Key Points
Question
Is persistent pain associated with accelerated cognitive decline in the elderly?
Findings
In this longitudinal, population-based cohort study, reporting pain in 2 successive interviews 2 years apart was associated with a statistically significant increase in the rate of memory decline and the probability of dementia over the subsequent 12 years, compared with controls who did not report persistent pain.
Meaning
Persistent pain, which may reflect chronic pain, may help identify elders at risk of accelerated cognitive decline.
This longitudinal, population-based cohort study uses Health and Retirement Study data to investigate the association between persistent pain and memory decline and dementia.
Abstract
Importance
Chronic pain is common among the elderly and is associated with cognitive deficits in cross-sectional studies; the population-level association between chronic pain and longitudinal cognition is unknown.
Objective
To determine the population-level association between persistent pain, which may reflect chronic pain, and subsequent cognitive decline.
Design, Setting, and Participants
Cohort study with biennial interviews of 10 065 community-dwelling older adults in the nationally representative Health and Retirement Study who were 62 years or older in 2000 and answered pain and cognition questions in both 1998 and 2000. Data analysis was conducted between June 24 and October 31, 2016.
Exposures
“Persistent pain,” defined as a participant reporting that he or she was often troubled with moderate or severe pain in both the 1998 and 2000 interviews.
Main Outcomes and Measures
Coprimary outcomes were composite memory score and dementia probability, estimated by combining neuropsychological test results and informant and proxy interviews, which were tracked from 2000 through 2012. Linear mixed-effects models, with random slope and intercept for each participant, were used to estimate the association of persistent pain with slope of the subsequent cognitive trajectory, adjusting for demographic characteristics and comorbidities measures in 2000 and applying sampling weights to represent the 2000 US population. We hypothesized that persistent pain would predict accelerated memory decline and increased probability of dementia. To quantify the impact of persistent pain on functional independence, we combined our primary results with information on the association between memory and ability to manage medications and finances independently.
Results
Of the 10 065 eligible HRS sample members, 60% were female, and median baseline age was 73 years (interquartile range, 67-78 years). At baseline, persistent pain affected 10.9% of participants and was associated with worse depressive symptoms and more limitations in activities of daily living. After covariate adjustment, persistent pain was associated with 9.2% (95% CI, 2.8%-15.0%) more rapid memory decline compared with those without persistent pain. After 10 years, this accelerated memory decline implied a 15.9% higher relative risk of inability to manage medications and an 11.8% higher relative risk of inability to manage finances independently. Adjusted dementia probability increased 7.7% faster (95% CI, 0.55%-14.2%); after 10 years, this translates to an absolute 2.2% increase in dementia probability for those with persistent pain.
Conclusions and Relevance
Persistent pain was associated with accelerated memory decline and increased probability of dementia.
Introduction
Chronic pain affects 25% to 33% of older adults, and its prevalence increases with age. The elderly are less likely to recover from chronic pain, compared with younger patients. Even after adjustment for comorbidities, patients with pain—including the elderly—are more than twice as likely to report poor subjective health status. More recently, population-based studies have demonstrated an association between pain and geriatric syndromes such as falls, functional impairment, and cognitive decline and dementia.
Because of the implications for functional independence and quality of life, the association between chronic pain and cognitive impairment deserves further attention. Cross-sectional studies using detailed neuropsychological testing have revealed significant deficits in attention, memory, information processing, and executive function in patients with chronic pain. In particular, memory and executive function—with which functional independence is tightly correlated—are significantly poorer in elders with chronic pain.
There remain important gaps in our understanding of the longitudinal impact of chronic pain on cognition in the elderly, however. Studies with prospective assessments of pain and cognitive follow-up are essential to establish temporal order, and existing cross-sectional studies cannot evaluate for the presence of accelerated cognitive decline. Accordingly, we undertook the present analysis of the Health and Retirement Study (HRS), a longitudinal population-based cohort of elder Americans, to evaluate the impact of reported pain on longitudinal changes in memory performance and dementia probability. Because cognitive deficits often result in difficulty managing daily functional tasks, we also investigated potential impact of any pain-associated memory decrement on the cognitively intensive tasks of medication and financial management.
Methods
Study Design and Participants
The HRS is a population-based cohort of community-dwelling older Americans who undergo detailed in-person or telephone interviews approximately every 2 years from cohort entry until death or dropout. All participants in the HRS give verbal informed consent for their participation in the study; HRS data collection is approved by the Health Sciences and Behavioral Sciences institutional review board at the University of Michigan. The present study of HRS data was approved by the University of California, San Francisco, Committee on Human Research. Participants for this analysis answered questions about pain and cognition without proxy in the 1998 and 2000 evaluation waves and were at least 60 years old at the 1998 wave. Participants were followed until death, dropout, or the 2012 evaluation wave (see eFigure 1 in the Supplement for data flow). The HRS is sponsored by the National Institute on Aging and is conducted by the University of Michigan.
Coprimary Outcome Definitions
The 2 primary outcomes were memory score and dementia probability score, assessed according to the methods of Wu and colleagues. The memory score is a quantitative summary metric that combines results from several cognitive tests, including those completed by a proxy respondent for severely impaired HRS participants, into a single scale, with lower scores reflecting worse cognition. Dementia probability incorporates direct and proxy cognitive test responses to estimate the chance that an individual would meet Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) or Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) diagnostic criteria for dementia. Both scores are continuous, which improves statistical power and therefore sensitivity to small cognitive changes, and may reduce misclassification.
Briefly, the memory score and dementia probability score were developed using core HRS questionnaire items calibrated against the Aging, Demographics, and Memory Study (ADAMS) sample, a subset of HRS participants who underwent detailed in-person neuropsychological batteries; this or similar dementia assessments have been used frequently in prior work on population patterns of dementia. ADAMS participants underwent direct memory assessments and formal evaluation of clinical dementia ratings. These were compared against participants’ prior-wave HRS core interview performance, incorporating direct and proxy responses. Using the models derived from the ADAMS cohort, memory score and dementia probability were estimated for all HRS participants based on the HRS core component measures.
Primary Predictor and Adjustment Variables
The HRS does not evaluate presence of chronic pain directly. Rather, at each interview, participants respond to pain-related questions: “Are you often troubled with pain?” and “How bad is the pain most of the time: mild, moderate or severe?” Participants reporting that they were often troubled with moderate or severe pain at both the 1998 and 2000 interviews were considered to have persistent pain. Participants reporting no or mild pain at both interviews, and those reporting pain at 1 interview but not both, provided the comparison group. Analysis revealed no clinically relevant differences in modeled results between those reporting pain at a single wave and those who denied pain. Accordingly, the primary comparison is between a group of respondents reporting pain in both the 1998 and 2000 interviews (persistent pain) and all other respondents. To aid in interpretation of the respondents’ pain report, prevalence of “arthritis or rheumatism,” as the HRS question is phrased, is reported in the Results.
Model adjustment variables included demographic measures (baseline age, sex, race/ethnicity [white, black, Hispanic, other]), education (less than high school, high school, some/completed college, Masters’ or professional degree), tobacco use (current, former, never smoker), and medical comorbidities (self-report of a physician’s diagnosis of hypertension, diabetes, cancer [excluding minor skin cancers], chronic lung disease, heart disease, and stroke). An additional group of covariates were considered likely to be affected by pain (and therefore may be mediators): total household financial assets, marital status, current alcohol use (none, low-risk, or binge), depressive symptoms assessed with a modified 8-item Centers for Epidemiologic Studies Depression Scale, and report of any limitation in activities of daily living. The hypothesized relationships among model covariates, including those identified as potential mediators vs those considered confounders, are diagrammed in eFigure 2 in the Supplement. All covariates were assessed at the 2000 interview according to participant report. When available, cleaned and processed variables prepared by the RAND Center for the Study of Aging were used.
The eligible sample included 104 participants (1.0%) who were missing a value for at least 1 covariate. We found no systematic differences of importance to the research question (eTable 1 in the Supplement). Given the small number of incomplete cases, complete case analysis was used.
Statistical Analysis
Bivariate associations between categorical predictors and pain group were obtained using Pearson χ2 statistics corrected for survey design with the second-order correction of Rao and Scott. For continuous variables, t tests with sampling weights were used to generate P values, and median and interquartile range, or mean and standard deviation, are reported according to degree of normality. A P value of .05 was considered the threshold for statistical significance; all reported P values reflect 2-sided significance tests.
Multivariable linear mixed-effects models were developed for the coprimary outcomes of memory score and dementia probability. Adjustment covariates were selected without regard to statistical significance in bivariate tests of association and were entered sequentially according to an a priori modeling plan. Results were qualitatively similar when adjusting for a minimal vs a comprehensive set of covariates. The primary coefficient of interest was the interaction between elapsed time since the year 2000 and the persistent pain classification to describe differences in the slope of the cognitive trajectory for participants with vs without persistent pain at baseline. Individual participants’ slopes and intercepts for the cognitive trajectory were allowed to vary as random effects. Memory score was modeled without transformation; dementia probability was modeled on the log-odds scale and back-transformed using the smearing method of Duan. We computed estimates and confidence intervals (CIs) for the linear predictor from the mixed-effects model at different time points using the mean values of the other covariates in the model. To back-transform to the original scale for dementia probability, we computed the first 2 moments and associated 95% CI of the logit-normal distribution by numerical integration. Complex survey design was taken into account with probability weights specified at the respondent level using a clustered sandwich variance estimator.
The unadjusted model incorporated terms for slope and intercept associated with persistent pain, and slope associated with advancing years in the study (ie, mean cognitive decline over time). The final multivariable model (“fully-adjusted model”) incorporated main effects and interactions with time elapsed for those covariates not hypothesized to be potential mediators. We included only main effects terms for covariates identified as potential mediators.
We explored the unequal follow-up duration between the pain and comparison groups with univariate Cox proportional hazards modeling of time to death. Given evidence that memory score and/or dementia probability may be correlated with survival or study dropout, a post hoc joint model for longitudinal and survival data was used to evaluate whether results were sensitive to selective survival or dropout.
To assist with interpretation of the modeled memory score results, we investigated the correspondence between the accelerated memory loss in those with persistent pain and limitations in 2 instrumental activities of daily living: independent management of medications and of finances. To do this, we calculated the absolute decrement in memory score at the end of a 10-year period for a 73-year-old white woman with less than a high school education and no health comorbidities associated with persistent pain. A hierarchical logistic regression model was used to estimate the absolute and relative increased probability of self-reported inability to manage money and inability to manage finances associated with that magnitude of memory score decrement, based on observed rates in the study cohort. Estimates were adjusted by age because there was a significant interaction between memory score and age at evaluation in the prediction of financial or medication independence.
Analyses were performed with SAS, version 9.4 (SAS Institute Inc), Stata, version 14.1 (StataCorp LP), or R, version 3.3.2 (R Foundation for Statistical Computing) statistical packages.
Results
Of the 10 065 eligible HRS sample members, 60% were female, and median baseline age was 73 years (interquartile range, 67-78 years). Among these, 1120 (10.9% of the weighted sample) reported persistent pain (Table 1). Baseline prevalence of participant-reported arthritis was 91.4% in the persistent pain group and 60.0% in the comparison group. Participants with persistent pain reported more depressive symptoms, a greater prevalence of limitations in activities of daily living, and more comorbid medical conditions. Pain phenotypes were relatively stable over time: participants with persistent pain reported pain at 69% of subsequent interviews whereas the comparison group reported pain at only 20% of subsequent interviews. Median follow-up included 4 subsequent biennial cognitive evaluations after the 2000 interview, but the persistent pain group had significantly shorter follow-up due to an increased rate of death (Cox proportional hazards model P < .001).
Table 1. Baseline Characteristics and Follow-up in Health and Retirement Study (HRS) Participants Included in the Analyses.
| Characteristic | Groupa | P Value | |
|---|---|---|---|
| Comparison | Persistent Pain | ||
| Raw No. (weighted percentage) | 8945 (89.1) | 1120 (10.9) | |
| Weighted Percentages | |||
| HRS entry year | |||
| 1992 | 39.2 | 36.3 | .39 |
| 1993 | 37.1 | 38.7 | |
| 1994-1996 | 0.3 | 0.5 | |
| 1998 | 23.4 | 24.6 | |
| Age, mean (SD), y | 73.6 (5.2) | 73.8 (5.4) | .53 |
| Male sex | 41.9 | 24.0 | <.001 |
| Race/ethnicity | |||
| White | 85.6 | 85.7 | .31 |
| Black | 8.0 | 7.7 | |
| Hispanic | 4.5 | 5.4 | |
| Other | 1.9 | 1.2 | |
| Education | |||
| Less than high school or GED | 29.0 | 39.6 | <.001 |
| High school | 49.4 | 47.0 | |
| College | 14.0 | 10.9 | |
| Master’s or professional degree | 7.6 | 2.5 | |
| Ever smoker | 57.6 | 59.1 | .42 |
| Assets less than median ($169 000) | 48.2 | 64.5 | <.001 |
| Marital status | |||
| Nonpartnered | 42.6 | 47.9 | .007 |
| Comorbidities | |||
| Hypertension | 48.4 | 60.9 | <.001 |
| Diabetes | 14.2 | 19.7 | <.001 |
| Cancer | 14.0 | 15.3 | .21 |
| Lung disease | 7.7 | 14.3 | <.001 |
| Heart disease | 24.6 | 36.7 | <.001 |
| Stroke | 7.8 | 12.9 | <.001 |
| Depression (Center for Epidemiologic Studies Depression Scale score >3) | 20.2 | 50.0 | <.001 |
| Current smoking | 10.7 | 13.5 | .02 |
| Current alcohol use | |||
| None | 53.1 | 68.4 | <.001 |
| Low-risk | 45.6 | 31.3 | |
| Binge | 1.3 | 0.4 | |
| Difficulty in ≥1 activity of daily living | 12.3 | 46.0 | <.001 |
| Pain in subsequent waves, % (SD) | 20.0 (20.9) | 69.1 (24.4) | <.001 |
| No. of follow-up interviews, median (IQR) | 5 (2-6) | 4 (2-6) | <.001 |
| Follow-up time, y, % (95% CI) | 11.8 (6.3-12.2) | 8.6 (4.3-12.1) | <.001 |
| Baseline memory score, mean (SD) | 0.90 (0.36) | 0.88 (0.36) | .36 |
| Baseline dementia probability, % (95% CI) | 0.13 (0.003-2.0) | 0.13 (0.006-2.8) | .003 |
Abbreviations: GED, general educational development; IQR, interquartile range.
Values take complex survey design into account.
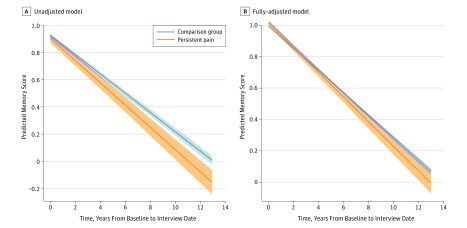
Respondents varied in their baseline memory score (SD of random intercept, 0.47 memory units) and in their rates of memory score decline over time (SD of random slope, 0.07 memory units per year). In unadjusted models, participants with persistent pain experienced 15.0% more rapid decline in memory score than the comparison group (Table 2), even with adjustment for the increased rate of death in the persistent pain group by use of a joint model for longitudinal and survival data. After full covariate adjustment, the interindividual variability in baseline memory score was lower (SD of random intercept, 0.24 memory units) but the variability in memory score decline over time was largely unchanged (SD of random slope, 0.05 memory units per year). Participants with persistent pain had a mean 9.2% (95% CI, 2.8%-15.0%) more rapid decline in memory score than participants without persistent pain (Table 2 and Figure 1; full model coefficients presented in eTable 2 in the Supplement).
Table 2. Selected Coefficients From Hierarchical Linear Mixed-Effects Models for Memory Score Incorporating Random Effects for Individual Participant Intercepts and Slopes.
| Characteristic | Coefficient (95% CI) | P Value |
|---|---|---|
| Unadjusted Modela | ||
| Difference in intercept (or predicted baseline value) for individuals with persistent pain | −0.023 (−0.056 to 0.010) | .17 |
| Mean annual memory score decline in those without persistent pain | −0.071 (−0.073 to −0.069) | <.001 |
| Additional annual memory decline for individuals with persistent pain | −0.011 (−0.017 to −0.004) | .001 |
| Fully-adjusted Modelb | ||
| Difference in intercept (or predicted baseline value) for individuals with persistent pain | 0.018 (−0.002 to 0.039) | .08 |
| Mean annual memory score decline in those without persistent pain | −0.081 (−0.084 to −0.077) | <.001 |
| Additional annual memory decline for individuals with persistent pain | −0.007 (−0.012 to −0.002) | .004 |
The unadjusted model describes the population-level cognitive slope over time and population intercept.
The fully-adjusted model includes main effects and interactions with time for demographic and health factors not hypothesized to be on the causal pathway between pain and cognition, and, for factors potentially on the causal pathway (eg, depression, activities of daily living difficulty), incorporates only adjustment for main effects (ie, influences on the intercept). Coefficients for all variables in the fully-adjusted model are available in eTable 2 in the Supplement.
Figure 1. Memory Score Trajectories From Linear Mixed-Effects Models.
Trajectories displayed are at the mean of adjustment covariates, where relevant. Shaded area indicates 95% confidence interval.
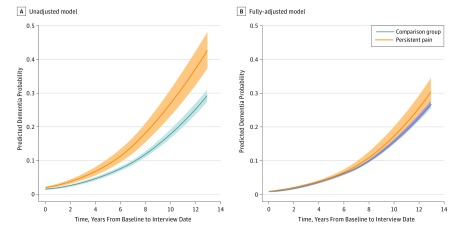
Adjusted dementia probability also increased 7.7% (95% CI, 0.55%-14.2%) faster in the persistent pain group vs the comparison group (Figure 2). The absolute predicted probability of dementia after 10 years of follow-up is 9.0 percentage points higher for patients with persistent pain prior to covariate adjustment (26.7%; 95% CI, 22.8%-30.9% vs 17.7%; 95% CI, 16.4%-19.1%), and 2.2 percentage points higher in the fully-adjusted model (22.1%; 95% CI, 18.8%-25.7% vs 19.9%; 95% CI, 18.1%-21.8%).
Figure 2. Dementia Probabilities Over Time From Back-Transformed Linear Mixed-Effects Modeling of Log Odds of Dementia Probability.
Trajectories displayed are at the mean of adjustment covariates, where relevant. Shaded areas indicate 95% confidence interval.
After 10 years, the population-level decrement in memory score associated with persistent pain corresponds to an absolute 2.3 percentage point higher risk (95% CI, 0.8%-3.7%) of being unable to manage finances independently and a 1.4 percentage point increased risk (95% CI, 0.3%-2.5%) of being unable to manage medications independently, relative to age-adjusted peers. Age-adjusted population prevalence of difficulty with financial and medication management was, respectively, 19.5% and 9.0%; the absolute increased risk therefore reflects a relative risk increase of 11.8% for financial, and 15.9% for medication, difficulties.
Discussion
This study uses population-level cognitive data to estimate the prognostic effect of persistent reports of pain on longitudinal cognitive trajectory in the elderly. Over time, participants with persistent pain experienced a 9.2% more rapid decline in memory score. This translated to a relative 11.8% to 15.9% increased risk of inability to manage medications or finances independently at the end of 10 years, compared with age-adjusted HRS peers. The accelerated memory decline is also compatible with the finding that population-level dementia probability increased 7.7% faster in those with persistent pain compared with those without.
Elderly participants who reported persistent pain in 1998 and 2000 also reported pain in 69% of subsequent HRS interviews, suggesting that this approximates a chronic pain phenotype. Chronic pain is variably defined as pain lasting beyond the usual course of acute illness or injury, or more than 3 to 6 months. Whereas it is known that chronic pain is associated with poorer cognitive performance in cross-sectional studies, this study newly demonstrates accelerated memory decline and increased probability of developing dementia year-on-year at a population level. Not merely a cross-sectional effect, the presence of persistent pain has long-term implications for memory performance and dementia in the elderly.
For the elderly, maintenance of cognition is crucial for quality of life and functional independence. As many as 1 in 3 elders experience chronic pain, and the social and medical burden of cognitive impairment and dementia in this population is enormous. Elucidating the nature of the relationship between pain and cognitive decline is the first step toward developing strategies to mitigate it. We consider 3 non–mutually exclusive possibilities: first, that the association is mediated by another factor in the causal pathway (eg, opioid consumption); second, that pain directly compromises cognitive functioning; and third, that the association is an artifact of residual confounding.
Severe chronic pain is commonly treated with opioid analgesics. Central nervous system depression from opioid use may be implicated in attentional and memory deficits seen in patients with chronic pain. In the context of the present opioid abuse epidemic, it is tempting to declare that opioid weaning and liberation could mitigate the cognitive impact of chronic pain. However, the direct effect of opioid use on cognition is potentially confounded by the coexistence of chronic pain, depression, disability, and other factors; most studies lack an adequate control group. A recent epidemiologic study refutes the role of opioids as a direct cause of cognitive decline: whereas patients with chronic pain receiving opioid treatment had an elevated rate of progression to dementia compared with controls, patients treating their chronic pain with only nonsteroidal anti-inflammatory drugs experienced nearly identical increased dementia risk. This opioid-independent association prompts consideration of potential direct effects of chronic pain on cognition.
Although our study was not designed to demonstrate causality, many hypotheses have been advanced to explain potential mechanisms by which chronic pain may directly impair cognition. The hallmarks of cognitive dysfunction associated with chronic pain are (1) diminished attentional capacity and (2) impaired memory function, particularly short-term memory. Pain may directly compete for cognitive processing resources, diverting attention, especially when pain is severe and in patients with a tendency to ruminate about their pain. Attentional impairment correlates with degree of memory impairment, suggesting that as attention is diverted elsewhere, memory encoding may be incomplete. Furthermore, the affective stress of chronic pain may be, as other stressful exposures are, implicated in faster cognitive decline via putative cortisol-based pathways. If these mechanisms fully explain the accelerated cognitive decline that we demonstrate here, resolution of pain would mitigate the detrimental cognitive effects. Classic studies do describe cognitive improvement after initiation of opioid treatment, although these studies were not performed in an elderly population.
The potential role of epiphenomena, such as confounding, in the relationship between chronic pain and cognitive decline is plausible, given the major baseline differences between those who reported persistent pain and those who did not. Participants with persistent pain had more depressive symptoms, and more frequently experienced limitations in activities of daily living. This suggests that simple questions about pain, such as the HRS uses, may provide important insight for clinicians into high-prevalence coexisting issues—such as depression and activities of daily living limitations—and potentially, subsequent problems such as accelerated cognitive decline in this population. This remains a clinically relevant issue even if the association between chronic pain and accelerated cognitive decline is epiphenomenal. Asking about chronic pain as part of a care visit opens discussion not only on potential pain management topics, but also opportunities to introduce mitigation strategies—such as assistive devices or other physical or occupational therapy interventions to address pain-related functional limitations, or self-efficacy and mindfulness strategies to reduce the affective impact of chronic pain.
Limitations
This study has important limitations. First, patients with chronic pain underwent significantly fewer evaluations due to death or dropout in comparison with the control group; although our conclusions were not sensitive to this feature, the confidence bounds for the pain group toward the end of the follow-up period are wide and long-term conclusions are necessarily limited. Second, HRS provides little information about the source, nature, or treatment of pain. We were thus unable to stratify participants according to common criteria (eg, opioid use; joint vs back vs cancer-related pain) that would otherwise have been of interest. Finally, the relationship between pain and cognitive decline is likely multifactorial and involves many potential mediators and moderators that were not measured in the HRS or not used in modeling, for example, opiate, psychotropic, or anticholinergic medication use, physical activity, and social participation. A formal mediation analysis, incorporating time-varying assessments of pain, disability, and cognition, is an important topic for future research. Due to the observational nature of these data, we cannot draw any causal conclusions about the impact of pain on subsequent cognition, or whether strategies to reduce the functional and affective impact of pain might also improve long-term cognition.
Conclusions
In summary, we demonstrate that a persistent report of moderate to severe pain, which may reflect chronic pain, is associated with accelerated cognitive decline and increased dementia probability in a large population-representative data set of elders. Clinicians should be aware of this association, which persisted after extensive statistical adjustment for confounding health and demographic factors. Patients reporting ongoing pain may be at higher risk for current and incident cognitive impairment and physical debility.
eFigure 1. Study flow diagram
eFigure 2. Conceptual framework incorporating hypothesized relationships among the adjustment variables, including those treated as confounders versus potential mediators
eTable 1. Distribution of missing data, by variable, and comparison of baseline characteristics between those with missing data and those without
eTable 2. Coefficients for fully-adjusted model for memory score
References
- 1.Mäntyselkä PT, Turunen JH, Ahonen RS, Kumpusalo EA. Chronic pain and poor self-rated health. JAMA. 2003;290(18):2435-2442. [DOI] [PubMed] [Google Scholar]
- 2.Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186):1248-1252. [DOI] [PubMed] [Google Scholar]
- 3.Elliott AM, Smith BH, Hannaford PC, Smith WC, Chambers WA. The course of chronic pain in the community: results of a 4-year follow-up study. Pain. 2002;99(1-2):299-307. [DOI] [PubMed] [Google Scholar]
- 4.Reyes-Gibby CC, Aday L, Cleeland C. Impact of pain on self-rated health in the community-dwelling older adults. Pain. 2002;95(1-2):75-82. [DOI] [PubMed] [Google Scholar]
- 5.Stubbs B, Binnekade T, Eggermont L, Sepehry AA, Patchay S, Schofield P. Pain and the risk for falls in community-dwelling older adults: systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95(1):175-187.e9. [DOI] [PubMed] [Google Scholar]
- 6.Shega JW, Weiner DK, Paice JA, et al. . The association between noncancer pain, cognitive impairment, and functional disability: an analysis of the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2010;65(8):880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dublin S, Walker RL, Gray SL, et al. . Prescription opioids and risk of dementia or cognitive decline: a prospective cohort study. J Am Geriatr Soc. 2015;63(8):1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Leeuw G, Eggermont LH, Shi L, et al. . Pain and cognitive function among older adults living in the community. J Gerontol A Biol Sci Med Sci. 2016;71(3):398-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93(3):385-404. [DOI] [PubMed] [Google Scholar]
- 10.Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: the Freedom House study. J Am Geriatr Soc. 2004;52(3):346-352. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Tchetgen Tchetgen EJ, Osypuk TL, White K, Mujahid M, Maria Glymour M. Combining direct and proxy assessments to reduce attrition bias in a longitudinal study. Alzheimer Dis Assoc Disord. 2013;27(3):207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement study and the Aging, Demographics, and Memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i162-i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langa KM, Kabeto M, Weir D 2010 Alzheimer's Disease Facts and Figures; Note A15:63-64. http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf. Accessed January 24, 2017.
- 14.Wu Q, Tchetgen Tchetgen EJ, Osypuk T, et al. . Estimating the cognitive effects of prevalent diabetes, recent onset diabetes, and the duration of diabetes among older adults. Dement Geriatr Cogn Disord. 2015;39(3-4):239-249. [DOI] [PubMed] [Google Scholar]
- 15.Marden JR, Mayeda ER, Walter S, et al. . Using an Alzheimer disease polygenic risk score to predict memory decline in black and white Americans over 14 years of follow-up. Alzheimer Dis Assoc Disord. 2016;30(3):195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marden JR, Walter S, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. Validation of a polygenic risk score for dementia in black and white individuals. Brain Behav. 2014;4(5):687-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC, Heyman A, Mohs RC, et al. . The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). part I. clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159-1165. [DOI] [PubMed] [Google Scholar]
- 18.Elwood RW. The Wechsler Memory Scale-Revised: psychometric characteristics and clinical application. Neuropsychol Rev. 1991;2(2):179-201. [DOI] [PubMed] [Google Scholar]
- 19.Langa KM, Plassman BL, Wallace RB, et al. . The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25(4):181-191. [DOI] [PubMed] [Google Scholar]
- 20.Brandt J, Spencer M, Folstein MF. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111-117. [Google Scholar]
- 21.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145-153. [DOI] [PubMed] [Google Scholar]
- 22.National Institue on Alcohol Abuse and Alcoholism Drinking levels defined. 2017. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed January 24, 2017.
- 23.RAND Center for the Study of Aging. RAND HRS Data, Version P, produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration. Santa Monica, CA; August 2016.
- 24.Rao JNK, Scott AJ. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat. 1984;12:46-60. [Google Scholar]
- 25.Duan N. Smearing estimate: a nonparametric retransformation method. J Am Stat Assoc. 1983;78(383):605-610. [Google Scholar]
- 26.Guo X, Carlin BP. Separate and joint modeling of longitudinal and event time data using standard computer packages. Am Stat. 2004;58:16-24. [Google Scholar]
- 27.National Institute of Neurological Disorders and Stroke Chronic Pain Information Page. 2016. https://www.ninds.nih.gov/Disorders/All-Disorders/Chronic-Pain-Information-Page. Accessed January 24, 2017.
- 28.Dhingra L, Ahmed E, Shin J, Scharaga E, Magun M. Cognitive effects and sedation. Pain Med. 2015;16(suppl 1):S37-S43. [DOI] [PubMed] [Google Scholar]
- 29.Mailis-Gagnon A, Lakha SF, Furlan A, Nicholson K, Yegneswaran B, Sabatowski R. Systematic review of the quality and generalizability of studies on the effects of opioids on driving and cognitive/psychomotor performance. Clin J Pain. 2012;28(6):542-555. [DOI] [PubMed] [Google Scholar]
- 30.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg. 2007;104(5):1223-1229. [DOI] [PubMed] [Google Scholar]
- 31.Schoth DE, Nunes VD, Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clin Psychol Rev. 2012;32(1):13-25. [DOI] [PubMed] [Google Scholar]
- 32.Peavy GM, Salmon DP, Jacobson MW, et al. . Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am J Psychiatry. 2009;166(12):1384-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munoz E, Sliwinski MJ, Scott SB, Hofer S. Global perceived stress predicts cognitive change among older adults. Psychol Aging. 2015;30(3):487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segerstrom SC, Geiger PJ, Boggero IA, Schmitt FA, Sephton SE. Endogenous cortisol exposure and declarative verbal memory: a longitudinal study of healthy older adults. Psychosom Med. 2016;78(2):182-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434-445. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz J, Beck H, Bromm B. Cognitive performance, mood and experimental pain before and during morphine-induced analgesia in patients with chronic non-malignant pain. Pain. 1997;73(3):369-375. [DOI] [PubMed] [Google Scholar]
- 37.Tassain V, Attal N, Fletcher D, et al. . Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non-cancer pain. Pain. 2003;104(1-2):389-400. [DOI] [PubMed] [Google Scholar]
- 38.Penninx BW, Messier SP, Rejeski WJ, et al. . Physical exercise and the prevention of disability in activities of daily living in older persons with osteoarthritis. Arch Intern Med. 2001;161(19):2309-2316. [DOI] [PubMed] [Google Scholar]
- 39.Turner JA, Anderson ML, Balderson BH, Cook AJ, Sherman KJ, Cherkin DC. Mindfulness-based stress reduction and cognitive behavioral therapy for chronic low back pain: similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. Pain. 2016;157(11):2434-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study flow diagram
eFigure 2. Conceptual framework incorporating hypothesized relationships among the adjustment variables, including those treated as confounders versus potential mediators
eTable 1. Distribution of missing data, by variable, and comparison of baseline characteristics between those with missing data and those without
eTable 2. Coefficients for fully-adjusted model for memory score