Abstract
We describe a case of a 23-year-old woman with a history of Crohn's disease (CD), who initially presented with sepsis-like symptoms, subsequently developed severe cholestasis and following extensive inpatient workup was found to have non-caseating granulomas on her liver biopsy. Infectious aetiologies were excluded and the patient was treated with oral corticosteroids, which ameliorated but did not completely reverse the cholestasis. We review the differential diagnosis of hepatic granulomas and discuss the potential difficulties in establishing their exact aetiology in patients with CD.
Keywords: Crohn's disease, pancreas and biliary tract, granulomatous hepatitis, sarcoidosis
Background
Cholestasis coupled with systemic symptoms can pose a diagnostic conundrum in the setting of coexisting Crohn's disease (CD). We briefly review the literature on hepatic granulomas, a relatively rare manifestation of both extraintestinal CD and extrapulmonary sarcoidosis. We discuss the etiopathogenetic similarities between CD and sarcoid hepatic granulomas and propose that further research is needed to better characterise the relationship between the two overlapping disorders.
Case presentation
A 23-year-old African-American woman with CD presented to the emergency department with nausea without vomiting, non-specific abdominal pain, chills and hypotension. There was no associated cough, dysuria or rashes.
Prior medical history was significant for Crohn’s ileocolitis and perianal disease diagnosed in 2013, fistulotomy and diverting loop ileostomy in 2014 and recurrent hospitalisations for Crohn’s flare-ups. The patient had been treated with azathioprine and prophylactic antibiotics (ciprofloxacin and metronidazole) with marginal success and was noted to be poorly compliant with her medications and follow-ups. She had been off her medications for several weeks prior to this presentation. Previous therapies included adalimumab (2013–2014), infliximab (discontinued after patient developed an infusion reaction to third induction dose in 2016) and corticosteroids, all of which resulted in inadequate disease control, in part due to poor medication compliance.
On examination, the patient’s temperature was 38.6oC, heart rate 139 bpm and blood pressures in the 70/40 mm Hg. Abdomen was diffusely tender to palpation without guarding or rigidity. Perianal ulceration with some purulence was noted. Laboratory findings were significant for white blood cell count of 14.6 ×109/L (normal 4.0–10.8), lactate 4.3 mmol/L (normal 0.7–2.1), haemoglobin 7.9 gm/dL (normal 11.0–14.5), sedimentation rate 66 mm/hour (normal 0–22), high-sensitivity C-reactive protein 62.6 mg/L (normal 0–3), creatinine 1.25 mg/dL (normal 0.52–1.04), albumin 2.1 g/dL (normal 3.5–5.0), total bilirubin 0.7 mg/dL (normal 0.2–1.3), alanine transaminase 26 U/L (normal 15–41), aspartate transaminase 55 U/L (normal 3–34), alkaline phosphatase 187 U/L (normal 45–117) and negative urinalysis.
The patient was admitted to the intensive care unit for presumed sepsis secondary to acute cholecystitis and was treated with aggressive fluid resuscitation and antibiotics (empiric intravenous vancomycin and piperacillin/tazobactam with subsequent addition of daptomycin and imipenem).
After haemodynamic improvement, the patient was transferred to the internal medicine floor for further care. She continued to spike fevers with intermittent tachycardia, while a rapid uptrend in her liver enzymes was noted (table 1). Examination was notable for marked jaundice, right upper quadrant tenderness and a grade II/VI systolic murmur. Ostomy site was intact with stable output.
Table 1.
Liver function tests during the two hospitalisations and subsequent outpatient follow-up visits
| Total bilirubin(mg/dL) | AST(U/L) | ALT(U/L) | Alkaline phosphatase(U/L) | |
| Normal ranges | 0.2–1.3 | 3–34 | 15–41 | 45–117 |
| First hospitalisation | ||||
| Admission | 0.7 | 55 | 26 | 187 |
| Day 12 | 11.6 | 342 | 176 | 1101 |
| Discharge | 2.4 | 96 | 113 | 717 |
| Second hospitalisation | ||||
| Discharge | 5.3 | 143 | 125 | 1112 |
| Hepatology | ||||
| 2-month follow-up | 0.7 | 65 | 48 | 410 |
| Gastroenterology | ||||
| 4-month follow-up | 0.8 | 41 | 41 | 410 |
| Gastroenterology | ||||
| 5-month follow-up | 0.7 | 93 | 85 | 246 |
ALT, alanine transaminase; AST, aspartate transaminase.
Investigations
Despite extensive workup, the source of the presumed sepsis remained elusive. Ultrasonography of the right upper quadrant was notable for non-specific gallbladder wall thickening, negative sonographic Murphy sign and absence of biliary obstruction or gallstones. In contrast, hepatobiliary scintigraphy (hepatobiliary iminodiacetic acid scan) demonstrated high-grade biliary obstruction with absence of extrahepatic biliary transit of the radiopharmaceutical. CT of the abdomen and pelvis with intravenous contrast revealed oedema around the gallbladder without calcified gallstones and no evidence of abscess or Crohn's flare. Crohn's flare was further excluded via magnetic resonance enterography. The patient underwent endoscopic retrograde cholangiopancreatography with sphincterotomy, which redemonstrated a normal biliary duct system with normal intrahepatic, common hepatic and common bile ducts (the latter two measuring 2–3 mm in diameter). Opacification of the cystic duct (1 mm) and partial gallbladder opacification were noted.
Blood cultures were positive for Peptostreptococcus on admission, but all subsequent cultures revealed no growth of microorganisms. Although the patient’s fevers and leucocytosis gradually resolved with antibiotic therapy, the cholestasis persisted. On hospital day 8, the patient underwent a percutaneous biopsy of the liver, which revealed numerous non-caseating granulomas with surrounding chronic inflammation, mild portal fibrosis and microsteatosis (figure 1). A percutaneous cholecystostomy tube was placed out of concern for acalculous cholecystitis; bile cultures, however, yielded no bacterial growth.
Figure 1.
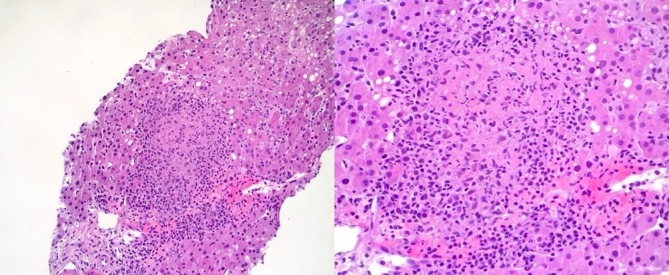
Patient’s liver biopsy specimen showing a non-caseating granuloma (low and high magnifications). Granuloma is a circumscribed lesion that forms in response to chronic inflammation in body tissues. It consists of a central aggregation of modified (epithelioid) macrophages and a peripheral rim of lymphocytes and fibroblasts.
Acid-fast, fungal and Gram staining of the liver biopsy were negative for microorganisms, as were acid-fast bacilli blood cultures, urine Histoplasma antigen and serologies for hepatitides A/B/C, Epstein-Barr virus (EBV), herpes simplex virus (HSV), cytomegalovirus (CMV), HIV and rapid plasma reagin (RPR). Repeat urinalysis, cultures and echocardiography were negative. CT of the chest with intravenous contrast was normal except for two very small ground-glass nodular opacities within the upper lobe anteriorly and within the posterior basal segment of the right lower lobe (figure 2). No enlargement of mediastinal or hilar lymph nodes was noted. Pulmonary function tests were normal. Tests for antismooth muscle, antimitochondrial M2 and antinuclear antibodies were negative.
Figure 2.
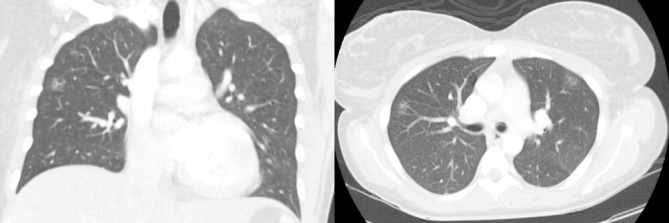
CT of the chest with intravenous contrast showing small ground-glass nodular opacities within the upper lobes. No pleural collections, mediastinal or hilar lymph node enlargement were noted.
Differential diagnosis
The differential diagnosis for non-caseating hepatic granulomas includes drug-induced, infectious, sarcoid-related and autoimmune aetiologies.
Infliximab has been used to treat hepatic granulomas and sarcoidosis in patients not responding to corticosteroids.1–3 However, some authors have described a paradoxical development of granulomatous hepatitis and extrapulmonary sarcoidosis in patients treated with tumour necrosis factor alpha (TNFα) inhibitors.4 5 The fact that our patient developed cholestasis during her hospital stay and had been off infliximab for several months prior to presentation argued strongly against a drug-associated aetiology for her liver granulomas.
The exclusion of infectious causes in symptomatic granulomatous hepatitis is crucial, since inappropriate corticosteroid therapy may promote the spread of an underlying infection. Infectious aetiologies were excluded based on the patient’s clinical history, demographic characteristics, radiographic findings and infectious disease workup. Acid-fast, fungal and Gram staining of the liver biopsy were negative for microorganisms, as were acid-fast bacilli blood cultures, urine Histoplasma antigen and serologies for hepatitides A/B/C, EBV, HSV, CMV, HIV and RPR.
Autoimmune hepatitis and primary biliary cholangitis (PBC) were excluded based on the histopathological characteristics of the liver biopsy and negative antismooth muscle, antimitochondrial M2 and antinuclear antibodies. Primary sclerosing cholangitis (PSC) was ruled out based on the endoscopic retrograde cholangiopancreatography and liver biopsy findings.
CT of the chest showed no evidence of pulmonary sarcoidosis or other abnormalities, except for two small non-specific ground-glass opacities (figure 2). Pulmonary function tests were normal. Angiotensin-converting enzyme levels were not tested.
Based on the above findings, we concluded that the hepatic granulomas most likely represented either an extraintestinal manifestation of CD or an isolated extrapulmonary manifestation of sarcoidosis.
Treatment
Following the completion of her antibiotic course, the patient experienced a gradual improvement in her symptoms and her liver function enzymes began to downtrend (table 1). She was discharged on prednisone 40 mg and was instructed to follow-up with her CD specialist and a hepatologist.
Outcome and follow-up
The patient presented to a different local hospital 1 month later with similar sepsis-like symptoms. She was admitted and treated for presumed healthcare-acquired pneumonia based on pleuritic chest pain and new basilar opacities detected on CT, but, once again, no definite source of infection could be identified. As before, a marked elevation in liver enzymes followed by a gradual downtrend was noted. Following discharge, the patient was treated with prednisone for 2 months. Tests from subsequent visits continued to show downtrending liver markers (table 1).
Discussion
Hepatic granulomas
Granuloma is a circumscribed lesion that forms in response to chronic inflammation in body tissues. It consists of a central aggregation of modified (epithelioid) macrophages and a peripheral rim of lymphocytes and fibroblasts.6 The morphology of granulomas can be useful in refining the differential diagnosis. Four major histological variants of hepatic granulomas have been described: (1) caseating, such as those seen in tuberculosis; (2) non-caseating, as seen in sarcoidosis; (3) fibrin ring, caused by infections and vasculitides; and (4) lipogranulomas, often seen in hepatic steatosis and mineral oil ingestion. A variety of disorders can cause granulomas in the liver. In the USA, up to 75% of hepatic granulomas are associated with sarcoidosis, tuberculosis, PBC and drug reactions.7
The clinical manifestations of hepatic granulomas are related to their pathophysiology. Activated macrophages and lymphocytes release cytokines that may cause systemic symptoms (fever, anorexia night sweats) or direct hepatic injury. Occasionally, the finding of granulomas on liver biopsy may be the only clue to the presence of an underlying systemic disease. In most cases, however, laboratory evidence of inflammation, increased levels of alkaline phosphatase or hepatomegaly provides additional clues.6
The differential diagnosis of hepatic granulomas can be broadly categorised into sarcoid-related, autoimmune, drug-induced, infectious, malignant and idiopathic aetiologies. In the remaining sections, we briefly review hepatic granulomas in the context of sarcoidosis and CD.
Hepatic sarcoidosis
Despite recent advances in immunogenetic research, the exact pathogenesis of sarcoidosis remains unknown.8 9 Virtually all (99%) sarcoid granulomas are non-caseating, accounting for up to 30% of all cases of granulomatous hepatitis.6 Additional histopathological findings include periportal fibrosis and chronic intrahepatic cholestasis.10 Such lesions, however, are not pathognomonic for sarcoidosis and may resemble, for example, those seen in patients with inflammatory bowel disease and PBS or PSC.11
Sarcoidosis is a multiorgan disorder most commonly affecting the lungs.12 Extrapulmonary manifestations of sarcoidosis include the lymph nodes, skin, eyes and liver.13 Isolated hepatic involvement, although well documented, remains a relatively rare finding.10 14 Most patients with hepatic sarcoidosis are asymptomatic. The most common symptoms are abdominal pain and hepatosplenomegaly. Occasionally, the main presenting feature is elevated liver enzymes.15–17
Hepatic sarcoidosis is a diagnosis of exclusion, since there are no pathognomonic signs on liver biopsy that can firmly establish the diagnosis. Other causes, such as infections, autoimmune disorders, drug-induced granulomas and malignancy, must be excluded. While the lack of extrahepatic involvement does not exclude the diagnosis of hepatic sarcoidosis, a definitive diagnosis requires evidence of sarcoid lesions in at least one other organ.18
Asymptomatic patients with non-infectious hepatic granulomas do not require treatment. The mainstay therapy for symptomatic patients consists of corticosteroids. Relapses may occur after corticosteroid discontinuation, requiring repeat courses.19 20 Ursodeoxycholic acid has been used to treat pruritus in patients with severe cholestasis and jaundice.10 In patients not responding to or not tolerating corticosteroids, alternative therapies, including azathioprine, methotrexate and TNFα inhibitors, have been used successfully.1 2
Hepatic granulomas in CDand overlap with sarcoidosis
Hepatic granulomas are a rare complication of CD.21 As with most hepatobiliary manifestations of CD, their presence is typically unrelated to intestinal disease activity. Patients may present with fever, hepatomegaly and elevated alkaline phosphatase levels.22
The prevalence of hepatic granulomas due to CD is unknown. Hilzenrat et al23 reported a case of granulomatous hepatitis in a patient with CD who presented with fever and cholestasis, closely resembling our case. Reviewing the literature, they found a total of 12 biopsy-confirmed cases of hepatic granulomas associated with CD. However, a rigorous exclusion of alternative causes may not have been feasible in some of the cited studies. McCluggage and Sloan,24 in their retrospective study of 163 liver biopsies, attributed another three cases of granulomatous hepatitis to CD.
The co-occurrence of sarcoidosis and CD is a very rare phenomenon, yet more frequent that would be expected by chance alone.25 This observation has led to the hypothesis that there may be an etiopathogenetic link between the two disorders.26 The coexistence of sarcoidosis and CD has been observed among different members of the same family, suggesting a possible genetic overlap.27–29 Genetic analyses have identified Nod2/CARD15 polymorphisms in both disorders,30 as well as common susceptibility loci on chromosome 10p12.2.31 A shared pathogenetic mechanism is also supported by the fact that both CD and sarcoidosis tend to respond to anti-TNF therapy.32 Clearly, further studies are needed to better understand these relationships.
In summary, the exact cause of hepatic granulomas can be difficult to ascertain. The diagnosis is particularly challenging in patients with suspected extraintestinal CD, which can mimic extrapulmonary sarcoidosis. Our case highlights some of the dilemmas that clinicians may face when treating such patients.
Learning points.
Cholestasis coupled with systemic symptoms can pose a diagnostic conundrum in the setting of coexisting Crohn’s disease (CD).
Exclusion of infectious causes in symptomatic granulomatous hepatitis is crucial, since inappropriate corticosteroid therapy may promote the spread of an underlying infection.
Hepatic granulomas are a relatively rare manifestation of both extraintestinal CD and extrapulmonary sarcoidosis.
CD and sarcoid hepatic granulomas share several etiopathogenetic features and further research is needed to better characterise the relationship between the two overlapping disease entities.
Footnotes
Contributors: Both authors have contributed equally to this work, including reviewing the patient's medical records, researching relevant medical literature, and preparing and editing the manuscript. The corresponding author obtained the patient's informed consent.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kennedy PT, Zakaria N, Modawi SB, et al. Natural history of hepatic sarcoidosis and its response to treatment. Eur J Gastroenterol Hepatol 2006;18:721–6. 10.1097/01.meg.0000223911.85739.38 [DOI] [PubMed] [Google Scholar]
- 2.Ayyala US, Padilla ML. Diagnosis and treatment of hepatic sarcoidosis. Curr Treat Options Gastroenterol 2006;9:475–83. 10.1007/s11938-006-0004-9 [DOI] [PubMed] [Google Scholar]
- 3.Farah M, Al Rashidi A, Owen DA, et al. Granulomatous hepatitis associated with etanercept therapy. J Rheumatol 2008;35:349–51. [PubMed] [Google Scholar]
- 4.Decock A, Van Assche G, Vermeire S, et al. Sarcoidosis-like lesions: Another Paradoxical Reaction to Anti-TNF Therapy? J Crohns Colitis 2017;11:jjw155 10.1093/ecco-jcc/jjw155 [DOI] [PubMed] [Google Scholar]
- 5.Takahashi H, Kaneta K, Honma M, et al. Sarcoidosis during infliximab therapy for Crohn's disease. J Dermatol 2010;37:471–4. 10.1111/j.1346-8138.2010.00861.x [DOI] [PubMed] [Google Scholar]
- 6.Coash M, Forouhar F, Wu CH, Ch W, et al. Granulomatous liver diseases: a review. J Formos Med Assoc 2012;111:3–13. 10.1016/j.jfma.2011.11.023 [DOI] [PubMed] [Google Scholar]
- 7.Culver EL, Watkins J, Westbrook RH. Granulomas of the liver. Clin Liver Dis 2016;7:92–6. 10.1002/cld.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol 2000;12:71–6. 10.1097/00002281-200001000-00012 [DOI] [PubMed] [Google Scholar]
- 9.Müller-Quernheim J, Prasse A, Zissel G. Pathogenesis of sarcoidosis. Presse Med 2012;41:e275–e287. 10.1016/j.lpm.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 10.Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol 2008;103:3184–92. 10.1111/j.1572-0241.2008.02202.x [DOI] [PubMed] [Google Scholar]
- 11.Devaney K, Goodman ZD, Epstein MS, et al. Hepatic sarcoidosis. Clinicopathologic features in 100 patients. Am J Surg Pathol 1993;17:1272–80. [PubMed] [Google Scholar]
- 12.Cozier YC, Berman JS, Palmer JR, et al. Sarcoidosis in black women in the United States: data from the black women’s health study. CHEST Journal 2011;139:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judson MA. Extrapulmonary sarcoidosis. In seminars in respiratory and critical care medicine. 2007;28:083–101. [DOI] [PubMed] [Google Scholar]
- 14.Jovicić I, Popović DDj, Toncev L, et al. Isolated hepatic sarcoidosis. Vojnosanit Pregl 2014;71:399–403. 10.2298/VSP1404399J [DOI] [PubMed] [Google Scholar]
- 15.Karagiannidis A, Karavalaki M, Koulaouzidis A. Hepatic sarcoidosis. Ann Hepatol 2006;5:251–6. [PubMed] [Google Scholar]
- 16.Cremers J, Drent M, Driessen A, et al. Liver-test abnormalities in sarcoidosis. Eur J Gastroenterol Hepatol 2012;24:17–24. 10.1097/MEG.0b013e32834c7b71 [DOI] [PubMed] [Google Scholar]
- 17.Bihari C, Rastogi A, Kumar N, et al. Hepatic Sarcoidosis: Clinico-pathological characterization of symptomatic cases. Acta Gastroenterol Belg 2015;78:306–13. [PubMed] [Google Scholar]
- 18.Govender P, Berman JS. The Diagnosis of Sarcoidosis. Clin Chest Med 2015;36:585–602. 10.1016/j.ccm.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 19.Tan CB, Rashid S, Rajan D, et al. Hepatic sarcoidosis presenting as portal hypertension and liver cirrhosis: case report and review of the literature. Case Rep Gastroenterol 2012;6:183–9. 10.1159/000338355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modaresi Esfeh J, Culver D, Plesec T, et al. Clinical presentation and protocol for management of hepatic sarcoidosis. Expert Rev Gastroenterol Hepatol 2015;9:349–58. 10.1586/17474124.2015.958468 [DOI] [PubMed] [Google Scholar]
- 21.Rojas-Feria M, Castro M, Suárez E, et al. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol 2013;19:7327 10.3748/wjg.v19.i42.7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memon MI, Memon B, Memon MA. Hepatobiliary manifestations of inflammatory bowel disease. HPB Surg 2000;11:363–71. 10.1155/2000/98384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilzenrat N, Lamoureux E, Sherker A, et al. Cholestasis in Crohn's disease: a diagnostic challenge. Can J Gastroenterol 1997;11:35–7. [DOI] [PubMed] [Google Scholar]
- 24.McCluggage WG, Sloan JM. Hepatic granulomas in Northern Ireland: a thirteen year review. Histopathology 1994;25:219–28. 10.1111/j.1365-2559.1994.tb01321.x [DOI] [PubMed] [Google Scholar]
- 25.Fries W, Grassi SA, Leone L, et al. Association between inflammatory bowel disease and sarcoidosis. Report of two cases and review of the literature. Scand J Gastroenterol 1995;30:1221–3. 10.3109/00365529509101635 [DOI] [PubMed] [Google Scholar]
- 26.McCormick PA, O'Donoghue DP, FitzGerald MX. Crohn's colitis and sarcoidosis. Postgrad Med J 1986;62:951–3. 10.1136/pgmj.62.732.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grönhagen-Riska C, Fyhrquist F, Hortling L, et al. Familial occurrence of sarcoidosis and Crohn's disease. Lancet 1983;1:1287–8. 10.1016/S0140-6736(83)92748-4 [DOI] [PubMed] [Google Scholar]
- 28.Bambery P, Kaur U, Bhusnurmath SR, et al. Familial idiopathic granulomatosis: sarcoidosis and Crohn's disease in two Indian families. Thorax 1991;46:919–21. 10.1136/thx.46.12.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Mayouf SM, Albuhairan I, Muzaffer M, et al. Familial aggregation of Crohn's disease and necrotizing sarcoid-like granulomatous disease. Eur J Rheumatol 2015;2:122–4. 10.5152/eurjrheum.2015.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damen GM, van Krieken JH, Hoppenreijs E, et al. Overlap, common features, and essential differences in pediatric granulomatous inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2010;51:690–7. 10.1097/MPG.0b013e3181dc0d73 [DOI] [PubMed] [Google Scholar]
- 31.Franke A, Fischer A, Nothnagel M, et al. Genome-wide association analysis in sarcoidosis and Crohn's disease unravels a common susceptibility locus on 10p12.2. Gastroenterology 2008;135:1207–15. 10.1053/j.gastro.2008.07.017 [DOI] [PubMed] [Google Scholar]
- 32.Chebib N, Piégay F, Traclet J, et al. Improvement with infliximab of a disseminated sarcoidosis in a patient with Crohn's disease. Case Rep Pulmonol 2014;2014:1–4. 10.1155/2014/368780 [DOI] [PMC free article] [PubMed] [Google Scholar]


