following mild traumatic brain injury. J Neurotrauma. 2013;30:642–52. doi: 10.1089/neu.2012.2393. [DOI] [PubMed] [Google Scholar]
- 37.Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry. 2009;166:768–76. doi: 10.1176/appi.ajp.2009.08101604. [DOI] [PubMed] [Google Scholar]
- 38.Yue JK, Vassar MJ, Lingsma HF, et al. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013;30:1831–44. doi: 10.1089/neu.2013.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho BC, Wassink TH, O’Leary DS, et al. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol Psychiatry. 2005;10:87–98. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- 40.Ohnishi T, Hashimoto R, Mori T, et al. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain. 2006;129:399–410. doi: 10.1093/brain/awh702. [DOI] [PubMed] [Google Scholar]
- 41.Manley GT, Diaz-Arrastia R, Brophy M, et al. Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch Phys Med Rehabil. 2010;91:1667–72. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Agren T, Furmark T, Eriksson E, et al. Human fear reconsolidation and allelic differences in serotonergic and dopaminergic genes. Transl Psychiatry. 2012;2:e76. doi: 10.1038/tp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill SY, Lichenstein S, Wang S, et al. Caudate volume in offspring at ultra high risk for alcohol dependence: COMT Val158Met, DRD2, externalizing disorders, and working memory. Adv Mol Imaging. 2013;3:43–54. doi: 10.4236/ami.2013.34007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong SB, Zalesky A, Park S, et al. COMT genotype affects brain white matter pathways in attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2015;36(1):367–77. doi: 10.1002/hbm.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang JI, Kim SJ, Song YY, et al. Genetic influence of COMT and BDNF gene polymorphisms on resilience in healthy college students. Neuropsychobiology. 2013;68:174–80. doi: 10.1159/000353257. [DOI] [PubMed] [Google Scholar]
- 46.Graham DP, Helmer DA, Harding MJ, et al. Serotonin transporter genotype and mild traumatic brain injury independently influence resilience and perception of limitations in veterans. J Psychiatr Res. 2013;47:835–42. doi: 10.1016/j.jpsychires.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YJ, Hsu YW, Chang CM, et al. The influence of BMX gene polymorphisms on clinical symptoms after mild traumatic brain injury. Biomed Res Int. 2014;2014:293687. doi: 10.1155/2014/293687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waters RJ, Murray GD, Teasdale GM, et al. Cytokine gene polymorphisms and outcome after traumatic brain injury. J Neurotrauma. 2013;30:1710–6. doi: 10.1089/neu.2012.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thurmond VA, Hicks R, Gleason T, et al. Advancing integrated research in psychological health and traumatic brain injury: common data elements. Arch Phys Med Rehabil. 2010;91:1633–6. doi: 10.1016/j.apmr.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Weathers F, Litz B, herman D, et al. The PTSD checklist (PCL): reliability, validity, and diagnostic utility; 9th Annual Convention of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. [Google Scholar]
- 51.Whyte J, Vasterling J, Manley GT. Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch Phys Med Rehabil. 2010;91:1692–6. doi: 10.1016/j.apmr.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 52.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–85. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 53.Brady KT, Killeen TK, Brewerton T, et al. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. 2000;61:22–32. [PubMed] [Google Scholar]
- 54.Hapke U, Schumann A, Rumpf HJ, et al. Post-traumatic stress disorder: the role of trauma, pre-existing psychiatric disorders, and gender. Eur Arch Psychiatry Clin Neurosci. 2006;256:299–306. doi: 10.1007/s00406-006-0654-6. [DOI] [PubMed] [Google Scholar]
- 55.Sandweiss DA, Slymen DJ, Leardmann CA, et al. Preinjury psychiatric status, injury severity, and postdeployment posttraumatic stress disorder. Arch Gen Psychiatry. 2011;68:496–504. doi: 10.1001/archgenpsychiatry.2011.44. [DOI] [PubMed] [Google Scholar]
- 56.Jacobs B, Beems T, Stulemeijer M, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma. 2010;27:655–68. doi: 10.1089/neu.2009.1059. [DOI] [PubMed] [Google Scholar]
- 57.Polusny MA, Kehle SM, Nelson NW, et al. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in National Guard soldiers deployed to Iraq. Arch Gen Psychiatry. 2011;68:79–89. doi: 10.1001/archgenpsychiatry.2010.172. [DOI] [PubMed] [Google Scholar]
- 58.Bombardier CH, Fann JR, Temkin N, et al. Posttraumatic stress disorder symptoms during the first six months after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2006;18:501–8. doi: 10.1176/jnp.2006.18.4.501. [DOI] [PubMed] [Google Scholar]
- 59.Clark R, DeYoung CG, Sponheim SR, et al. Predicting post-traumatic stress disorder in veterans: interaction of traumatic load with COMT gene variation. J Psychiatr Res. 2013;47:1849–56. doi: 10.1016/j.jpsychires.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 60.Lonsdorf TB, Weike AI, Nikamo P, et al. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychol Sci. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 61.Raczka KA, Mechias ML, Gartmann N, et al. Empirical support for an involvement of the mesostriatal dopamine system in human fear extinction. Transl Psychiatry. 2011;1:e12. doi: 10.1038/tp.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]


