Abstract
Background
The BREAST-Q Reduction module is a rigorously developed, well-validated patient reported outcome (PRO) instrument designed specifically for evaluating outcomes in reduction mammoplasty. However, there are currently no published normative scores, limiting the interpretation of BREAST-Q data.
Methods
The BREAST-Q Reduction module was administered via the Army of Women (AOW), an online community of women (with and without breast cancer) engaged in breast-cancer related research. Normative data were generated from women age 18 and older, without a prior history of breast cancer or breast surgery. Data analysis was performed using descriptive statistics and a linear multivariate regression. Generated normative data were then compared to previously published BREAST-Q Reduction findings.
Results
The preoperative version of the BREAST-Q Reduction module was completed by 1,206 women. Participant mean age was 55 ±13, mean body mass index (BMI) was 27 ±6, and 40% (n=481) had a bra cup ≥D. Mean normative scores were as follows: Satisfaction with Breasts 57 ±16, Psychosocial Well-being 68 ±19, Sexual Well-being 55 ±19, and Physical Well-being 76 ±11. Normative scores were lower in women with BMI ≥30 and bra cup ≥D. In comparison to normative AOW scores, published BREAST-Q scores for women undergoing reduction mammoplasty were lower (worse) for preoperative patients and higher (better) for postoperative patients.
Conclusions
These new AOW normative data provides insights into breast-related satisfaction and well-being in women not pursuing breast reduction, giving new clinical context to better understand the health burden of macromastia, and to demonstrate the value of reduction mammoplasty in certain patients.
Introduction
Over 100,000 women undergo reduction mammoplasty each year (1). Symptomatic relief is the primary motivating factor for many of these women (2, 3). It is these subjective factors, such as symptoms of back and neck pain, headaches, shoulder grooving, and upper extremity numbness, that drive the health burden of macromastia and motivate women to seek reduction mammoplasty, (4-6)as opposed to quantitative factors, such as body mass index (BMI) or breast cup size. These same symptoms have also been shown to improve after reduction mammoplasty (7-9), highlighting their importance as outcome metrics. Thus it is necessary to use research tools that are capable of appropriately capturing these constructs from the patient perspective when evaluating the outcomes of patients presenting for surgical relief of macromastia and the impact of intervention on their symptoms.
Breast surgery specific patient-reported outcome (PRO) instruments do just this and are frequently utilized in patients seeking reduction mammoplasty. The BREAST-Q Reduction module, one of the most widely used PRO instruments specific to reduction mammoplasty, allows researchers and clinicians to capture reproducible data regarding the impact of macromastia on symptoms and quality of life, and to track change over time (10, 11). However, unlike objective outcomes in surgical research, the interpretation of PRO results presents a unique challenge.
PRO instruments, including the BREAST-Q, generate a numeric score. This score is given meaning when compared over time or between intervention groups. However, the clinical relevance of these findings without such comparisons is not always readily apparent. Thus a current limitation to the BREAST-Q Reduction module is a lack of normative scores for breast-related satisfaction and well-being of women in the general population; such normative scores could be used to provide clinical context for both pre- and postoperative data points.
The primary aim of this study was to generate and describe population norms for the BREAST-Q Reduction module. The secondary aim was to compare AOW population norms to previously published BREAST-Q Reduction scores, to bring greater clinical context and understanding to previously determined findings describing women presenting for and undergoing reduction mammoplasty.
Methods
Study Population
Participants were recruited through the Army of Women (AOW), an online community started in 2008 by the Dr. Susan Love Research Foundation, with the goal of connecting breast cancer researchers to women with and without breast cancer. In order to recruit patients using the AOW, researchers must have funding, IRB approval from the home institution, and be accepted by the AOW Scientific Advisory Committee. After IRB exemption was granted from Dartmouth College, and acceptance by the AOW, an electronic recruitment email (e-blast) was circulated to AOW members. Interested women self-selected to participate if they met the following inclusion criteria: age 18 years or older, no personal history of breast cancer or breast surgery, and an ability to complete a questionnaire online in English.
Recruitment
The e-blast was sent to 121,688 AOW members. Those participants who were interested and self-screened themselves as eligible followed a link to electronically complete the BREAST-Q, which was administered using Qualtrics, an online, web-based software for questionnaire administration (Provo, UT; www.qualtrics.com). Participant recruitment was part of a larger study generating normative values for the three BREAST-Q modules (i.e., Reduction, Augmentation and Reconstruction). Participants did not know which BREAST-Q module was being completed. In addition to completing a pre-operative BREAST-Q module, data collection included demographic questions as well as bra cup size, height, and weight. A recruitment algorithm was written into Qualtrics that automatically rerouted participants to the next BREAST-Q module after 1,200 participants had completed one of the modules, starting with Reduction, followed by Reconstruction and then Augmentation. Normative data for the latter two will be reported in other manuscripts.
BREAST-Q
The BREAST-Q is a rigorously developed and well-validated PRO instrument designed for all types of breast surgery (11, 12). Utilized in research with over 22,000 women having different types of breast surgery, the BREAST-Q is one of the most widely used breast surgery specific PRO instruments (11-17). The BREAST-Q, first published in 2009, was developed following internationally accepted guidelines for PRO development (4, 18).
Development of the conceptual framework and set of scales included a literature review, 48 primary patient interviews, 46 cognitive debriefing interviews, and expert opinion from a panel of plastic surgeons and other healthcare professionals. The BREAST- Q was then tested in a sample of 2715 patients, 908 pre-surgery patients and 1807 post-surgery patients, with a response rate of 72%.
There are 4 pre-operative BREAST-Q Reduction scales: Satisfaction with Breasts (n=11 items), Psychosocial Well-being (n=9 items), Sexual Well-being (n=5 items), and Physical Well-being (n=14 items). Questions assess breast satisfaction including satisfaction with macromastia symptoms, as well as QOL and well-being as it relates to macromastia and reduction mammoplasty. For all BREAST-Q scales, items are summed and transformed on a scale from 0 (worst) to 100 (best) using Q-Score program (New York, NY; https://webcore.mskcc.org/breastq/scoring.html). In the BREAST-Q development sample (n=1950), Reduction scales had Cronbach's alpha scores between 0.83 and 0.95, mean item total correlations from 0.46 to 0.83, and test-retest reliability with intraclass correlation coefficients between 0.73 and 0.94. Additionally, the BREAST-Q has demonstrated validity and the ability to detect clinically meaningful change (14).
Data Analysis
Descriptive statistics were computed, including the mean, standard deviation (SD), and 95% confidence interval (CI) for continuous variables, and percentages listed for categorical variables. Backward-selection linear multivariate regression was used to determine variables associated with BREAST-Q scores. Variables were categorized to form dichotomous variables as follows: BMI = ≥30 vs. BMI <30, age = ≥40 vs. age <40, bra size = ≥D vs. <D, ethnicity = white non-Hispanic vs. other, education = college degree or higher vs. less than college degree, employment = full-time vs. other than full-time, income = ≥$40,000 vs. <$40,000/year, and marital status = married vs. other. Binomial variables with a probability of less than 0.2 were rejected and removed from the model, and the model was rerun with only the significant variables (p<0.05). Statistical significant difference was determined by use of 95% CIs in which there was a difference in the results if the CIs of the measures did not cross. Data analysis was performed using Stata/SE 11.0 (College Station, Texas).
A separate analysis compared the normative scores to published and unpublished BREAST-Q Reduction scores using 95% CIs. To identify published scores, we searched PubMed in January 2016 with “BREAST-Q” or “BREASTQ” as key terms, and then screened title and abstracts to identify publications using the Reduction module. A 2013 prospective study by Coriddi et al. was selected as it had the greatest number of participants and most complete prospective dataset from available studies. Coriddi et al. reported data for all of the Reduction scales in 38 pre-op patients and 38 patients at 6-weeks post-op (10). Mean age was 36 ±13 years, mean BMI 32 ±6. Pedicle was superomedial in 33% and inferior in 67% of patients. Skin incision was Wise pattern in 76% and vertical incision in 24% of patients. Given the small sample size in available published data, the authors additionally used an unpublished pre-operative BREAST-Q Reduction dataset of 279 patients presenting for breast reduction at Dartmouth–Hitchcock Medical Center. This dataset was collected as part of routine clinical care and was granted IRB exemption from Dartmouth's Committee for the Protection of Human Subjects (Study000280776). Mean age was 45 ±14 years, mean BMI 31 ±7. Surgical incision and pedicle information were not available as this is a preoperative cohort. From the selected studies, we extracted the following information: study design, sample size, and BREAST-Q scores. We contacted the authors as needed to obtain missing data. The sample size, mean BREAST-Q score, and standard deviation were used to calculate a 95% CI for each publication.
Results
There were 121,688 AOW members at the time of e-blast. Three months following the e-blast, a second e-blast was circulated to complete recruitment for the remaining 409 participants needed to reach the minimum 3,600 participants for all 3 BREAST-Q modules. Across all three modules, a total of 4,326 women self-selected as eligible participants meeting the study inclusion criteria, 3,618 women completed BREAST-Q pre-operative modules, and 142 women who were not included attempted to participate after the final module had reached capacity, prior to the AOW closing the study. The overall response rate across all three modules was 86.5%. In total, 1,206 women completed the BREAST-Q Reduction module pre-operative questionnaire.
For the Reduction sample, the mean age was 55 years ±13, mean BMI 27 ±6, and bra cup size of at least a D was present in 40% of women (n=481). The majority of participants were of white ethnicity (91%, n=1093), 84% had a college education or greater (n=1009), 43% were employed full-time (n=511), 44% had an annual gross household income of $100,000 or greater (n=505), and 69% were married (n=828). A chronic health condition was reported in 50% (n=596), with commonly cited conditions as follows: hypothyroidism, diabetes, hypertension, hyperlipidemia, asthma, gastroesophageal reflux disease, inflammatory bowel disease, irritable bowel syndrome, arthritis, psoriasis, and headaches. Full demographic values of the women who completed the Reduction module are listed in Table 1.
Table 1. BREAST-Q Reduction Module Demographics.
| Number | Percentage | |
|---|---|---|
| Sample Size | 1206 | |
| Age in years: mean ±SD | 55 ±13 | |
| BMI: mean ±SD | 27 ±6 | |
| Bra Cup Size | ||
| <A | 22 | 2% |
| A | 90 | 8% |
| B | 287 | 24% |
| C | 320 | 27% |
| D | 232 | 19% |
| DD | 149 | 12% |
| >DD | 100 | 8% |
| Ethnic/cultural group | ||
| South Asian or East Indian | 1 | 0.1 % |
| Asian or Pacific Islander | 11 | 0.9% |
| Black Non-Hispanic | 18 | 2% |
| Black Hispanic | 4 | 0.3% |
| White Non-Hispanic | 1093 | 91% |
| White Hispanic | 44 | 4% |
| Native Canadian/American | 14 | 1% |
| Other | 16 | 1% |
| Long-term health condition | ||
| Yes | 596 | 50% |
| Education | ||
| Some High School | 0 | 0.0 % |
| High School Diploma | 34 | 3% |
| Some College, Trade or University | 159 | 13% |
| College, Trade or University Diploma | 442 | 37% |
| Some Master or Doctoral | 98 | 8% |
| Master or Doctoral Degree | 469 | 39% |
| Employment | ||
| Full Time | 511 | 43% |
| Part Time | 168 | 14% |
| Voluntary Work | 33 | 3% |
| Homemaker | 85 | 7% |
| Student | 16 | 1% |
| Retired | 326 | 27% |
| Unable to Work or Disabled | 12 | 1% |
| Unemployed or Seeking Employment | 19 | 2% |
| Other | 32 | 3% |
| Annual Gross Household Income | ||
| <$20,000 | 32 | 3% |
| $20,000 - $39,999 | 90 | 8% |
| $40,000 - $59,999 | 161 | 14% |
| $60,000 - $79,999 | 202 | 18% |
| $80,000 - $99,999 | 159 | 14% |
| >$100,000 | 505 | 44% |
| Marital Status | ||
| Married | 828 | 69% |
| Living with Significant Other | 75 | 6% |
| Widowed | 47 | 4% |
| Separated | 13 | 1% |
| Divorced | 107 | 9% |
| Single, Never Married | 131 | 11% |
SD = standard deviation
The normative scores are shown in Table 2 and ranged from 55 to 76, with standard deviations 11 to 19. The Sexual Well-being scale was completed by 85% (n=1024), the lowest completion rate. Of note, the instructions specify not to complete the Sexual Well-being scale if the participant was uncomfortable with the content or felt items were not applicable.
Table 2. BREAST-Q Reduction Module Normative Values.
| N | Mean | SD | |
|---|---|---|---|
| Satisfaction with Breasts | 1205 | 57 | 16 |
| Psychosocial Well-being | 1205 | 68 | 19 |
| Sexual Well-being | 1024 | 55 | 19 |
| Physical Well-being Chest | 1205 | 76 | 11 |
N = number; SD = standard deviation
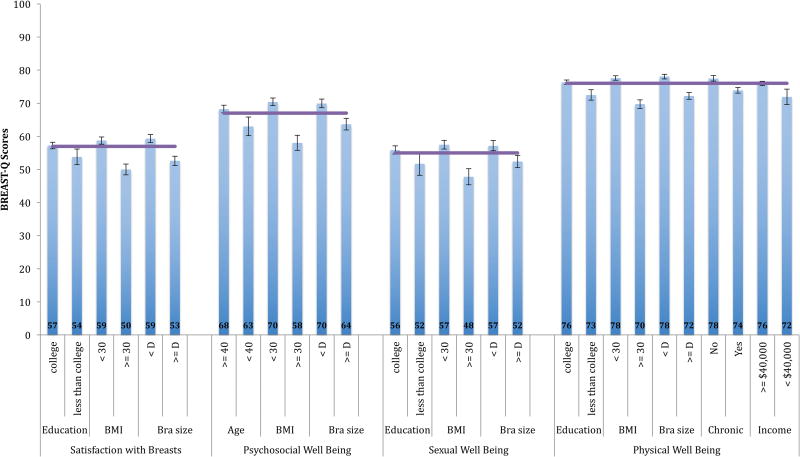
For the 4 BREAST-Q scales, the linear multivariate regression models generated between 3 and 5 demographic variables associated with Reduction scores. Figure 1 demonstrates these significant variables with 95% CIs. Common across the 4 BREAST-Q Reduction scales was the association of lower BREAST-Q scores for respondents with a BMI of 30 or more and bra cup size of D or greater compared to the corresponding reference groups (all p-values in the regression models for these 2 variables were less than 0.001).
Figure 1. BREAST-Q Reduction Module: Variable Demographics Factors using 95% Confidence Intervals.
- Purple line = mean score with 95% confidence intervals in brackets
- Chronic = chronic disease
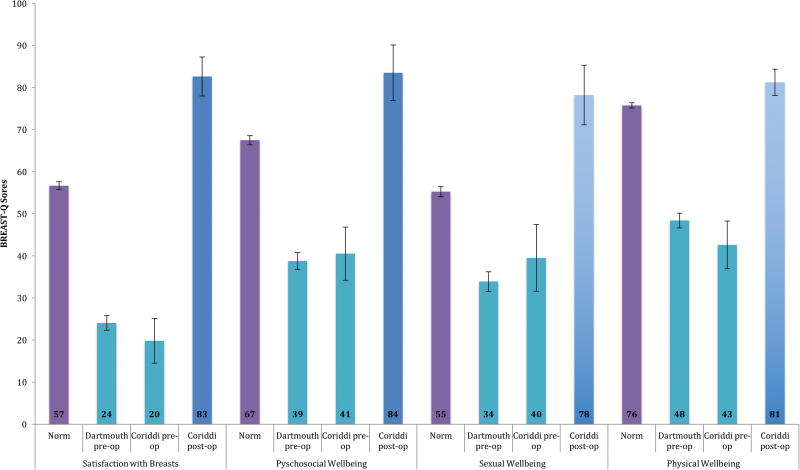
The normative scale scores generated in this study were compared to previously published (Coriddi, et al.) and unpublished (Dartmouth) BREAST-Q data in patients presenting for and undergoing reduction mammoplasty. Figure 2 shows the results of the pre-op studies and the post-op prospective study in comparison to the normative results. The two separate pre-operative means were not significantly different from each other, and both were significantly lower than the norm across all 4 scales. Post-operative means were significantly higher than the norm across all 4 scales.
Figure 2. BREAST-Q Normative Data vs. Previously Published Data using 95% Confidence Intervals.
Discussion
Relief of symptoms, such as neck, back, shoulder and arm pain, headaches, rashes, itching and bra strap grooving, are the primary motivators for most women pursuing reduction mammoplasty (5, 6). Generic PRO instruments have demonstrated that these women with symptomatic macromastia who undergo reduction mammoplasty report significant improvements in pain and quality of life, often to a level that is improved from the general population (6, 19). Furthermore, researchers have failed to explain these improvements in quality of life and symptoms using objective measures, such as BMI, bra cup size, and quantity of resected breast tissue (19-21). However, despite these well-established findings in the literature, stating that it is subjective rather than objective measures that matter most for patients, third party payers have yet to respond. Third party payers often require the inevitable failure of lengthy non-operative treatment attempts, size requirements of the initial bra cup size, and/or pre-determined quantities of breast tissue resected, continuing to rely on quantitative data to drive reimbursements for a procedure that is best evaluated in qualitative outcomes.
PRO instruments represent a potential bridge to this discrepancy. Furthermore, disease-specific PRO instruments, as opposed to generic instruments such as the SF-36 or EuroQOL that are used to assess general changes in PROs across multiple disease processes, have the potential to provide reproducible data, specific to a given disease process and capable of capturing change over time (22). The BREAST-Q Reduction module provides such data for patients presenting for and undergoing reduction mammoplasty (11). However, a key limitation of the current disease-specific PRO instruments for macromastia and reduction mammoplasty, and specifically the BREAST-Q Reduction module, is the lack of normative data. Whereas generic PRO instruments, such as the SF-36, are scaled to a normative population, prior to this analysis, it was not known what a normative BREAST-Q Reduction score was.
In this study, we successfully generated population norms for the Reduction module of the BREAST-Q. Normative BREAST-Q scores will help to demonstrate the health burden associated with macromastia and the impact of surgical intervention. Within the normative scores, larger BMI and bra cup sizes were associated with lower BREAST-Q scores when compared to reference groups with smaller BMI and bra cup sizes. This finding suggests that even women not seeking reduction mammoplasty but with higher BMI and/or bra-cup sizes may have lower associated well-being or quality of life in regards to their breasts.
Our comparison of normative BREAST-Q scores with previously collected data, highlights the extent to which pre-operative scores were below normative values, quantifying the health burden associated with macromastia. Additionally, post-operative scores were significantly higher than the norm, demonstrating the success of reduction mammoplasty. Of note, the normative BREAST-Q scores in women with a large BMI or breast cup size, while lower than a reference group of women with a small BMI or breast cup size, were significantly higher than the pre-operative scores in women presenting for reduction mammoplasty. This finding suggests that a large BMI or bra cup size do not alone explain the full health burden of disease associated with macromastia.
The strengths of this study are as follows. This is the first study to generate normative values for the BREAST-Q, one of the most widely used PRO instruments in breast surgery. Furthermore, the sample size is large, with over 1,200 participants. Lastly, given the standard scoring system of the BREAST-Q, the normative data presented here can be seamlessly integrated into ongoing and future clinical care and research, providing a normative reference point for BREAST-Q score interpretation.
The limitations of this study include our sample characteristics and method of selection. Our sample population is predominantly white, educated and wealthy, and while this is comparable to both the Reduction data presented from Dartmouth as well as the overall current usage of the BREAST-Q, (17) the AOW (23), and a common issue faced in large scale health outcomes research (24), it is not representative of the US population at large. In addition, women self-selected themselves to be participants in the study, without a clinician or researcher confirming eligibility. While the inclusion criteria were straightforward and did not rely on significant medical knowledge, it is possible that they were misinterpreted. It is also possible that women actively pursuing or planning for, yet prior to undergoing reduction mammoplasty were included in the analysis. The data collected for this study was collected as part of a larger study evaluating normative scores across all three pre-operative BREAST-Q modules. There were slight variances in demographic values as well as pre-operative BREAST-Q scores between the three samples (variance likely explained by differences in each module's question content). Each module was completed by 1200 participants in full prior to the algorithm moving to the next module. This algorithm may have introduced more bias than if participants were randomly assigned to a module and all three modules were completed simultaneously.
There were also limitations in our comparison to the literature. The post-operative outcomes described here were at 6 weeks, a relatively short follow-up period. It is possible that if this type of cohort completed the BREAST-Q months to several years postoperatively, the scores may return to population norms, and here we are merely presenting an exaggerated effect immediately following surgery. Additionally, despite the Coriddi study being the largest prospective study published to date, there were only 38 pre-operative and 38 post-operative patients, with only some overlap in individuals between these two groups, further limiting this data.
The normative values described here provide researchers and clinicians with a novel method of interpreting BREAST-Q Reduction data. Our hope is that this new normative clinical framework will inform future research working to better understand the health burden of macromastia and evaluate the outcomes of reduction mammoplasty. Additionally, we hope that these normative data could be used to better frame the discussion of appropriate surgical indications for mammoplasty, as determined by surgeons and third party payers, with the goal of better aligning the findings in the literature with payment behaviors. Future directions could include establishing normative data for populations with increased diversity.
Conclusions
The normative data generated in this analysis provides an essential, yet previously unavailable, clinically relevant reference point for the interpretation of the BREAST-Q Reduction module. We provide context to better delineate the health burden associated with macromastia, such as confirming that bra cup size and large BMI negatively impact health-related quality of life, yet not at the level of patients presenting for reduction mammoplasty. This data also confirms that women presenting for reduction mammoplasty have a quality of life significantly below that of the norm, and that this significantly improves to above the norm with surgical intervention. These normative values may be used to drive future research and clinical care regarding the health burden of macromastia, including appropriate indications for surgical intervention from the perspective of both the clinician and a third party payer, as well as to evaluate outcomes after reduction mammoplasty.
Acknowledgments
Funding for the study was provided from a discretionary account of Dr. Kerrigan's held by The Dartmouth Institute. The BREAST-Q is owned by Memorial Sloan-Kettering Cancer Center. Dr. Pusic and Dr. Klassen are co-developers. They receive a portion of licensing fees when the BREAST-Q is used in industry sponsored clinical trials. Dr. Andrea Pusic received support through the NIH/NCI Cancer Center Support Grant P30 CA008748. Drs. Mundy, Homa, and Kerrigan have no commercial associations or financial disclosures.
Footnotes
Author Contributions:
1) Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: All authors
2) Drafting the article or revising it critically for important intellectual content: All authors
3) Final approval of the version to be published: All authors
4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors
References
- 1.American Society for Aesthetic Plastic Surgery. ASAPS National Data Bank Statistics. [Accessed March 30, 2016]; Available at: http://www.surgery.org/sites/default/files/Stats2015.pdf.
- 2.Atterhem H, Holmner S, Janson PE. Reduction mammaplasty: Symptoms, complications, and late results. A retrospective study on 242 patients. Scand J Plast Reconstr Surg Hand Surg. 1998;32:281–286. doi: 10.1080/02844319850158615. [DOI] [PubMed] [Google Scholar]
- 3.Singh KA, Losken A. Additional benefits of reduction mammaplasty: A systematic review of the literature. Plast Reconstr Surg. 2012;129:562–570. doi: 10.1097/PRS.0b013e31824129ee. [DOI] [PubMed] [Google Scholar]
- 4.Netscher DT, Meade RA, Goodman CM, Brehm BJ, Friedman JD, Thornby J. Physical and psychosocial symptoms among 88 volunteer subjects compared with patients seeking plastic surgery procedures to the breast. Plast Reconstr Surg. 2000;105:2366–2373. doi: 10.1097/00006534-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kerrigan CL, Collins ED, Striplin D, et al. The health burden of breast hypertrophy. Plast Reconstr Surg. 2001;108:1591–1599. doi: 10.1097/00006534-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez MA, Glickman LT, Aladegbami B, Simpson RL. Quality of life after breast reduction surgery: A 10-year retrospective analysis using the Breast Q questionnaire: does breast size matter? Ann Plast Surg. 2012;69:361–363. doi: 10.1097/SAP.0b013e31824a218a. [DOI] [PubMed] [Google Scholar]
- 7.Glatt BS, Sarwer DB, O'Hara DE, Hamori C, Bucky LP, LaRossa D. A retrospective study of changes in physical symptoms and body image after reduction mammaplasty. Plast Reconstr Surg. 1999;103:76–82. doi: 10.1097/00006534-199901000-00013. discussion 83-75. [DOI] [PubMed] [Google Scholar]
- 8.Rogliani M, Gentile P, Labardi L, Donfrancesco A, Cervelli V. Improvement of physical and psychological symptoms after breast reduction. J Plast Reconstr Aesthet Surg. 2009;62:1647–1649. doi: 10.1016/j.bjps.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 9.Blomqvist L, Eriksson A, Brandberg Y. Reduction mammaplasty provides long-term improvement in health status and quality of life. Plast Reconstr Surg. 2000;106:991–997. doi: 10.1097/00006534-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Coriddi M, Nadeau M, Taghizadeh M, Taylor A. Analysis of satisfaction and well-being following breast reduction using a validated survey instrument: the BREAST-Q. Plast Reconstr Surg. 2013;132:285–290. doi: 10.1097/PRS.0b013e31829587b5. [DOI] [PubMed] [Google Scholar]
- 11.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 12.Pusic AL, Reavey PL, Klassen AF, Scott AM, McCarthy C, Cano SJ. Measuring patient outcomes in breast augmentation: introducing the BREAST-Q Augmentation module. Clin Plast Surg. 2009;36:23–32v. doi: 10.1016/j.cps.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Cano S, Klassen AF, Scott A, Thoma A, Feeny D, Pusic A. Health outcome and economic measurement in breast cancer surgery: Challenges and opportunities. Exp Rev Pharmacoecon Outcomes Res. 2010;10:583–594. doi: 10.1586/erp.10.61. [DOI] [PubMed] [Google Scholar]
- 14.Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. 2012;129:293–302. doi: 10.1097/PRS.0b013e31823aec6b. [DOI] [PubMed] [Google Scholar]
- 15.Pusic AL, Klassen AF, Cano SJ. Use of the BREAST-Q in clinical outcomes research. Plast Reconstr Surg. 2012;129:166e–167e. doi: 10.1097/PRS.0b013e3182362e65. author reply 167e. [DOI] [PubMed] [Google Scholar]
- 16.Cano SJ, Klassen AF, Scott AM, Pusic AL. A closer look at the BREAST-Q((c)) Clin Plast Surg. 2013;40:287–296. doi: 10.1016/j.cps.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Cohen WA, Mundy, LR, Ballard TN, et al. The BREAST-Q in surgical research: A review of the literature 2009-2015. J Plast Reconstr Aesthet Surg. 2016;69:149–62. doi: 10.1016/j.bjps.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11:193–205. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 19.Collins ED, Kerrigan CL, Kim M, et al. The effectiveness of surgical and nonsurgical interventions in relieving the symptoms of macromastia. Plast Reconstr Surg. 2002;109:1556–1566. doi: 10.1097/00006534-200204150-00011. [DOI] [PubMed] [Google Scholar]
- 20.Spector JA, Karp NS. Reduction mammaplasty: A significant improvement at any size. Plast Reconstr Surg. 2007;120:845–850. doi: 10.1097/01.prs.0000277660.49802.76. [DOI] [PubMed] [Google Scholar]
- 21.Spector JA, Singh SP, Karp NS. Outcomes after breast reduction: Does size really matter? Ann Plast Surg. 2008;60:505–509. doi: 10.1097/SAP.0b013e31816f76b5. [DOI] [PubMed] [Google Scholar]
- 22.Klassen AF, Stotland MA, Skarsgard ED, Pusic AL. Clinical research in pediatric plastic surgery and systematic review of quality-of-life questionnaires. Clin Plast Surg. 2008;35:251–267. doi: 10.1016/j.cps.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Bright EE, Petrie KJ, Partridge AH, Stanton AL. Barriers to and facilitative processes of endocrine therapy adherence among women with breast cancer. Breast Cancer Res Treat. 2016 Jun 24; doi: 10.1007/s10549-016-3871-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Stanton AL, Morra ME, Diefenbach MA, et al. Responding to a significant recruitment challenge within three nationwide psychoeducational trials for cancer patients. J Cancer Surviv. 2013;7:392–403. doi: 10.1007/s11764-013-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]