Abstract
Background
Anti-vascular endothelial growth factor (VEGF) therapy has shown promise in the treatment of high-grade gliomas (HGG). Aflibercept is a recombinant human fusion protein that acts as a soluble decoy receptor for VEGF-A, VEGF-B and placental growth factor (PlGF), depleting circulating levels of these growth factors.
Methods
The Adult Brain Tumor Consortium (ABTC) conducted a phase I trial of aflibercept and temozolomide (TMZ) in patients with newly diagnosed high-grade gliomas (HGG) with 2 dose levels and a 3+3 design. Three arms using aflibercept were examined; with radiation and concomitant temozolomide; with adjuvant temozolomide using the 5/28 regimen; and with adjuvant temozolomide using the 21/28 day regimen.
Results
Fifty-nine patients were enrolled, 21 in arm 1, 20 in arm 2 and 18 in arm 3. Median age was 56 years (24-69); median KPS 90 (60-100). The maximum tolerated dose (MTD) of aflibercept for all 3 arms was 4mg/kg every 2 weeks. Dose limiting toxicities (DLTs) at the MTD were: Arm 1: 0/21 patients; Arm 2: 2/20 patients (G3 deep vein thrombosis, G4 neutropenia; Arm 3: 3/18 patients (G4 biopsy-confirmed thrombotic microangiopathy, G3 rash, G4 thrombocytopenia). The median number of cycles of aflibercept was 5 (range, 1-16). All patients stopped treatment; 28 (47%) for disease progression, 21 (36%) for toxicities, 8 (14%) for other reasons, and 2 (3%) patients completed the full treatment course.
Conclusions
This study met its primary endpoint and the MTD of aflibercept with radiation and concomitant and adjuvant temozolomide is 4mg/kg every 2 weeks.
Keywords: newly diagnosed glioblastoma, aflibercept, dose-dense, temozolomide, VEGF Trap
Introduction
High-grade gliomas, particularly glioblastomas, are highly vascular tumors with elevated levels of vascular endothelial growth factor (VEGF) expression[1]. VEGF stimulates angiogenesis including endothelial cell proliferation, differentiation and migration, leading to formation of abnormal blood vessels, thus promoting tumor growth. This abnormal vasculature is also thought to induce tumor hypoxia, impair intratumoral delivery of chemotherapy and in turn, reduce the efficacy of chemoradiation.
There has been considerable interest in the utilization of angiogenesis targeting therapies to control tumor growth. Bevacizumab, a humanized monoclonal anti-VEGF-A antibody is approved by the Food and Drug Administration (FDA) for recurrent glioblastoma based on phase II trials which demonstrated improved response rates and progression-free survival (PFS)[2, 3]. Two randomized phase III trials in newly diagnosed glioblastoma that studied the upfront use of bevacizumab in conjunction with standard chemoradiation confirmed PFS prolongation, but found no significant difference in overall survival[4, 5]. Studies with small molecule inhibitors of VEGF receptor (VEGFR) have shown only modest results[6].
Aflibercept is a novel recombinant fusion protein consisting of the extracellular domains of VEGFR1 and VEGFR2 fused to an immunoglobulin Fc domain which acts as a soluble decoy for VEGF and placental growth factor (PlGF). Aflibercept has higher VEGF-A binding affinity (Kd = 0.47 pM) than bevacizumab (Kd ≈ 800 pM), and also binds VEGF-B and other related factors. PlGF has the ability to displace VEGF from VEGFR1 thus increasing bioavailability of VEGF which stimulates angiogenesis. Additionally, PlGF can contribute to the angiogenic switch which leads to transformation of anaplastic gliomas to glioblastoma, providing a rationale for targeting both VEGF and PlGF simultaneously using aflibercept. A phase II trial of aflibercept conducted by the North American Brian Tumor Consortium (NABTC) showed moderate toxicity and some activity in recurrent high-grade gliomas (HGG)[7].
Vascular normalization by VEGF and angiogenesis inhibition is known to increase drug delivery to the tumor tissue and leads to synergistic activity with radiation (RT) and cytotoxic chemotherapy[8]. This can also reduce radiation induced VEGF production and peritumoral edema. Preclinical models have shown that aflibercept in combination with RT significantly delayed the growth of subcutaneous xenografts compared to RT alone or aflibercept alone[9].
The Adult Brain Tumor Consortium (ABTC, formerly North American Brain Tumor Consortium/NABTC) conducted this phase I trial to study the safety of aflibercept in combination with RT and temozolomide.
Patients and Methods
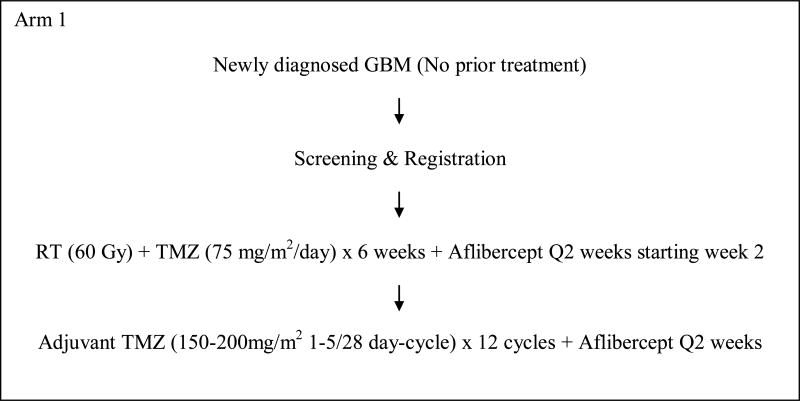
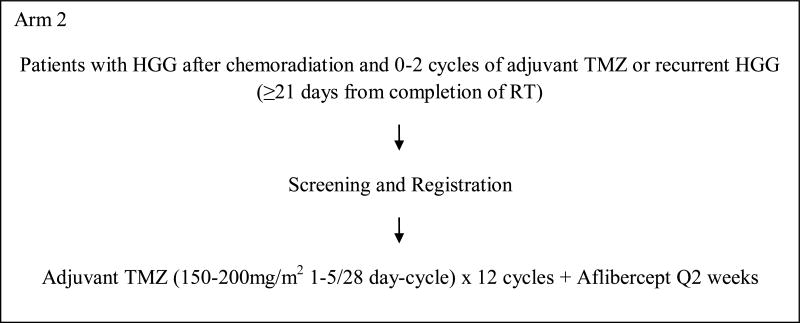
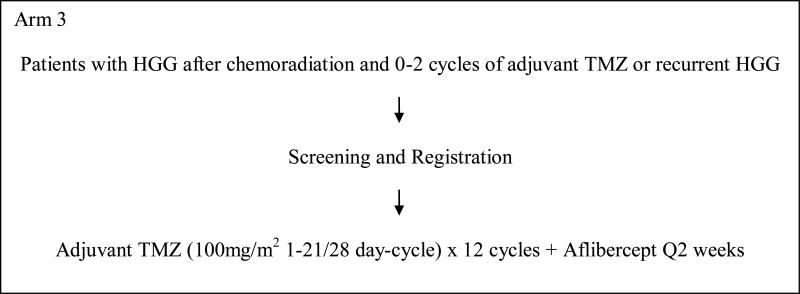
This was a 3-arm, phase I study to evaluate the safety of aflibercept with RT and temozolomide and determine the maximum tolerated dose (MTD) in each of the 3 arms (Figure 1). In arm 1, patients with newly diagnosed glioblastoma were to be treated with aflibercept in combination with radiation and temozolomide followed by adjuvant temozolomide. Arms 2 and 3 included patients with stable or recurrent HGG post-radiation who were to receive aflibercept with standard or dose-dense temozolomide regimen. This protocol was approved by the institutional review boards of all participating centers. All patients signed informed consent. This study was designed and started accrual before the results of 2 phase III trials were available which showed no benefit of dose-dense temozolomide over standard schedule[10, 11].
Figure 1. Schema.
Patients
Patients who were 18 years of age or older and had a Karnofsky performance status (KPS) of ≥60, with histologically proven intracranial glioblastoma or gliosarcoma were eligible. Patients with anaplastic glioma, including anaplastic astrocytoma, anaplastic oligodendroglioma, anaplastic mixed oligoastrocytoma or malignant astrocytoma not otherwise specified were eligible for arms 2 and 3 of the study. Additionally, patients were required to have a life expectancy of over 12 weeks and adequate bone marrow, renal and liver function. Patients who had received prior carmustine (Gliadel) wafers were excluded. Patients with known hypersensitivity to Chinese Hamster Ovary (CHO) cell products, recombinant human antibodies, or other chemical or biologic agents used in the study were excluded. Patients with uncontrolled medical illnesses, history of abdominal fistula, gastrointestinal perforation, intraabdominal abscess, gastrointestinal bleeding or diverticulitis within 6 months of treatment were considered ineligible. Patients with clinically significant cardiovascular or cerebrovascular disease in the past 6 months were excluded, as were those with bleeding diathesis, coagulopathy, or on anticoagulants. Patients with significant intratumoral or peritumoral hemorrhage were excluded, except those with post-operative intracavitary blood. Pregnant women were excluded. Those with history of any other cancer except non-melanomatous skin cancer or carcinoma in situ of the cervix were considered ineligible unless in complete remission and off all treatment for at least 3 years.
Study Design and Treatment
This was a 3-arm study (Figure 1).
Patients enrolled on arm 1 of the study received involved field radiation to 60 Gy with concurrent temozolomide orally at a dose of 75mg/m2/day for 6 weeks, and subsequently in 4 weeks temozolomide at 150mg/m2/day orally on days 1-5 of the 1st 28 day-cycle followed by 200mg/m2 orally on days 1-5 of all subsequent cycles (maximum total 12 cycles). Aflibercept was initiated after 2 weeks of radiation and administered intravenously every 2 weeks. Patients who had been previously treated with radiation and concurrent temozolomide followed by 0 to 2 cycles of adjuvant temozolomide were enrolled on arms 2 and 3. On arm 2, patients received temozolomide at 150mg/m2/day orally on days 1-5 of the 1st 28 day-cycle followed by 200mg/m2/day orally on days 1-5 of all subsequent cycles (maximum total 12 cycles). On arm 3, patients received temozolomide at 100mg/m2/day orally on days 1-21 of every 28 day-cycle. Aflibercept was administered intravenously on days 1 and 15 of every cycle on arms 2 and 3. Aflibercept was administered at a starting dose of 2mg/kg intravenously every 2 weeks. If no dose limiting toxicities were observed, the dose was increased to 4mg/kg in the next cohort of patients. Only 2 dose levels were planned for the 3 arms and no further dose escalation was permitted.
Patients were enrolled in arm 1 and either arm 2 or 3 simultaneously. Arms 2 and 3 enrolled patients sequentially such that 3 patients enrolled in arm 2 and then 3 patients in arm 3. A standard ‘3 + 3’ dose-escalation design was used for each arm. At least 18 patients were planned to be enrolled in each arm. MTD was defined as the dose at which fewer than one-third of patients experienced a DLT due to aflibercept and/or temozolomide.
Treatment was continued until tumor progression, development of unacceptable toxicity or completion of 12 cycles.
Patient Evaluation and Safety Assessment
For arm 1, patients underwent clinical evaluation for the first 9 weeks with aflibercept during radiation and concurrent temozolomide, and then prior to first adjuvant temozolomide cycle (after week 10). For arms 2 and 3, weekly clinical assessments were performed for the first 5 weeks. For all 3 arms, patients were evaluated prior to every 4-week adjuvant cycle. Neuro-imaging was done 4 weeks after radiation prior to starting adjuvant cycles for arm 1, and every 8 weeks (2 cycles) thereafter for all 3 arms.
Toxicities were graded according to the National Cancer Institute common terminology criteria for adverse events (CTCAE), version 4.0. Dose limiting toxicity (DLT) was defined as any ≥ grade 3 thrombocytopenia, grade 4 anemia or grade 4 neutropenia for >7 days, any non-hematologic toxicity ≥ grade 3, except alopecia, despite maximal medical therapy, any grade 4 radiation-induced skin changes, and failure to recover from toxicities to restart treatment with aflibercept and temozolomide within 14 days of last dose of either drug. DLTs in arm 1 were based on toxicities during the 1st 8 weeks of treatment starting with the 1st aflibercept infusion. DLTs in arms 2 and 3 were based on toxicities during the 1st 4 weeks of treatment.
Endpoints and Statistical Analysis
The primary endpoint of this study was to define the MTD and characterize the safety profile of aflibercept in each of the 3 arms. The exploratory endpoint was to evaluate the effect of treatment with aflibercept, radiation and temozolomide on neurocognitive outcomes.
Baseline characteristics and treatment administration were described for all enrolled patients. Safety variables were summarized by descriptive statistics.
Neurocognitive Testing
Neurocognitive testing was assessed in arm 1 patients at baseline and at every visit when patient had an MRI. The neurocognitive Clinical Trial Battery (CTB) included the Hopkins Verbal Learning Test- Revised (HVLT-R), Trail Making Test (TMT) and Controlled Oral Word Association (COWA). All health care professionals administering the tests were pre-certified by Dr. Jeffrey Wefel from MD Anderson Cancer Center. Standardized scores were computed for each test adjusting for relevant demographic factors [12-14]. The CTB Composite (CTB COMP) score was computed by obtaining the arithmetic mean of the standardized test scores at each time point for each patient that had completed at least 5 out of 6 CTB tests. Descriptive analyses were performed at baseline. The reliable change index (RCI) for each test was used to determine the frequency of changes in test performance relative to each patient's baseline that are statistically unlikely to be due to chance based on the psychometric properties of each test [13-15].
There was central review of pathology performed by Dr. Kenneth Aldape at University of Texas, MD Anderson Cancer Center, and of neuroimaging at University of California, San Francisco.
Results
Patient Characteristics
A total of 59 patients were enrolled from September 2008 to June 2011; 21 in arm 1, 20 in arm 2 and 18 in arm 3. Of these, 51 patients carried a histopathologic diagnosis of glioblastoma and 8 anaplastic glioma. Patient characteristics are provided in Table 1.
Table 1. Patient Characteristics.
| ARM 1 | ARM 2 | ARM 3 | |
|---|---|---|---|
| Number of patients | 21 | 20 | 18 |
| DL0 (2mg/kg) | 3 | 4 | 3 |
| DL1 (4mg/kg) | 18 | 16 | 15 |
| Men/Women | 8/13 | 14/6 | 15/3 |
| Glioblastoma | 100% | 75% | 72% |
| Anaplastic Glioma | 0% | 25% | 28% |
| Median Age (years, range) | 58 (44-69) | 56 (24-69) | 56 (29-65) |
| Median KPS | 90 (70-100) | 80 (60-100) | 90 (70-100) |
| Median no. of cycles (range) | 5 (1-14) | 4 (1-15) | 5.5 (1-16) |
DL: Dose level
MTD and Safety
The MTD of aflibercept for all 3 arms was 4 mg/kg every 2 weeks. The DLTs at MTD were Grade 3 deep vein thrombosis (DVT) and Grade 4 neutropenia in arm 2; and Grade 3 rash, Grade 4 thrombotic microangiopathy (biopsy-confirmed) and Grade 4 thrombocytopenia in arm 3. In arm 1, 2 patients did not receive aflibercept due to serious adverse events in the 2 week-period before starting the 1st dose of aflibercept and were replaced. No DLTs were observed in arm 1 at maximal administered dose of 4 mg/kg every 2 weeks. One patient on arm 2 received incorrect dosing (lower dose) and was replaced. Dose expansion and number of DLTs are provided in Table 2. Tables 3A, B and C summarize the grade 3 or 4 adverse events related to aflibercept and temozolomide per arm. The most common serious adverse events considered possibly, probably or definitely related to aflibercept and temozolomide were lymphopenia, neutropenia, thrombocytopenia, seizures, fatigue and hypertension. There were no deaths related to the study treatment.
Table 2. Dose Expansion and DLT.
| Dose | Arm 1 | Arm 2 | Arm 3 |
|---|---|---|---|
| DL0 (2mg/kg) | 0/3 | 0/3 | 0/3 |
| DL1 (4mg/kg) | 0/7* | 1/8** | 1/6 |
| Dose expansion (4mg/kg) | 0/11* | 1/9 | 2/9 |
DL : Dose level,
1 patient was replaced for serious adverse event before starting aflibercept,
1 patient was replaced for incorrect dosing.
Table 3A. Grade 3 and 4 toxicities attributed to aflibercept and temozolomide for Arm 1.
| Adverse Event | Aflibercept DL0 | Aflibercept DL1 | Temozolomide (DL0) | Temozolomide (DL1) |
|---|---|---|---|---|
| Abdominal pain | 1 | |||
| Alanine aminotransferase increase | 1 | |||
| Aspartate aminotransferase increase | 1 | |||
| Alkaline phosphatase increase | 1 | |||
| Bilirubin increase | 2 | 1 | ||
| Gamma glutamyl transferase increase | 1 | |||
| Arthralgia | 1 | 1 | ||
| Colonic perforation | 1 | |||
| Colitis | 1 | |||
| Dehydration | 1 | |||
| Fatigue | 1 | 1 | ||
| Headache | 1 | |||
| Hypertension | 1 | |||
| Hypokalemia | 1 | |||
| Hyponatremia | 1 | |||
| Seizure | 3 | 3 | ||
| Nausea | 1 | |||
| Lung infection | 1 | |||
| Peripheral nerve infection | 1 | |||
| Urinary tract infection | 1 | |||
| Vascular access complication | 1 | 1 | ||
| Leukopenia | 1 | 1 | ||
| Lymphopenia | 3 | 4 | ||
| Neutropenia | 2 | 2 | ||
| Thrombocytopenia | 2 | 2 | ||
| Investigations (other) | 1 |
Table 3B. Grade 3 and 4 toxicities attributed to aflibercept and temozolomide for Arm 2.
| Adverse Event | Aflibercept DL0 | Aflibercept DL1 | Temozolomide (DL0) | Temozolomide (DL1) |
|---|---|---|---|---|
| Biliary anastomotic leak | 1 | |||
| Aspartate aminotransferase increase | 1 | |||
| Cholecystitis | 1 | 1 | ||
| Diarrhea | 1 | |||
| Fatigue | 1 | 1 | ||
| Dysphasia | 1 | |||
| Hypertension | 5 | 2 | ||
| Hyponatremia | 1 | 1 | ||
| Palmar-plantar erythrodysesthesia syndrome | 1 | |||
| Thromboembolic event | 1 | |||
| Leukopenia | 2 | 2 | ||
| Lymphopenia | 4 | 4 | ||
| Neutropenia | 2 | 1 | 2 | |
| Thrombocytopenia | 2 | 3 |
Table 3C. Grade 3 and 4 toxicities attributed to aflibercept and temozolomide for Arm 3.
| Adverse Event | Aflibercept DL0 | Aflibercept DL1 | Temozolomide (DL0) | Temozolomide (DL1) |
|---|---|---|---|---|
| Aspartate aminotransferase increase | 1 | 1 | ||
| Acute kidney injury | 1 | |||
| Creatinine increase | 1 | |||
| Anorexia | 1 | 1 | ||
| Fatigue | 3 | 3 | ||
| Headache | 1 | 1 | ||
| Hypertension | 2 | |||
| Vagus nerve disorder | 1 | |||
| Pain in extremity | 1 | 1 | ||
| Palmar-plantar erythrodysesthesia syndrome | 2 | 2 | ||
| Thromboembolic event | 3 | |||
| Leukopenia | 2 | 3 | ||
| Lymphopenia | 1 | 4 | 1 | 7 |
| Neutropenia | 2 | 3 | ||
| Thrombocytopenia | 1 | 1 | ||
| Investigations (other) | 1 |
Outcomes
The median number of cycles of aflibercept for arms 1, 2 and 3 were 5, 4 and 5.5, respectively (Table 1). All patients stopped treatment on protocol, of whom 28 (47%) came off for disease progression and 21 (36%) for adverse events. Five patients (8%) withdrew consent and 3 other patients (5%) came off based on treating physician's discretion. Two patients completed treatment as specified in protocol (completion of 12 cycles) without progression or adverse events.
Neurocognitive Outcomes
The number of patients in Arm 1 that completed neurocognitive testing at each time point was 12 (baseline), 9 (week 11), 5 (week 19), 4 (week 27), 4 (week 35), 3 (week 43), 3 (week 51), and 3 (off study). At baseline, the majority of patients were performing in the impaired range based on the CTB COMP. The frequency and severity of impairment was highest on measures of learning and memory (HVLT-R TR, HVLT-R DR) and executive function (TMTB) (Table 4). Cognitive decline from baseline to week 11 based on the CTB COMP occurred in 44% of patients with relatively equal frequency across all CTB tests except the COWA; more severe decline was evident on tests of memory (HVLT-R DR) and executive function (TMTB) (Table 5). We included in Table 5 cognitive decline as demonstrated in a phase III randomized Radiation Therapy Oncology Group (RTOG) trial, RTOG 0525, which evaluated the efficacy of dose-dense temozolomide in newly diagnosed glioblastoma patients[10, 16].
Table 4. Baseline Clinical Trial Battery (CTB) Test Scores.
| Mean (SD) | Median | Range | % Impaired* | |
|---|---|---|---|---|
| CTB COMP (n=12) | -1.63 (1.91) | -1.12 | -4.65, 1.00 | 75 |
| Learning and Memory | ||||
| HVLT-R TR (n=12) | -1.54 (1.32) | -1.51 | -4.16, 0.58 | 50 |
| HVLT-R DR (n=12) (n=11) | -1.92 (1.80) | -1.94 | -4.29, 1.22 | 64 |
| HVLT-R R (n=12) | -0.52 (1.28) | -0.57 | -2.91, 0.86 | 25 |
| Processing Speed | ||||
| TMTA (n=12) | -1.72 (5.14) | 0.08 | -14.75, 2.20 | 25 |
| Executive Function | ||||
| TMTB (n=12) | -3.41 (-0.74) | -0.74 | -13.13, 1.00 | 42 |
| COWA (n=12) | -0.70 (1.09) | -0.87 | -2.28, 1.29 | 25 |
CTB COMP: Clinical Trial Battery Composite, HVLT-R : Hopkins Verbal Learning Test- Revised, TMT : Trail Making Test, COWA : Controlled Oral Word Association
Impairment was defined as a score ≤ -1.5 for all CTB tests and as a score ≤ -0.70 for the CTB COMP
Table 5. Clinical Trial Battery (CTB) Test Change Scores from Baseline to Week 11 and Comparison with RTOG 0525.
| Mean (SD) | Median | Range | % Decline* | % Decline RTOG 0525**[16] | |
|---|---|---|---|---|---|
| CTB COMP (n=9) | -0.71 (1.70) | -0.40 | -3.86, 2.11 | 44 | 29 |
| Learning and Memory | |||||
| HVLT-R TR (n=9) | -0.79 (1.13) | -0.23 | -2.33, 0.53 | 44 | 28 |
| HVLT-R DR (n=8) | -0.97 (1.65) | -1.66 | -3.53, 1.29 | 63 | 22 |
| HVLT-R R (n=9) | -0.78 (1.89) | 0.0 | -4.54, 1.82 | 33 | 22 |
| Processing Speed | |||||
| TMTA (n=9) | -0.13 (4.53) | -0.70 | -4.73, 11.06 | 44 | 24 |
| Executive Function | |||||
| TMTB (n=9) | -1.84 (6.03) | -1.39 | -16.64, 5.54 | 44 | 28 |
| COWA (n=9) | 0.34 (0.60) | 0.54 | -0.32, 1.25 | 0 | 12 |
Decline was defined by the reliable change index (RCI) for all CTB tests and as worsening in the CTB COMP z-score of ≥ 0.50
Change from Baseline to Prior to Cycle 4 average across both arms
Discussion
This phase I study evaluated the combination of aflibercept with radiation therapy and temozolomide in patients with HGG. This trial met its primary endpoint of safety and the recommended phase II dose of aflibercept with radiation and temozolomide was deemed to be 4mg/kg every 2 weeks. However, by the time of trial completion, results from the phase II trial of aflibercept in recurrent HGG patients became available and did not show a meaningful clinical benefit with overall response rate of 18% and a six month progression-free survival rate (PFS6) of 7.7%[7]. Moreover, in that trial 24% of the patients came off study for toxicity, which may have also contributed to the relatively poor PFS. Although many patients progressed while on study treatment in the current trial, a relatively large number (36%) of the patients stopped treatment for side-effects. DLTs were observed in a total of 5 patients and the majority of serious (≥ grade 3) toxicities were seen at MTD. Some of the common grade 1-2 toxicities attributable to aflibercept at MTD that may have contributed to intolerance were fatigue, anorexia, constipation, mucositis, headache, hypertension, dysarthria, elevated transaminases, leukopenia and thrombocytopenia. While hypertension and headaches were common, intracranial hemorrhage and stroke were not noted in this population.
Baseline neurocognitive testing was completed in 12 out of the 21 patients enrolled in arm 1 and scores were compared from baseline to week 11 for 9 patients. In general, the scores were low at baseline, likely related to the impact of tumor, and deteriorated further at 11 weeks in 44% of the patients. When compared to the control arm of RTOG 0525 that did not receive anti-angiogenic therapy there was numerically more frequent cognitive decline in patients treated with aflibercept across 6 out 7 cognitive outcomes[16]. Given the small sample size and issues of comparing outcomes between 2 separate trials, we did not analyze further for statistical significance. The compliance for neurocognitive testing was poor and this highlights the challenges including long duration of the tests and dropouts that are often encountered in such assessments in patients with brain tumors[17, 18].
As this was a phase I study, patients were not followed for survival after discontinuation from study. Patients completed a median number of 5 cycles of study treatment which implies that there was likely limited benefit from addition of aflibercept to radiation therapy and temozolomide, although this study was not designed to assess efficacy and response.
Hypoxia mediated by VEGFR inhibition leading to PlGF upregulation is thought to be one of the mechanisms of resistance to angiogenesis inhibitors. PlGF recruits bone marrow derived cells which then release proangiogenic factors in to the tumor microenvironment. Based on these considerations, dual targeting of VEGF and PlGF in addition to potent VEGF inhibition by aflibercept seems attractive. However, this did not appear to provide more benefit over VEGF inhibition alone with bevacizumab in the phase II trial in recurrent HGG. Additionally, de Groot and colleagues demonstrated that while VEGF levels decreased significantly after treatment with aflibercept, PlGF levels increased after an initial decrease which suggests that continuous anti-VEGF treatment induces PlGF expression[19]. In a phase I study of RO5323441 (anti-PlGF antibody) in combination with bevacizumab in patients with recurrent glioblastoma, there was no improvement in response rate or survival compared to single-agent bevacizumab and there was no association between baseline PlGF levels and response.[20] It is possible that PlGF inhibition may not be beneficial or that higher doses of aflibercept may be required to block VEGF in the setting of increasing PlGF levels.
In summary, the MTD of aflibercept in combination with radiation therapy and concomitant temozolomide, and with adjuvant temozolomide is 4mg/kg every 2 weeks. The therapy was only moderately-well tolerated with many patients coming off study for toxicities. A subsequent efficacy study in newly diagnosed glioblastoma was not pursued due to the moderate toxicity and lack of efficacy in recurrent disease. The results of 2 randomized phase III trials demonstrated lack of advantage with the 21/28 days adjuvant arm of temozolomide, and this schedule was not pursued further [10, 11].
Acknowledgments
This study was supported by Grant No. UM1 CA137443 from the Adult Brain Tumor Consortium.
Footnotes
Conflict of Interest/Disclosures: LN, JdG, FL, JD, MG, KA, JF, XY, AC, MP: None related to the current study.
JW: Advisory board for AbbVie, Angiochem, Bayer, Genetech, Juno, Novocure, Roche.
TC: Advisory board for Roche/Genentech, Novartis, Pfizer, Novocure, Celldex, Agios, VBL, Tocagen, Notable labs, Upshire Smith, Cytrx, Cortice, Pronia, Medqia, Oxigene, Nektar, Newgen, AbbVie
AO: Advisory board for Bristol Meyer Squibb, Oxigene, Stemline
LMD: Advisory board for Sapience, Juno Therapeutics
AWKY: Advisory board for DNAtrix
SC: Research support from Agios, Quest, Roche and Novartis; Advisory Board for Agios, Tocagen, NeOnc, Egde Therapeutics, Blaze
TTB: Advisory board for Merck, Proximagen/Upsher, Roche, Oxigene, Cavion, Foundation Medicine, Accerta, Champions Biotechnology. CME Lectures for Up to Date, Oakstone Medical Publishing, Imedex. Research support from Pfizer.
SG: Research support from Erimos, Advisory board for Midatech, Stock/other in Axxia, Honararia from Roche/Genentech, Medimmune.
PW: Research Support from Agios, Angiochem, Astra Zeneca, Exelixis, Genentech/Roche, GlaxoSmith Kline, Karyopharm, Novartis, Sanofi-Aventis, Regeneron Pharmaceuticals Inc., Vascular Biogenics; Advisory Board for AbbVie, Cavion, Celldex, Genentech/Roche, Midatech, Momenta, Novartis, Novocure, SigmaTau, Vascular Biogenics; Speakers Bureau for Merck.
References
- 1.Chi AS, Sorensen AG, Jain RK, Batchelor TT. Angiogenesis as a therapeutic target in malignant gliomas. The oncologist. 2009;14:621–636. doi: 10.1634/theoncologist.2008-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Mehta MP., Jr A randomized trial of bevacizumab for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 6.Arrillaga-Romany I, Reardon DA, Wen PY. Current status of antiangiogenic therapies for glioblastomas. Expert opinion on investigational drugs. 2014;23:199–210. doi: 10.1517/13543784.2014.856880. [DOI] [PubMed] [Google Scholar]
- 7.de Groot JF, Lamborn KR, Chang SM, Gilbert MR, Cloughesy TF, Aldape K, Yao J, Jackson EF, Lieberman F, Robins HI, Mehta MP, Lassman AB, Deangelis LM, Yung WK, Chen A, Prados MD, Wen PY. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2689–2695. doi: 10.1200/JCO.2010.34.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Wachsberger PR, Burd R, Cardi C, Thakur M, Daskalakis C, Holash J, Yancopoulos GD, Dicker AP. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. International journal of radiation oncology, biology, physics. 2007;67:1526–1537. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ, Mehta MP., Jr Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brada M, Stenning S, Gabe R, Thompson LC, Levy D, Rampling R, Erridge S, Saran F, Gattamaneni R, Hopkins K, Beall S, Collins VP, Lee SM. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4601–4608. doi: 10.1200/JCO.2009.27.1932. [DOI] [PubMed] [Google Scholar]
- 12.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 13.Benedict RHB, Schretien D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 14.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 1996;11:329–338. [PubMed] [Google Scholar]
- 15.Levine AJ, Miller EN, Becker JT, Selnes OA, Cohen BA. Normative data for determining significance of test-retest differences on eight common neuropsychological instruments. Clin Neuropsychol. 2004;18:373–384. doi: 10.1080/1385404049052420. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong TS, Wefel JS, Wang M, Gilbert MR, Won M, Bottomley A, Mendoza TR, Coens C, Werner-Wasik M, Brachman DG, Choucair AK, Mehta M. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4076–4084. doi: 10.1200/JCO.2013.49.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin NU, Wefel JS, Lee EQ, Schiff D, van den Bent MJ, Soffietti R, Suh JH, Vogelbaum MA, Mehta MP, Dancey J, Linskey ME, Camidge DR, Aoyama H, Brown PD, Chang SM, Kalkanis SN, Barani IJ, Baumert BG, Gaspar LE, Hodi FS, Macdonald DR, Wen PY. Response Assessment in Neuro-Oncology g (2013) Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. The Lancet Oncology. 14:e407–416. doi: 10.1016/S1470-2045(13)70308-5. [DOI] [PubMed] [Google Scholar]
- 18.van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJ, Jaeckle K, Junck L, Armstrong T, Choucair A, Waldman AD, Gorlia T, Chamberlain M, Baumert BG, Vogelbaum MA, Macdonald DR, Reardon DA, Wen PY, Chang SM, Jacobs AH. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. The Lancet Oncology. 2011;12:583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 19.de Groot JF, Piao Y, Tran H, Gilbert M, Wu HK, Liu J, Bekele BN, Cloughesy T, Mehta M, Robins HI, Lassman A, DeAngelis L, Camphausen K, Chen A, Yung WK, Prados M, Wen PY, Heymach JV. Myeloid biomarkers associated with glioblastoma response to anti-VEGF therapy with aflibercept. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4872–4881. doi: 10.1158/1078-0432.CCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassen U, Chinot OL, McBain C, Mau-Sorensen M, Larsen VA, Barrie M, Roth P, Krieter O, Wang K, Habben K, Tessier J, Lahr A, Weller M. Phase 1 dose-escalation study of the antiplacental growth factor monoclonal antibody RO5323441 combined with bevacizumab in patients with recurrent glioblastoma. Neuro-oncology. 2015;17:1007–1015. doi: 10.1093/neuonc/nov019. [DOI] [PMC free article] [PubMed] [Google Scholar]