Abstract
Introduction
The best methods for inducing analgesia and sedation for gastroscopy are still debated but finding an adequate regimen of sedation/analgesia is important. Stimulation of the larynx under sedation can cause reflex responses. Propofol with opioids has been recommended for gastroscopy sedation but the effects on cough reflex suppression remain unclear. This trial will evaluate the effects of propofol combined with small doses of dezocine, oxycodone, sufentanil or fentanyl for gastroscopy. We hypothesise that better performance may be obtained with a combination of propofol and oxycodone. We will observe the incidence and degree of reflex coughing and gagging under sedation when using propofol combined with one of the above drugs or propofol alone.
Methods and analysis
This will be a prospective, randomised, double-blind, controlled trial. ASA I–II level patients aged 18–65 years and scheduled for gastroscopy will be included. It is planned that 500 subjects will be randomised to intravenously receive 2–2.2 mg/kg propofol plus 0.5–0.8 μg/kg fentanyl (fentanyl group), 2–2.2 mg/kg propofol plus 0.05–0.08 μg/kg sufentanil (sufentanil group), 2–2.2 mg/kg propofol plus 0.04–0.05 mg/kg dezocine (dezocine group), 2–2.2 mg/kg propofol plus 0.04–0.05 mg/kg oxycodone (oxycodone group), or 2.4–3 mg/kg propofol plus 2–2.5 mL saline (control group) for sedation. The primary endpoint is the incidence and degree of reflex coughing and gagging. The secondary endpoints include the occurrence of discomfort or side effects, the use of jaw thrust, assisted ventilation or additional propofol, recovery time, duration of procedure and Steward score.
Ethics and dissemination
This study has been approved by the Institutional Ethics Committee for Clinical Research of Zhongda Hospital, Affiliated to Southeast University (No. 2015ZDSYLL033.0). The results of the trial will be published in an international peer-reviewed journal.
Trial registration
This study has been registered with the Chinese Clinical Trial Register (No. ChiCTR-ICR-15006952).
Trial status
At the time of manuscript submission, the study was in the recruitment phase.
Keywords: sedation, endoscopy, propofol, opioids, cough
Strengths and limitations of this study.
This study is the first randomised controlled trial investigating the effect of dezocine and oxycodone on cough reflex suppression when combined with propofol during gastroscopy.
This study focuses on the antitussive effects of a range of opioids which few previous studies have examined.
Study strengths include an appropriate sample size, stratified randomization and the double-blind placebo-controlled design.
This is a single-centre trial, which could be a limitation of this study.
The dose of propofol for this study could be a limitation, as it is relatively high and might abolish the effects of the opioids examined.
Introduction
Gastroscopy is an important and common endoscopic method for the diagnosis and treatment of digestive disease. However, during gastroscopy, patient anxiety and discomfort such as throat irritation, cough and nausea may result in low examination quality and consequently decreased patient willingness to undergo a repeat procedure. Pharyngeal anaesthesia and sedation/anaesthesia ranging from minimal sedation to general anaesthesia have been used to relieve anxiety and discomfort, resulting in a successful procedure.1–5
Endoscopic sedation is widely used in routine practice, with propofol sedation beingendorsed.5–12Propofol is an intravenously administered sedative with a rapid onset and short duration of action.13 It has a favourable sedative effect and a wide range of inhibition effects on the central nervous system.14 It also strongly inhibits the contraction of gastrointestinal smooth muscle, antagonises the vomiting reflex and reduces cough and physical movement.5 15 16 Postoperative headache occurs infrequently and in addition propofol reduces nausea and vomiting, so their incidence is also low.17 Therefore, propofol has been widely used for sedation for gastroscopic procedures. Propofol is often used as a single agent but has a short duration of clinical effect. Inadequate sedation with propofol alone occurs sometimes and requires additional doses. The duration of gastroscopy is relatively short, usually lasting for about 10 min, but repeated addition of propofol can significantly prolong the recovery time, increasing the risk of post-procedure respiratory depression and hypoxaemia, and the workload of recovery management. The use of propofol in combination with opioids has been proposed to improve sedation and analgesia regarding recovery time, sedative effect, pain and discomfort.18–22
Cough is a defensive airway reflex. The cough receptors in the epithelium are sensitive to both mechanical and chemical stimuli. Sedatives and analgesics have inhibitory effects on airway reflexes, but propofol may still cause cough.23 24 Moreover, stimulation of the larynx during propofol anaesthesia can cause various types of reflex responses.25 Although opioids exert favourable analgesic and sedative effects,20 26 and can inhibit the pharyngeal reflex and stress response, intravenous fentanyl- and sufentanil-induced coughing is not uncommon.27 28 Induced cough can be reduced by propofol29 and interestingly, by another opioid, dezocine.30 Dezocine is an opioid analgesic acting on μ-, δ- and κ-opioid receptors and has been used in propofol sedation.31–33 Oxycodone is an opioid alkaloid acting on μ- and κ-opioid receptors. It shows good performance for relieving visceral pain with slight respiratory depression and is known to depress the cough reflex. Unlike fentanyl or sufentanil, dezocine and oxycodone have rarely been studied in combination with propofol for gastroscopy.
The best methods to induce analgesia and sedation for gastroscopy are still debated, so finding an adequate regimen of sedation/analgesia is important as it can influence the quality of the examination, patient cooperation, and the patient’s and physician’s satisfaction with the sedation.5 16 34 Based on our preliminary observations in clinical practice, we hypothesise that a combination of propofol and low-dose dezocine, oxycodone or sufentanil for gastroscopy may decrease the incidence of cough and that specifically, the combination of propofol and oxycodone may show the best performance.
In order to verify our hypothesis, we designed this clinical study, aiming to investigate the effect of a combination of propofol and opioids on cough reflex suppression in gastroscopy.
Methods and analysis
Study objective
The primary objective of this study is to investigate the incidence and degree of reflex coughing and gagging under sedation with a combination of propofol and fentanyl, sufentanil, dezocine or oxycodone during gastroscopy. The secondary objective is to assess the effect of the combination regimens on sedative performance.
Study location
A prospective, single-centre, randomised, double-blinded, controlled trial will be conducted in patients undergoing gastroscopy in the Affiliated Zhongda Hospital of Southeast University, China.
Study population
Participants will be recruited voluntarily according to the inclusion and exclusion criteria below.
Inclusion criteria
ASA I–II level patients scheduled for gastroscopy, aged 18–65 years, and willing to participate after reading and signing an informed consent form.
Exclusion criteria
Body mass index (BMI) ≥30 kg/m2
Patients with preoperative circulatory, respiratory or nervous system disease
Preoperative haemoglobin level less than 70 g/L or albumin level less than 30 g/L
Patients with sleep apnoea
Patients with upper respiratory tract infection symptoms
Patients with a history of dry cough
Patients with a drug allergy.
Randomization and blinding
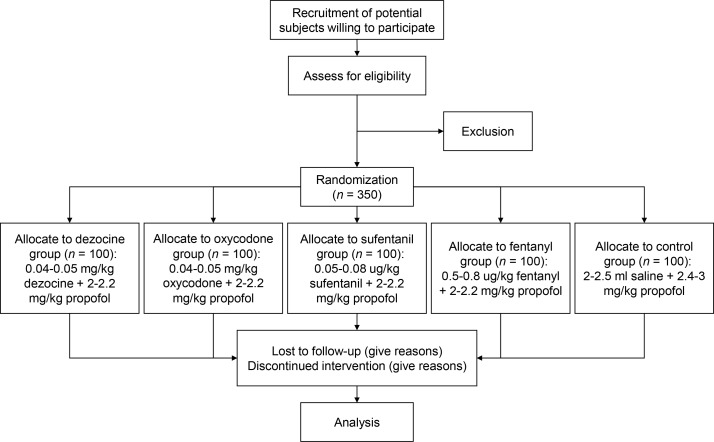
Stratified randomization will be used to assign the candidate subjects to five groups (figure 1) according to sex and BMI (two groups). Computer-generated random group numbers will be printed and placed in separate sealed envelopes. Whenreceiving a subject who meets the inclusion criteria, the anaesthesiologist will assign the newly recruited subject to a group according to the number in the envelope. Both anaesthesiologists and patients will be blinded to the regimen. The drugs will be prepared by nurse anaesthetists, labelled with numbers and then injected by the anaesthesiologist. The nurse anaesthetists will be responsible for recording patients' physiological characteristic . The anaesthesiologist will be notified of the study group by the nurse anaesthetist in case of emergency.
Figure 1.
Flow chart of the study.
Current sample size justification
It is planned that a total of 500 subjects will be recruited. The subjects will be randomly divided into five groups: a dezocine group, fentanyl group, oxycodone group, sufentanil group and control group, with 100 cases in each group. The sample size was estimated according to the χ2 test of the incidence of the primary categorical outcome, incidence of cough (30%) within 5 min after endoscope insertion. The number of observations will allow detection of a small to moderate effective size (approximately 0.20) with a 5% chance of a type I error and 90% power. The test of power will remain at 80% or higher if up to 20% of subjects drop out from the study.
Statistical analysis
All data will be analysed using SAS 9.3 or other statistical software packages as needed. The statistical methods will include descriptive statistics, the t-test, χ2 test, analysis of variance, univariate unconditional logistic regression analysis and multivariate linear regression analysis. The significance level will be set at 5%.
Sedation
Patients will fast for 12 hours before gastroscopy. Dyclonine will be administered orally 10 min before sedation. Right upper extremity venous access will be established before the patients enter the operating room. In the operating theatre, the patients will lie in the left-lateral position, with a blood pressure cuff fitted around the left upper arm, and receive oxygen via a nasal cannula (3–5 L/min). After placement of a bite block, blood pressure, heart rate and SpO2 will be measured non-invasively (Philips MP50, Germany) to establish baselines and will be monitored throughout the procedure. Blood pressure will be measured at 1 min intervals. Patients will then intravenously receive 2–2.2 mg/kg propofol (Fresenius Kabi AB, Germany) plus 0.5–0.8 μg/kg fentanyl (Humanwell Pharmaceutical, China), 2–2.2 mg/kg propofol plus 0.05–0.08 μg/kg sufentanil (Humanwell Pharmaceutical), 2–2.2 mg/kg propofol plus 0.04–0.05 mg/kg dezocine (Yangtze River Pharmaceutical, China), 2–2.2 mg/kg propofol plus 0.04–0.05 mg/kg oxycodone (Monti Pharmaceutical, China), or 2.4–3 mg/kg propofol plus 2–2.5 mL saline (figure 1). All drugs except propofol will be diluted with saline. The doses of dezocine and oxycodone have been defined according to our preliminary study. Subjects weighing ≤70 kg will be administered 2 mL of normal saline, while subjects weighing >70 kg will receive 2.5 mL of normal saline. Fentanyl will be diluted to 20 μg/mL, sufentanil to 2.5 μg/mL, and dezocine and oxycodone to 1 mg/mL. Drugs will be delivered by the Aespire 7900 anaesthesia delivery system (GE Healthcare, USA). We will insert the probe when the bispectral index (BIS) reaches 40–60. A BIS of 40–60 is usually considered to indicate sufficient depth of general anaesthesia. The jaw thrust manoeuvre will be performed in case of respiratory depression and oxygen desaturation. If this is not effective, assisted ventilation will be used. In severe situations, tracheal intubation assisted respiration will performed. After gastroscopy, all patients will remain in the post-anaesthesia care unit (PACU) and will be followed up until discharge from the PACU.
Adverse events
All adverse events, such as nausea, vomiting, dyspnoea, hypopnoea, apnoea, hypotension, oxygen desaturation and bradycardia, will be recorded and closely monitored. The medical strategy will be adjusted if necessary. Unexpected severe adverse events will be reported to the ethics committee.
Data collection and management
Demographic variables and clinical data will be collected from all patients. Furthermore, during the procedure, blood pressure, heart rate and oxygen saturation will be monitored. Any swallowing, coughing or gagging, physical movements, adverse events, jaw thrust manoeuvre, assisted ventilation, additional propofol use and duration of endoscopy will be recorded. Theduration of calls for eyes to open and the Steward score after eyes open will also be recorded. Data will be collected throughout the study and will be securely managed under conditions of confidentiality. Automatic data collection will be performed by the vital signs monitor and anaesthesia information system. Manual data collection will be performed by a nurse anaesthetist. The participants will be referred to by their participant number rather than their name throughout the study unless otherwise specified. All relevant documents and files will be archived for 5 years. Data can be only accessed by the investigators who sign the confidential disclosure agreement and by institutional or governmental auditors during the study. Data without patient identifiers will be publicly accessible after the study. The process will be monitored by the Institutional Ethics Committee (ICE) for Clinical Research of Zhongda Hospital.
Endpoints
The primary endpoint is the incidence and degree of reflex coughing and gagging, which will be recorded within 5 min after endoscope insertion and throughout the procedure. The severity of cough is defined according to cough intensity and whether it causes failure of endoscope insertion. The secondary endpoints include (1) the occurrence of swallowing, physical movement and adverse events, and whether the jaw thrust manoeuvre, assisted ventilation or additional propofol is used, which will be recorded within 5 min after endoscope insertion and throughout the procedure, and (2) duration of calls for eyes to open, the duration of the procedure and the Steward score35 after eyes open. Blood pressure, heart rate and SpO2 will be monitored throughout the procedure. ‘Patient satisfaction’ is not included as a secondary outcome since patients can not recall coughing or gagging.
Protocol amendments
The current protocol is version 1.5 (11 June 2017). Any changes in the protocol during the trial that may affect the conduct of the trial, the safety and the benefit to patients will require a formal amendment to the protocol.
Discussion
It is important to improve analgesia and sedation for gastroscopy. Opioids, which exert a favourable analgesic and sedative effect and inhibit the stress response, are an important part of surgical anaesthesia. A combination of opioids and propofol is the most commonly used regimen for general anaesthesia. Currently, a small dose of fentanyl combined with propofol is used for sedation for gastroscopy, but fentanyl may cause respiratory depression, choking and stiff chest wall muscles. Clinical studies of other opioids for gastroscopy, such as oxycodone, dezocine and sufentanil, are rare. As it is essential to find an adequate regimen, we will conduct a trial to evaluate the combination of small doses of dezocine, oxycodone or sufentanil with propofol for gastroscopy. This study will observe the incidence and degree of reflex coughing under sedation. However, the study has some limitations. It is a single-centre study, which may limit its generalisability so a future multiple-centre large-sample size study will be needed. Also the dose of propofol for this study is relatively high and may abolish the effects of the examined opioids. The result of this clinical trial may confirm the favourable effects of a combination of propofol with small-dose opioids, and may identify a satisfactory regimen for sedation for gastroscopy.
Supplementary Material
Footnotes
Contributors: NY conceived of the study. NY and JXia participated in its design and coordination. NY, JX, Y-ZC, XL, JY and JX collected references and developed the protocol. XL, Y-ZC, JY and JX performed statistics analysis. NY and JXia drafted the manuscript. All authors read and approved the final manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not–for–profit sectors.
Competing interests: The authors declare that they have no competing interests. The committee mentioned is independent from the sponsor and competing interests.
Ethics approval: This study has been approved by the Institutional Ethics Committee for Clinical Research of Zhongda Hospital, Affiliated to Southeast University (No. 2015ZDSYLL033.0) and is registered with the Chinese Clinical Trial Registry (ChiCTR–ICR–15006952). Only patients who give written informed consent will be recruited. An informed consent form has been provided. The results of the trial will be published in an international peer–reviewed journal.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Not applicable.
References
- 1. Lichtenstein DR, Jagannath S, Baron TH, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc 2008;68:815–26. 10.1016/j.gie.2008.09.029 [DOI] [PubMed] [Google Scholar]
- 2. Soma Y, Saito H, Kishibe T, et al. Evaluation of topical pharyngeal anesthesia for upper endoscopy including factors associated with patient tolerance. Gastrointest Endosc 2001;53:14–18. 10.1067/mge.2001.111773 [DOI] [PubMed] [Google Scholar]
- 3. Froehlich F, Schwizer W, Thorens J, et al. Conscious sedation for gastroscopy: patient tolerance and cardiorespiratory parameters. Gastroenterology 1995;108:697–704. 10.1016/0016-5085(95)90441-7 [DOI] [PubMed] [Google Scholar]
- 4. Evans LT, Saberi S, Kim HM, et al. Pharyngeal anesthesia during sedated EGDs: is "the spray" beneficial? A meta-analysis and systematic review. Gastrointest Endosc 2006;63:761–6. 10.1016/j.gie.2005.11.059 [DOI] [PubMed] [Google Scholar]
- 5. Triantafillidis JK, Merikas E, Nikolakis D, et al. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol 2013;19:463–81. 10.3748/wjg.v19.i4.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Porostocky P, Chiba N, Colacino P, et al. A survey of sedation practices for colonoscopy in Canada. Can J Gastroenterol 2011;25:255–60. 10.1155/2011/783706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fanti L, Agostoni M, Gemma M, et al. Sedation and monitoring for gastrointestinal endoscopy: a nationwide web survey in Italy. Dig Liver Dis 2011;43:726–30. 10.1016/j.dld.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 8. Paspatis GA, Manolaraki MM, Tribonias G, et al. Endoscopic sedation in Greece: results from a nationwide survey for the Hellenic Foundation of gastroenterology and nutrition. Dig Liver Dis 2009;41:807–11. 10.1016/j.dld.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 9. Cohen LB, Wecsler JS, Gaetano JN, et al. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol 2006;101:967–74. 10.1111/j.1572-0241.2006.00500.x [DOI] [PubMed] [Google Scholar]
- 10. Riphaus A, Rabofski M, Wehrmann T. Endoscopic sedation and monitoring practice in Germany: results from the first nationwide survey. Z Gastroenterol 2010;48:392–7. 10.1055/s-0028-1109765 [DOI] [PubMed] [Google Scholar]
- 11. Baudet JS, Borque P, Borja E, et al. Use of sedation in gastrointestinal endoscopy: a nationwide survey in Spain. Eur J Gastroenterol Hepatol 2009;21:882–8. 10.1097/MEG.0b013e328314b7ca [DOI] [PubMed] [Google Scholar]
- 12. Wehrmann T, Triantafyllou K. Propofol sedation in gastrointestinal endoscopy: a gastroenterologist's perspective. Digestion 2010;82:106–9. 10.1159/000285554 [DOI] [PubMed] [Google Scholar]
- 13. Byrne MF, Chiba N, Singh H, et al. Propofol use for sedation during endoscopy in adults: a Canadian Association of Gastroenterology position statement. Can J Gastroenterol 2008;22:457–9. 10.1155/2008/268320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inada T, Murao K, Shingu K, et al. Effects of propofol and thiopental on the central nervous system during nociceptive stimulation in cats. J Anesth 2001;15:159–63. 10.1007/s005400170019 [DOI] [PubMed] [Google Scholar]
- 15. Lee TL, Ang SB, Dambisya YM, et al. The effect of propofol on human gastric and colonic muscle contractions. Anesth Analg 1999;89:1246–9. 10.1213/00000539-199911000-00031 [DOI] [PubMed] [Google Scholar]
- 16. Fanti L, Testoni PA. Sedation and analgesia in gastrointestinal endoscopy: what's new? World J Gastroenterol 2010;16:2451–7. 10.3748/wjg.v16.i20.2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen LB, Delegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology 2007;133:675–701. 10.1053/j.gastro.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 18. Ho WM, Yen CM, Lan CH, et al. Comparison between the recovery time of alfentanil and fentanyl in balanced propofol sedation for gastrointestinal and colonoscopy: a prospective, randomized study. BMC Gastroenterol 2012;12:164. 10.1186/1471-230X-12-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poulos JE, Kalogerinis PT, Caudle JN. Propofol compared with combination propofol or midazolam/fentanyl for endoscopy in a community setting. Aana J 2013;81:31–6. [PubMed] [Google Scholar]
- 20. Zhang L, Bao Y, Shi D. Comparing the pain of propofol via different combinations of fentanyl, sufentanil or remifentanil in gastrointestinal endoscopy. Acta Cir Bras 2014;29:675–80. 10.1590/S0102-8650201400160008 [DOI] [PubMed] [Google Scholar]
- 21. Xu ZY, Wang X, Si YY, Zy X, Yy S, et al. Intravenous remifentanil and propofol for gastroscopy. J Clin Anesth 2008;20:352–5. 10.1016/j.jclinane.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 22. LaPierre CD, Johnson KB, Randall BR, et al. A simulation study of common propofol and propofol-opioid dosing regimens for upper endoscopy: implications on the time course of recovery. Anesthesiology 2012;117:252–62. 10.1097/ALN.0b013e31825fb1b2 [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto S, Motokawa K, Toyooka H. Propofol may cause coughing when used as a sedative in patients undergoing orthopedic surgery under spinal anesthesia. Anesth Analg 1998;86:529S. 10.1097/00000539-199802001-00527 [DOI] [Google Scholar]
- 24. Mitra S, Sinha PK, Anand LK, et al. Propofol-induced violent coughing. Anaesthesia 2000;55:707–8. 10.1046/j.1365-2044.2000.01557-22x./ [DOI] [PubMed] [Google Scholar]
- 25. Tagaito Y, Isono S, Nishino T. Upper airway reflexes during a combination of propofol and fentanyl anesthesia. Anesthesiology 1998;88:1459–66. 10.1097/00000542-199806000-00007 [DOI] [PubMed] [Google Scholar]
- 26. Koshy G, Nair S, Norkus EP, et al. Propofol versus midazolam and meperidine for conscious sedation in GI endoscopy. Am J Gastroenterol 2000;95:1476–9. 10.1111/j.1572-0241.2000.02080.x [DOI] [PubMed] [Google Scholar]
- 27. Yemen TA. Small doses of sufentanil will produce violent coughing in young children. Anesthesiology 1998;89:271–2. 10.1097/00000542-199807000-00043 [DOI] [PubMed] [Google Scholar]
- 28. Böhrer H, Fleischer F, Werning P. Tussive effect of a fentanyl bolus administered through a central venous catheter. Anaesthesia 1990;45:18–21. 10.1111/j.1365-2044.1990.tb14496.x [DOI] [PubMed] [Google Scholar]
- 29. Sedighinejad A, Naderi Nabi B, Haghighi M, et al. Propofol is effective to depress fentanyl-induced cough during induction of anesthesia. Anesth Pain Med 2013;2:170–3. 10.5812/aapm.8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu XS, Xu GH, Shen QY, et al. Dezocine prevents sufentanil-induced cough during general anesthesia induction: a randomized controlled trial. Pharmacol Rep 2015;67:52–5. 10.1016/j.pharep.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 31. Ramirez-Ruiz M, Smith I, White PF. Use of analgesics during propofol sedation: a comparison of ketorolac, dezocine, and fentanyl. J Clin Anesth 1995;7:481–5. 10.1016/0952-8180(95)00058-P [DOI] [PubMed] [Google Scholar]
- 32. Lu Y, Ye Z, Wong GT, et al. Prevention of injection pain due to propofol by dezocine: a comparison with lidocaine. Indian J Pharmacol 2013;45:619–21. 10.4103/0253-7613.121376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng M, Guo Y, Shan S, et al. Dezocine for anesthesia and stress reduction in induced abortion. Patient Prefer Adherence 2015;9:369–72. 10.2147/PPA.S76507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bell GD. Preparation, premedication, and surveillance. Endoscopy 2004;36:23–31. 10.1055/s-2004-814117 [DOI] [PubMed] [Google Scholar]
- 35. Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J 1975;22:111–3. 10.1007/BF03004827 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.