Abstract
The RSV vaccine field suffered a major set-back when children were vaccinated with a formalin-inactivated RSV vaccine (FI-RSV). Unexpectedly, the vaccinated children fared worse than unvaccinated children when they were naturally infected with RSV. Mouse models were then developed that implicated the CD4+ T helper cell population as a contributor to adverse events. Today, the T cell is viewed with much caution in the RSV field, and its induction by vaccination is sometimes discouraged. Here we re-emphasize the beneficial role of the CD4+ T cell. Experiments were performed with RSV-infected nude mice that received CD4+ T cells by adoptive transfer. Data demonstrated that CD4+ T cells were necessary for the induction of mucosal and systemic RSV-specific antibodies, for the establishment of RSV-specific IgG and IgA antibody secreting cells in the upper and lower respiratory tract, and for RSV clearance.
Keywords: Respiratory Syncytial Virus, CD4+ T cells, Antibody secreting cells, T helper function, Risk-benefit
INTRODUCTION
Respiratory syncytial virus continues to threaten the lives of children, particularly the lives of infants in the developing world [1–3]. Currently, there is no licensed vaccine for RSV [4–6]. The unfortunate outcome of a clinical study with a formalin-inactivated RSV vaccine (FI-RSV) in the 1960s is well remembered [7, 8]. This vaccine was not protective against RSV, and in fact, caused morbidity and mortality when vaccinated children were subsequently exposed naturally to RSV. Although the precise explanation for the outcome remains a topic of debate, it is likely that RSV-specific CD4+ T cells played some role. The formalin treatment of RSV altered key neutralizing antibody binding sites [9, 10] so that antibodies could not serve as a first line of defense against RSV in the respiratory tract. RSV entered the lung and was likely followed by a vigorous cellular response inclusive of T cells, neutrophils, and eosinophils that blocked airways and rendered children susceptible to asphyxiation.
Today, there is much concern associated with the induction of CD4+ T cells (and eosinophils) in the context of an RSV infection. The study described here emphasizes that RSV-specific CD4+ T cells can be beneficial. As an extension of previous literature [11], we now show that when mice are infected with RSV, but lack CD4+ T cells, they fail to (i) generate RSV-specific serum antibodies, (ii) generate RSV-specific IgG and IgA antibody secreting cells (ASCs) in the upper and lower respiratory tract (URT and LRT), and (iii) clear virus from the lung. Each of these deficits can be remedied by the adoptive transfer of RSV-specific CD4+ T cells, demonstrating the beneficial role that T helpers play during an RSV infection.
MATERIALS AND METHODS
Mice and infections
Adult, female, BALB/c mice and nude mice (NU-Foxn1nu) were purchased from Charles River Laboratory. BALB/c mice were infected intranasally with 2 × 106 plaque forming units (pfu) of RSV (A2 strain, received from ATCC) and rested for at least 1 month to serve as a source of RSV-specific T cells. To test immune responses in nude mice, mice were first infected with 2 × 106 pfu RSV intranansally. Test groups of nude mice then received intravenous cell transfers from memory, wildtype BALB/c animals (see details below) on day 14 relative to infection, based on the experience of Cannon et. al.[11]. Nude mice were sacrificed and samples were taken 5 weeks after the RSV infection (3 weeks after cell transfers) for analyses by ELISAs and ELISPOT assays. Additional, RSV-infected BALB/c or nude mice were sacrificed 24 days after RSV infections (10 days after cell transfers) to test for persistent virus in the lungs. All mouse experiments were repeated to ensure reproducibility.
Cell preparations for adoptive transfers
Spleens from sacrificed, RSV-infected BALB/c mice were collected and suspended in Hanks Balanced Salt Solution (HBSS, Gibco). Cells were incubated in 1 ml HBSS and 3 ml sterile Geys Solution (4.15g NH4Cl, 0.5g KHCO3 and 0.5ml 0.5% Phenol Red brought to 500 ml H2O) for 3 min at room temperature to lyse red blood cells, and then washed twice with HBSS at 4°C. Cells were suspended in 10 ml fresh RPMI 1640 (Gibco), supplemented with 10% heat-inactivated FCS (Atlanta Biologicals), 5 × 10−5 M 2ME (Gibco), 2mM L-Glutamine (Gibco), and antibiotics (termed ‘complete RPMI’). To enrich CD4+ T cells, two ml anti-MHC class II antibody-containing supernatants (from M5/114.15.2 cell cultures) and 2 ml anti-CD8α antibody-containing supernatants (from 53.6.7 cell cultures) were added to cells for a 30′ incubation on ice. Cells were then washed 1X with PBS (Biowhittaker) followed by centrifugation at 4°C. Pellets were resuspended in 20 ml complete RPMI with 200 μl/spleen pre-blocked sheep anti-rat Ig Dynabeads (Invitrogen Cat#11035) and 200 μl/spleen pre-blocked sheep anti-mouse Ig Dynabeads (Invitrogen Cat#11031). Cells were rotated at 4°C for 30′ and the tube was then placed on a Dynal Magnet for 10′. Unbound cells were collected and washed. Cells were then split into equal aliquots of 7 ml complete RPMI to serve as test (‘CD4s’) and control (‘Depleted CD4s’) cells.
The tube designated for ‘Depleted CD4s’ received anti-CD4 antibody-containing supernatants (from GK1.5 cell cultures) and was placed on ice for 30′. After 1X wash with PBS, cells were centrifuged and resuspended in 7 ml complete RPMI + 500 μl sheep anti-mouse Ig Dynabeads and 500 ul sheep anti-rat Ig Dynabeads for rotation at 4°C. The tube was again placed on a Dynal magnet for 10′ and non-adherent cells were collected. The tube designated for ‘CD4s’ received no GK1.5 supernatants, but was otherwise treated similarly. Cells were washed in PBS and depletions were confirmed by the staining of cell samples with antibodies for membrane markers (APC-conjugated anti-CD19 antibody [BD Pharmingen Cat# 550992], PE-conjugated anti CD8α antibody [Pharmingen Cat#553033] and FITC-conjugated anti-CD4 antibody [BD Pharmingen Cat# 553047]) for analyses by flow cytometry. The CD4-enriched population (‘CD4s’) were typically >80% CD4+ and <2% CD19+ or CD8+ among FSC/SSC-gated lymphocytes. The control cells (‘Depleted CD4s’) were <2% CD4+. Nude mice received intravenous injections with 5 × 106 ‘CD4s’ per animal or an equal aliquot of control ‘depleted CD4s’ (i.e. aliquots for injection were based on counts of ‘CD4s’ and were not readjusted based on the lower cell counts of ‘depleted CD4s’).
ELISAs
96-well ELISA plates were coated with a detergent-disrupted lysate of RSV (A2)-infected Hep2 cells or with purified RSV fusion protein (F, Sino Biologicals, diluted to 0.1 μg/ml). Plates were incubated overnight at 4°C. Plates were washed 3X with 150 μl PBS and then blocked with 100 μl 3% BSA in PBS for 2 hours at room temperature. Mouse serum or bronchoalveolar lavage (BAL) samples were serially diluted in 3% BSA and 0.1% Tween 20 in PBS. After removing blocking buffer from wells, 50 μl of sample were added per well in replicate and incubated for 1 hour at 37°C. Plates were washed 6X with 0.1% Tween 20 in PBS. Next, goat anti-mouse IgG (H chain specific; Southern Biotechnology Associates, Inc. [SBA]) or goat anti-mouse IgA (H chain, SBA) were diluted to1:1000 and added to plates at 50 μl per well. Plates were incubated for 1 hour at 37°C and then washed 6X with PBS with 0.1% Tween 20 in PBS. To each well, 100 μl of 4-nitrophenyl phosphate (Sigma-Aldrich) at 1mg/ml in diethanolamine buffer was added. OD 405 nm readings were recorded after 15 minutes. Titers were defined using a non-linear regression program (GraphPad Prism) with a cut-off for positivity of 0.1. T tests were conducted using GraphPad Prism Software. A sampling of one set of control BALB/c mice (n=5) several weeks after RSV infection showed that RSV-specific serum antibody titers ranged from 1,500 to 9,000.
ELISPOTs
RSV-A2 infected Hep2 frozen cell lysates were detergent-disrupted. Antigen was then diluted 1:1000 and wells of a 96 well MAIPS4510 plate were coated for overnight incubation at 4°C. Plates were washed 3X with PBS and 100 μl RPMI supplemented with glutamine and 10% FCS was added for a 1–2 hour incubation. Cells were isolated from nasal tissues (URT) and lungs (LRT) as described previously [12, 13] and concentrations were adjusted to 106 cells/ml. Blocking medium was removed from plates and cells were added to wells at 100 μl per well in replicate. Plates were incubated for 3 hours at 37°C. and washed 3X with PBS. Plates were then incubated with 100 μl alkaline phosphatase conjugated goat anti-mouse IgG (H chain) or goat anti-mouse IgA (H chain, SBA). Incubation was overnight at 4°C. Plates were washed 6X with PBS prior to adding 100 μl/well bromo-chloro-indolyl phosphate/nitro blue tetrazolium (Sigma-Aldrich). Plates were washed with water and dried. Spots were counted and recorded. T tests were conducted using GraphPad Prism Software.
RSV Plaque Assays
On day 24 after infection with RSV, mice were euthanized and lungs were collected in 2 ml Dulbecco’s phosphate buffered saline (DPBS) on ice. Lungs were homogenized using a PowerGen 125 Homogenizer (Fisher Scientific) and kept on ice. Serial dilutions of homogenates were made in EMEM with 10% fetal bovine serum (FBS). Virus titrations were in 12 well tissue culture plates that were 80–90% confluent with HEp-2 cells. Briefly, medium was removed from plates and serial dilutions of homogenates were plated in duplicate, 100 μl per well. Plates were incubated at 37°C, 5% CO2 for 1 hour with shaking every 15 minutes. One ml of pre-warmed 0.75% methyl cellulose in EMEM plus 10% FBS was added to each well for further incubation for 5 days. The medium with methyl cellulose was removed by aspiration. Cells were fixed with 1 ml 10% buffered formalin (Fisher Scientific) for 1 hour at room temperature. Formalin was removed and plates were washed with tap water. Then, 1 ml Hematoxylin solution (Sigma-Aldrich) was added for 20 minutes at room temperature. Plates were washed with tap water, air dried, and plaques were counted to determine plaque forming units (pfu) per lung.
RESULTS AND DISCUSSION
Cannon et. al. [11] previously used a cell transfer system to demonstrate that CD4+ T cells were required for the generation of serum antibody responses against RSV, and for RSV clearance from the respiratory tract. We employed a similar system for the testing of URT and LRT ASCs. Briefly, wildtype BALB/c mice were inoculated with RSV intranasally (i.n.) and rested for at least 1 month. Nude mice were then inoculated with RSV by the i.n. route. Fourteen days later, the nude mice received an intravenous injection with: 1) spleen cells depleted of CD8+ T cells and MHC Class II+ cells (B cells, monocytes, macrophages, and dendritic cells) from the primed BALB/c animals (an enriched RSV-specific CD4+ T cell source termed ‘CD4s’), or 2) the same cells additionally depleted of CD4+ T cells (negative control cells termed ‘Depleted CD4s’). Another negative control group was RSV-infected nude mice that were not injected with any cells. Serum and BAL antibodies and ASCs from nasal tissues (URT) and lungs (LRT) of nude mice were monitored 5 weeks after RSV infections (and 3 weeks after cell transfers). The day 14 transfer was selected based on the experience of Cannon et. al.[11].
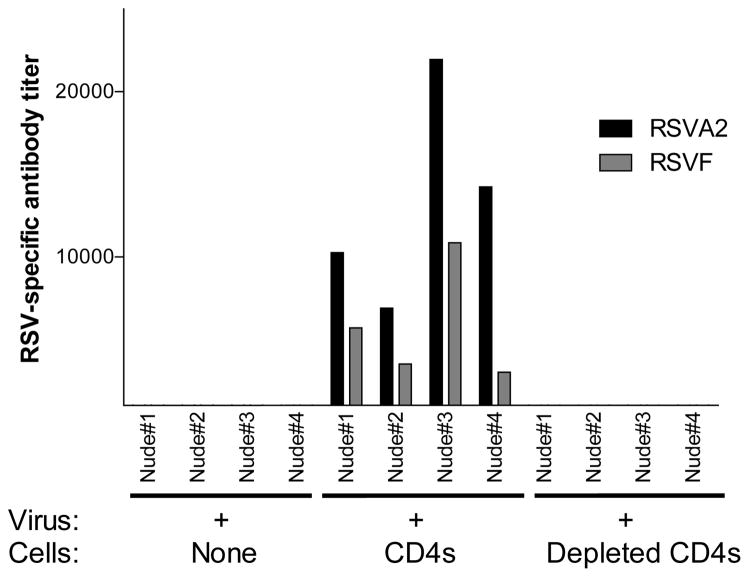
As expected, and comparable to the results of Cannon et. al., anti-RSV antibodies (scored as RSV-specific or RSV fusion [F] protein-specific antibodies) were recognized in the sera of animals that received the enriched CD4+ T cell populations. In the absence of CD4+ T cells, serum RSV-specific antibodies were not detected (Figure 1). Statistical analyses showed that enriched CD4+ T cell transfers were necessary to induce significant RSV-specific serum antibody responses (One sample T test; p<.05 for both types of ELISA).
Figure 1. Serum antibodies in RSV-infected mice.
Nude mice were infected with RSV and 14 days later received no cells, splenocytes from RSV-primed BALB/c mice enriched for CD4+ T cells (depleted of CD8+ and MHC class II+ cells including B cells, monocytes, macrophages, and dendritic cells [‘CD4s’]), or the same splenocytes additionally depleted of CD4+ T cells (‘Depleted CD4s’). Antibody responses against RSV or a purified RSV fusion protein (F) were analyzed by ELISAs 5 weeks after RSV infections.
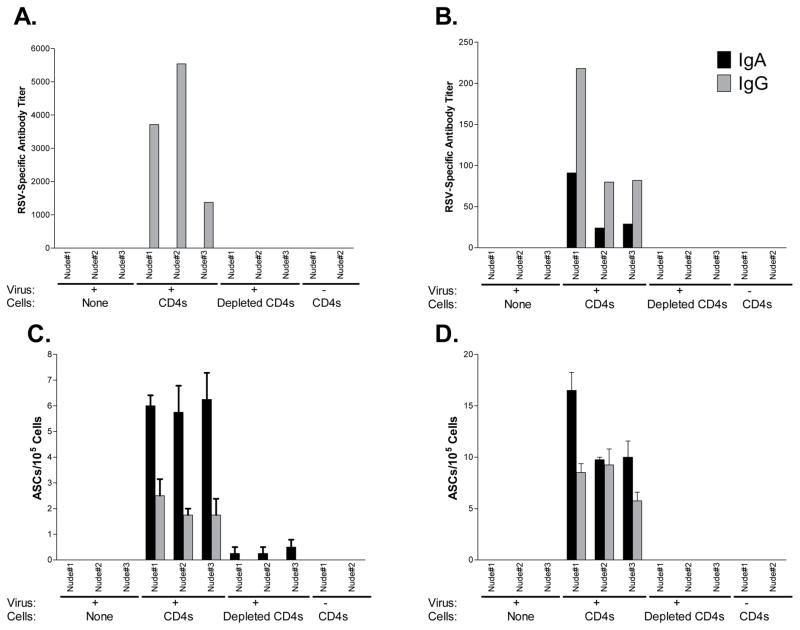
We next turned our attention to the respiratory tract, asking if CD4+ T cells were necessary for the induction of local RSV-specific IgA and IgG responses, a first line of defense against virus at its point of entry. Mouse groups were as described above. An additional group of nude mice received enriched CD4+ T cells, but no RSV. As shown in Figure 2, whereas RSV-specific IgG (not IgA) responses were measureable in sera (panel 2A), both IgA and IgG antibodies were measureable in the BAL (panel 2B), and both responses were dependent on CD4+ T cells. Next we examined ASCs in the URT and LRT, because these cells are well positioned to secrete antibodies into the respiratory tract lumen. Results are shown in Figures 2C and 2D. The only robust RSV-specific IgG and IgA ASC responses were in RSV-infected mice that received enriched CD4+ T cells. We note that the relationship of IgA and IgG ASCs in the lung (IgA>IgG) was inverted compared to the relationship of antibody titers in BAL (IgG>IgA). This was likely because serum IgG readily traffics to the LRT [14]. One-sample T tests demonstrated statistical significance for both IgG and IgA ASC responses (p<.05). Small and insignificant numbers of ASCs appeared in animals that received cells lacking the CD4+ T cell population.
Figure 2. ASCs in the URT and LRT of RSV-infected mice.
RSV-specific IgG and IgA antibody titers were measured in sera (panel A) and bronchoalveolar lavage fluids (panel B) of RSV-infected nude mice, which were treated as described in Figure 1. Additional controls received CD4+ T cells from RSV-primed BALB/c mice, but no RSV. IgG and IgA ASCs were measured in the URT (panel C) and LRT (panel D).
As shown in supplementary Figure 1, CD4+ T cells were also required for virus clearance. In the absence of CD4+ T cells, RSV was present in the lungs of all nude host animals on day 24 after virus inoculation. When CD4+ T cells were transferred on day 14, all mice exhibited virus clearance on day 24. The differences between mouse groups were significant (p<.05, Fisher’s Exact Test). Altogether, results confirmed and advanced those of Cannon et. al. [11], and demonstrated the importance of CD4+ T cells for the establishment of RSV-specific ASCs and antibodies in the respiratory tract.
Debates will continue concerning the types of immune cells or effector molecules that may be beneficial or harmful in the context of RSV vaccination and infection in children. Following murine experiments with the FI-RSV vaccine, it was suggested that a subset of RSV G-specific CD4+ Th2 cells and eosinophils associated with disease, and that inhibition of a Th2 cytokine abrogated bronchiolar histopathology [15, 16]. This and similar research prompted the suggestion that Th2 cells or perhaps all T cell responses toward RSV should be avoided [17]. On the other hand, not all research supports the idea that T cells and eosinophils are harmful in the context of RSV vaccinations and infections. For example, it has been noted that individuals who lack T cells can experience great difficulty in RSV clearance (as is also the case in nude mice [11]), and suffer worse outcomes than their immunocompetent counterparts [2, 18, 19]. Biased Th2 cytokine profiles may associate with pathology in some mouse models, but this is not necessarily the case in humans [20]. Results in the literature are also contradictory in that RSV-specific CD8+ T cells were described as harmful in one case, but beneficial in another [21–23]. Furthermore, contrary to previous expectations, the eosinophil can be shown to benefit an RSV-infected host. Specifically, when eosinophils are transferred to the lungs of RSV-infected mice, they improve RSV clearance and inhibit airway hyper-reactivity [24].
Taken together, results encourage a cautious and comprehensive view of the benefits and risks of immune populations. In the context of any lung infection, a balance must be achieved; immune cells can clear virus at the site of infection to avoid tissue destruction, but the same cells may obstruct airways if they infiltrate tissues in large numbers. Fine differences in context (e.g. virus dose, virus strain, cell clone size, antigen specificity, B-T ratio, lymphocyte-granulocyte ratio, infection site, airway integrity, and/or airway size) may tip the balance between benefit and harm. A focus on (or exoneration of) any one cell type as the prime mediator of RSV-associated morbidity may not be warranted, and may hamper the development of successful products in the vaccine field. Results in this report re-emphasize the beneficial role of CD4+ T cells in the context of RSV infection and a healthy B cell response.
The dependence of virus-specific B cells on CD4+ T cell help is apparent in our studies, but is not always the rule. In the influenza virus field for example, CD4+ T cells support B cell responses, but under some circumstances B cells can respond, at least transiently, in the absence of CD4+ T cell help [25–27].
CD4+ T cells normally support a plethora of activities that can lend to virus clearance. These include induction of interferons, help for CD8+ T cell activities, help for serum antibody responses, and help for mucosal antibody responses. Previous literature shows that antibodies administered either systemically or mucosally can clear a respiratory virus infection [11, 14, 28–30], and that interferon-producing CD4+ T cells, even in the absence of B cells, are protective [31]. Vaccines that induce some or all of these seemingly redundant activities: (i) can induce durable immune responses [13, 32], (ii) have proven highly successful in other fields [33, 34], and (iii) may ultimately serve to protect children from RSV.
Supplementary Material
SUPPLEMENTARY Figure 1. Virus clearance requires CD4+ T cells
BALB/c (control) and nude mice were infected with RSV by intranasal inoculations and tested 24 days later for RSV titers in the lungs. Results are shown for groups of BALB/c control mice, nude mice that received no CD4+ T cells, and nude mice that were adoptively transferred with CD4+ T cells from control, primed animals. A comparison of BALB/c mice and nude mice, or nude mice with or without transfers, yielded significant differences using the Fisher’s Exact Test (p<.05)
HIGHLIGHTS.
Adoptive T cell transfers were performed using RSV-infected athymic animals as hosts to define CD4+ T cell functions.
RSV-specific serum antibodies required CD4+ T cells for development.
The establishment of IgG and IgA-producing antibody secreting cells (ASCs) in the upper and lower respiratory tract required CD4+ T cells.
RSV clearance required CD4+ T cells.
Results emphasize the importance of CD4+ T cell functions in the context of an RSV infection.
Acknowledgments
This study was supported in part by funding from NIH NIAID R01 AI088729, NIH NCI CA 21765, and the American Lebanese Syrian Associated Charities (ALSAC).
Dr. Hurwitz is named on a provisional patent that describes Sendai virus as a vaccine vector.
Abbreviations
- URT
upper respiratory tract
- LRT
lower respiratory tract
- RSV
respiratory syncytial virus
- FI-RSV
formalin-inactivated RSV vaccine
- FCS
Fetal calf serum
- ELISA
Enzyme-linked immunosorbent assay
- HBSS
Hanks balanced salt solution
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson LJ, Parker RA, Strikas RL. Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. JInfectDis. 1990;161:640–6. doi: 10.1093/infdis/161.4.640. [DOI] [PubMed] [Google Scholar]
- 2.Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. CurrTopMicrobiolImmunol. 2013;372:3–38. doi: 10.1007/978-3-642-38919-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins PL, Crowe JE. Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, et al., editors. Fields Virology. Philadelphia, PA: Lippincott Williams&Wilkins; 2007. pp. 1601–46. [Google Scholar]
- 4.Hurwitz JL. Respiratory syncytial virus vaccine development. Expert review of vaccines. 2011;10:1415–33. doi: 10.1586/erv.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudraraju R, Jones BG, Sealy R, Surman SL, Hurwitz JL. Respiratory syncytial virus: current progress in vaccine development. Viruses. 2013;5:577–94. doi: 10.3390/v5020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BS, Anderson LJ. Challenges and opportunities for respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:391–404. doi: 10.1007/978-3-642-38919-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. AmJ Epidemiol. 1969;89:449–63. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 8.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. AmJEpidemiol. 1969;89:435–48. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 9.Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. JClinMicrobiol. 1988;26:1595–7. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy BR, Prince GA, Walsh EE, Kim HW, Parrott RH, Hemming VG, et al. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J ClinMicrobiol. 1986;24:197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon MJ, Stott EJ, Taylor G, Askonas BA. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology. 1987;62:133–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology. 2011;410:429–36. doi: 10.1016/j.virol.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sealy R, Jones BG, Surman SL, Hurwitz JL. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine. 2010;28:6749–56. doi: 10.1016/j.vaccine.2010.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. Journal of virology. 1985;55:517–20. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15:637–46. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 16.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. Journal of virology. 1994;68:5321–5. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLellan JS, Correia BE, Chen M, Yang Y, Graham BS, Schief WR, et al. Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus. J MolBiol. 2011;409:853–66. doi: 10.1016/j.jmb.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. JPediatr. 1980;96:179–86. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 19.Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, et al. Respiratory syncytial viral infection in children with compromised immune function. NEnglJ Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 20.Melendi GA, Laham FR, Monsalvo AC, Casellas JM, Israele V, Polack NR, et al. Cytokine profiles in the respiratory tract during primary infection with human metapneumovirus, respiratory syncytial virus, or influenza virus in infants. Pediatrics. 2007;120:e410–5. doi: 10.1542/peds.2006-3283. [DOI] [PubMed] [Google Scholar]
- 21.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J ExpMed. 1997;186:421–32. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. JImmunol. 2007;179:5415–24. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg HF, Dyer KD, Domachowske JB. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res. 2009;83:1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–86. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 25.Scherle PA, Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. The Journal of experimental medicine. 1986;164:1114–28. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. The Journal of experimental medicine. 2003;198:1011–21. doi: 10.1084/jem.20021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. Journal of immunology. 2005;175:5827–38. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 28.Mazanec MB, Lamm ME, Lyn D, Portner A, Nedrud JG. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 1992;23:1–12. doi: 10.1016/0168-1702(92)90063-f. [DOI] [PubMed] [Google Scholar]
- 29.Renegar KB, Small PA., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. Journal of immunology. 1991;146:1972–8. [PubMed] [Google Scholar]
- 30.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. Journal of immunology. 2004;173:1978–86. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 31.Surman SL, Brown SA, Jones BG, Woodland DL, Hurwitz JL. Clearance of HIV type 1 envelope recombinant sendai virus depends on CD4+ T cells and interferon-gamma but not B cells, CD8+ T cells, or perforin. AIDS Res Hum Retroviruses. 2010;26:783–93. doi: 10.1089/aid.2009.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sealy R, Webby RJ, Crumpton JC, Hurwitz JL. Differential localization and function of antibody-forming cells responsive to inactivated or live-attenuated influenza virus vaccines. International immunology. 2013;25:183–95. doi: 10.1093/intimm/dxs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–37. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 34.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. Journal of immunology. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY Figure 1. Virus clearance requires CD4+ T cells
BALB/c (control) and nude mice were infected with RSV by intranasal inoculations and tested 24 days later for RSV titers in the lungs. Results are shown for groups of BALB/c control mice, nude mice that received no CD4+ T cells, and nude mice that were adoptively transferred with CD4+ T cells from control, primed animals. A comparison of BALB/c mice and nude mice, or nude mice with or without transfers, yielded significant differences using the Fisher’s Exact Test (p<.05)