Abstract
Background
The purpose of this investigation was to evaluate the effect of probiotic lactobacilli on Streptococcus mutans (MS) and multispecies biofilms isolated from children with severe caries.
Material/Methods
Twenty children with active caries (DMFS ≥6) were selected as the experimental group and Streptococcus mutans (MS) were isolated from their saliva. After identification the MS strains were mixed with lactobacilli at 37°C, following which viable MS colonies were counted. At the same time dental plaques from the children were mixed with lactobacilli in vitro to form biofilms, and the population of nine common strains in the biofilms was enumerated after 24 hours of growth.
Results
Lactobacillus casei Shirota, L. casei LC01, L. plantarum ST-III and L. paracasei LPC37 all had strong inhibitory effects on the majority the MS isolated from children with active caries, with the inhibition rate reaching approximately 70–90% (p<0.05). L. casei Shirota, L. casei LC01, L. plantarum ST-III, L. paracasei LPC37 also significantly reduced the numbers of MS, Streptococcus spp., S. sanguinis and total bacteria in mixed biofilms compared with the control group (p<0.05).
Conclusions
The four strains of lactobacilli were able to inhibit the growth of Streptococcus mutans and had effects on the composition of bacterial biofilms in vitro. Ingestion of probiotics may be a promising method of caries prevention.
MeSH Keywords: Biofilms, Dental Caries, Probiotics, Streptococcus Mutans
Background
Probiotics are beneficial bacteria that can improve the micro-ecological balance of the host [1]. Most probiotic bacteria belong to the genera Lactobacillus or Bifidobacterium and these bacteria are commonly added to probiotic drinks or yogurt products. Recent studies have shown that probiotics play a positive role in oral health [2], and can affect the oral micro-ecology by changing the protein composition of dental plaque [2]. This has stimulated research to study the anti-caries effect of probiotics.
The use of fluoride products and xylitol chewing gum has greatly reduced the prevalence of dental caries in children [3,4]. However, the use of these products can result in the generation of fluoride resistant bacteria [5], and the ingestion of xylitol chewing gum may be lethal for children [6]. In China, 76.6% of children at the age of five suffer from dental caries [7], therefore, safe and effective methods for the prevention of caries needs to be explored. As probiotics can produce different antibacterial compounds [8], improve the oral microbial ecology, and rarely cause infections in humans, these organisms represent a safe and promising way to control caries.
Many studies have shown that caries are caused by ecological imbalance in the oral cavity [9]. When caries-related bacteria increase and beneficial bacteria are reduced, the dental plaque can be transformed from non-cariogenic plaque to cariogenic plaque. Thus, the control of caries should be aimed at maintaining effective ecological balance of the oral flora. Cariostatic agents should not just have antibacterial activity against Streptococcus mutans (MS) and lactobacilli, but also affect the aerobes and anaerobes present in dental plaque. MS are one of the most important cariogenic bacteria and various in vitro studies have shown that Lactobacillus rhamnosus and L. paracasei can reduce the quantity of MS significantly [10]. However, as with most probiotics research, the interpretation generally focuses solely on the levels of standard MS, while there are great different between the standard strains and clinical strains. Therefore, in this study we extracted dental plaque from children with active caries and mixed these organisms with probiotic lactobacilli, calculating the number of MS, oral Streptococcus (OS) and Lactobacillus (LB) in biofilms.
Our previous study has shown that five probiotic lactobacilli strains were able to inhibit growth and biofilm formation of MS, likely through the production of an acid environment, bacteriocin-like polypeptides or both [11]. Here we compared the effect of four common commercial probiotic LB on clinically isolated MS strains and mixed biofilms from children with active caries to explore the practical value of probiotics in caries prevention.
Material and Methods
Identification of MS from saliva of children with active caries
Experienced dentists in the Affiliated Hospital of Stomatology of Zhejiang University examined the children’s oral health according to the International Caries Detection and Assessment System (ICDAS) standards. A total of 20 children with active caries (10 females, 10 males; range: 3–5 year of age) were asked to participate in the study after signing an informed consent form by their parents. The inclusion criteria were: DMFS ≥6, no antibiotic treatment in the past three months, good oral hygiene, and no systemic disease or abnormal tooth structures. The project was approved by the Ethics Committee of the Department of Conservative Dentistry and Periodontics, Affiliated Hospital of Stomatology, College of Medicine, Zhejiang University. All parents of the volunteers provided written informed consent.
Unstimulated whole saliva (1 mL) was collected from the children before a meal. Subgingival plaque was removed from the tooth with a sterile curette. All samples were sent to a microbiology laboratory and cultured within 2 hours.
The saliva specimens were vortexed for 1 minute, serially diluted with PBS buffer, and 50 μL of diluted saliva was cultured on mitis salivarius bacitracin agar (MSB) at 37°C in a microaerophilic atmosphere (5% O2, 10% CO2 and 85% N2) for 24 hours for MS. MS were verified by Gram-staining and their species identity was confirmed by using an automated mass spectrometry microbial identification system (Vitek, BioMérieux, France). After identification, the MS strains were purified on MSB again and were kept frozen at −80°C until used.
Interference test of the MS stains from saliva with four probiotic LB strains
Four probiotic LB strains were used in this study (L. casei Shirota, L. casei LC01, L. plantarum ST-III, and L. paracasei LPC37). The LB were grown in DeMan Rogosa Sharpe agar (MRS) (Oxoid, UK) under microaerophilic conditions at 37°C for 48 hours. The isolated MS were cultured in brain heart infusion (BHI) medium (Oxoid, UK). After incubation, the concentrations of the LB and MS cultures were adjusted with VITEK Densichek (BioMérieux, France) to match the McFarland 0.5 standard (1.5×108 CFU/mL) in PBS. Finally, 50 μL of the MS and LB cultures were combined with 2 mL BHI broth and incubated under microaerophilic conditions at 37°C for 24 hours. The S. aureus ATCC 25923 culture was used as the negative control, while 50 μL PBS was served as blank control.
After incubation, the suspensions were vortexed for one minute and serially diluted to 10−3. Then 100 μL of the suspension was plated on MSB and incubated under microaerophilic conditions at 37°C for 24 hours, after which the number of cells was counted. The inhibition rate was calculated according to the following formula:
where A and B represent the colony number of experimental group and negative control respectively.
Interference test of the biofilm of dental plaque with four probiotic LB strains
In this study, the dental plaque of each patient was extracted to carry out the biofilm experiment. The dental plaque collected from children with active caries were first cultured in BHI broth at 37°C under microaerophilic conditions for 24 hours, then the concentration of the mixed bacteria was adjusted to 1.5×108 CFU/mL. Biofilm formation was conducted according to the method of Bueno-Silva B et al. [12]. Each well in six-well plates was filled with 2 mL of BHI broth supplemented with 1% sucrose, and a piece of sterile hydroxyapatite (HA) was added to each well. 20 μL of the mixed bacterial suspension was added to each well along with 20 μL of LB suspension (1.5×108 CFU/mL). As a control, 20 μL PBS was used. All six-well plates were incubated under microaerophilic conditions at 37°C for 24 hours.
The analyze of biofilm formation on HA from dental plaque by Quantitative real-time polymerase chain reaction
During the 24 hours of biofilm formation, the pH of the biofilms was measured every three hours. After incubation, the HA pieces were put in 1 mL of PBS buffer and vortexed for 90 seconds, then the culture was diluted in PBS to 10−3. Finally bacterial RNA from 100 uL of the suspension was prepared and analyzed by previously described methods [13]. The qPCR primers used in this study are shown in Table 1. Real time PCR was performed in triplicate in a 10 μL reaction volume containing 1×SYBR Green Master Mix (DBI, China), 100 nM specific primer and 50 ng template DNA on an ABI PRISM 7900HT system (Applied Biosystems Inc, USA) in 384-well PCR plates.
Table 1.
qPCR Primers used in this study.
| Species | Sequence (5′ to 3′) | Target gene | Amplicon size(bp) |
|---|---|---|---|
| Streptococcus spp. | F: GTACAGTTGCTTCAGGACGTATC | tuf | 197 |
| R: ACGTTCGATTTCATCACGTTG | |||
| S. mutans | F: AGTGCCAAGACTGACGCTTT | dexA | 141 |
| R: GGGCTGACTGCTTCTGGAGT | |||
| S. sobrinus | F: TGCCATCAACACTCTCTTGC | gtfT | 162 |
| R: TGACCGAAACGAACCGATAC | |||
| S. gordonii | F: CGGATGATGCTAATCAAGTGACC | gtfG | 177 |
| R: GTTAGCTGTTGGATTGGTTGCC | |||
| S. sanguinis | F: GTGTCATCAATTCCCAGAAAAG | sodA | 104 |
| R: ATTATTGGCTGATGTGGAGTC | |||
| S. oralis | F: AAAGGCTGCTGTTGCTGAAG | gtfR | 193 |
| R: GGGCAAGCGATCTTTCTTTG | |||
| S. salivarius | F: CAGTGGGTTACTTTGGCTGTC | gtfK | 133 |
| R: CCGACCGTAGTTGTTGAAGG | |||
| P. gingival | F: GGAAGAGAAGACCGTAGCACAAGGA | rpoB | 143 |
| R: GAGTAGGCGAAACGTCCATCAGGTC | |||
| A. naeslundii | F: GTCTCTTCGCCCAGATCGAG | ureC | 143 |
| R: GTTGGTGATGACGGTGTCG | |||
| Total Bacteria | F: CCATGAAGTCGGAATCGCTAG | 16S rRNA | 89 |
| R: GCTTGACGGGCGGTGT |
Statistical methods
The data were analyzed with SPSS 14.0 software for Windows and data comparisons were performed with Dunnett’s two-sided t-test. A p-value <0.05 was considered as statistically significant.
Results
Growth inhibition of MS
A total of 12 S. mutans strains were isolated from 20 children with active caries, then they were analyzed to determine if four strains of Lactobacillus possessed potential inhibitory effects on the growth of MS. L. casei Shirota, L. casei LC01, L. plantarum ST-III, and L. paracasei LPC37; all had a strong inhibitory effect on the majority of the MS isolated from the children with active caries and the inhibition rate reached approximately 70–90%. (p<0.05; Tables 2–5).
Table 2.
Inhibitory effects of L. casei Shirota on clinical isolated MS after 24 h (×108 CFU/ml, means ±SD).
| Strains | L. casei Shirota | Blank control | Inhibition rate (%) |
|---|---|---|---|
| 1 | 1.73±0.38 | 6.24±1.37 | 72.27 |
| 2 | 1.68±0.54* | 7.74±1.54 | 60.60 |
| 3 | 3.65±1.24 | 7.58±2.21 | 51.84 |
| 4 | 2.41±0.87* | 8.01±2.45 | 69.91 |
| 5 | 1.21±0.22* | 6.74±1.41 | 82.05 |
| 6 | 1.74±0.41* | 6.66±0.74 | 73.87 |
| 7 | 1.87±0.33* | 7.14±1.09 | 73.81 |
| 8 | 4.41±1.77 | 9.04±3.24 | 51.22 |
| 9 | 1.01±0.24** | 7.11±0.87 | 85.79 |
| 10 | 3.14±1.01 | 8.14±1.99 | 61.42 |
| 11 | 1.00±0.31** | 6.14±2.01 | 83.71 |
| 12 | 1.66±0.65* | 8.45±2.35 | 80.36 |
P<0.05;
P<0.01.
Table 3.
Inhibitory effects of L. casei LC01 on clinical isolated MS after 24 h (×108 CFU/ml, means ±SD).
| Strains | L. casei Shirota | Blank control | Inhibition rate (%) |
|---|---|---|---|
| 1 | 2.45±0.78* | 6.66±1.35 | 63.21 |
| 2 | 2.47±1.11* | 7.45±2.21 | 66.85 |
| 3 | 3.47±1.56 | 7.14±1.98 | 51.40 |
| 4 | 1.45±0.87* | 7.87±1.54 | 81.57 |
| 5 | 2.01±0.88* | 8.84±3.74 | 77.26 |
| 6 | 1.35±0.65* | 7.75±2.20 | 82.58 |
| 7 | 1.39±0.54* | 7.24±2.14 | 80.80 |
| 8 | 1.22±0.41* | 6.87±1.11 | 82.24 |
| 9 | 1.14±0.11** | 6.78±0.98 | 83.18 |
| 10 | 3.33±1.42 | 8.98±3.21 | 62.92 |
| 11 | 1.36±0.77** | 7.77±2.39 | 82.49 |
| 12 | 1.87±0.98* | 8.12±3.12 | 76.97 |
P<0.05;
P<0.01.
Table 4.
Inhibitory effects of L. plantarum ST-III on clinical isolated MS after 24 h (×108 CFU/ml, means ±SD).
| Strains | L. plantarum ST-III | Blank control | Inhibition rate (%) |
|---|---|---|---|
| 1 | 2.24±1.01* | 9.78±3.34 | 77.10 |
| 2 | 2.54±0.87* | 8.89±2.21 | 71.43 |
| 3 | 4.58±2.01 | 10.45±4.53 | 56.17 |
| 4 | 1.89±0.78** | 7.98±2.19 | 76.31 |
| 5 | 2.11±0.99* | 8.98±3.01 | 76.50 |
| 6 | 1.45±1.01** | 7.25±1.25 | 80.00 |
| 7 | 3.54±1.87 | 8.01±2.87 | 55.81 |
| 8 | 2.78±0.98* | 9.01±3.78 | 69.15 |
| 9 | 2.47±0.79* | 9.58±4.01 | 74.22 |
| 10 | 2.22±1.11* | 8.88±3.21 | 75.00 |
| 11 | 3.88±2.11 | 10.14±4.12 | 61.73 |
| 12 | 2.01±0.56* | 9.98±3.33 | 79.86 |
P<0.05;
P<0.01.
Table 5.
Inhibitory effects of L. paracasei LPC37 on clinical isolated MS after 24 h (×108 CFU/ml, means ±SD).
| Strains | L. paracasei LPC37 | Blank control | Inhibition rate (%) |
|---|---|---|---|
| 1 | 1.05±0.77 | 6.98±2.10 | 84.95 |
| 2 | 2.25±1.21 | 9.27±2.26 | 75.73 |
| 3 | 1.87±0.89 | 8.45±1.21 | 77.87 |
| 4 | 2.38±1.24 | 8.45±1.39 | 71.83 |
| 5 | 4.05±2.01 | 8.12±2.22 | 50.12 |
| 6 | 1.49±0.78 | 6.98±0.79 | 78.65 |
| 7 | 1.68±0.44 | 8.84±1.25 | 80.99 |
| 8 | 3.98±1.59 | 7.58±0.88 | 47.49 |
| 9 | 2.37±1.05 | 8.47±1.58 | 72.02 |
| 10 | 2.39±1.02 | 7.87±2.01 | 69.45 |
| 11 | 1.54±0.47 | 7.87±2.09 | 80.05 |
| 12 | 2.07±1.24 | 9.01±3.21 | 77.03 |
P<0.05;
P<0.01.
Co-culture with lactobacilli influences different bacteria in biofilm
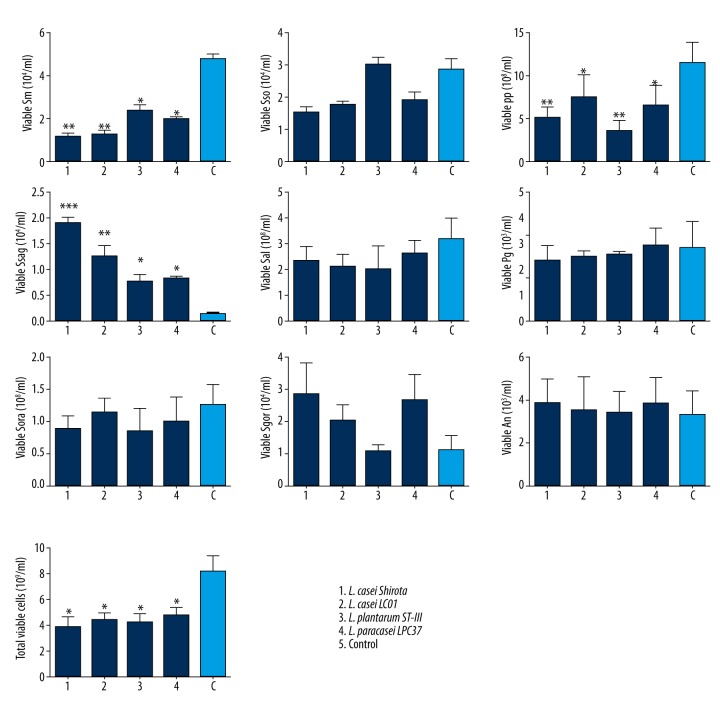
The real-time PCR of the biofilm showed that L. casei Shirota, L. casei LC01, L. plantarum ST-III, L. paracasei LPC37 significantly reduced the numbers of MS, Streptococcus spp, S. sanguinis and total bacteria in the mixed biofilm cultures compared with the control group (p<0.05; Figure 1). In the presence of the four Lactobacillus strains, the count of S. sobrinus, S. salvarius, Porphorymonas gingivalis, S. oralis, and Actinomyces naeslundii in the multispecies biofilms showed no significant difference compared with the control group (p>0.05; Figure 1). The L. casei Shirota and L. paracasei LPC37 appeared to increase the total count of S. gordonii in the mixed biofilm (p<0.05; Figure 1).
Figure 1.
Viable Streptococcus mutans (Sm), S. spp (Spp), S. sanguinis (Ssag), S. sobrinus (Sso), S. salvarius (Sal), Porphorymona gingivalis (Pg), S. oralis (Soral), S. gordonii (Sgor) and Actinomyces naeslundii (An) in the biofilms (1) Lactobacillus casei Shirota, (2) L. casei LC01, (3) L. plantarum ST-III, (4) L. paracasei LPC37, and (C) control (S. aureus)). Data are expressed as the mean ±SD; * p<0.05; ** p<0.01.
Discussion
Our current in vitro study results suggest that probiotic lactobacilli have an effect on the growth and inhibition of at least four out of 12 MS isolated from children with active caries. This finding agrees with the results in vivo from the study that showed taking probiotic yogurt or beverages can significantly reduce the level MS in saliva [14,15]. We selected clinical MS strain for our study, as different MS strains have different cariogenic potential [16]. Understanding the effect of probiotics on clinically isolated MS strains has important significance for the prevention of caries in children.
LB are usually considered to be caries-related bacteria. However, in recent years, studies have shown that L. plantarum and L. casei can play a beneficial role in oral health rather than a cariogenic effect [17]. The concentration of lactobacilli in probiotic products is usually between 1×108/mL to 3×108/mL and in our experiments the concentration of four LB strains were adjusted to 1.5×108/mL. At this concentration, the four strains of LB had a significant inhibitory effect on MS, which is consistent with the in vitro results of Ahmed et al. [18].
Antibacterial substances produced by probiotic lactobacilli include lactic acid, which can inhibit microbial growth by lowering the pH; hydrogen peroxide, which can inhibit bacterial DNA synthesis [19]; and bacteriocins, which can destroy bacterial cell membranes to kill gram-positive bacteria. As MS is acid-tolerant and hydrogen peroxide production by LB is low, it is possible that the antibacterial substances in probiotics may be primarily bacteriocins or bacteriocin-like proteins. Studies have shown that L. reuteri and L. plantarum showed significant inhibitory effects on MS due to bacteriocin production [20]. However, at present the antimicrobial mechanism of probiotic lactobacilli against MS is still not fully understood and requires further research.
Dental plaque is a biofilm structure which gradually sediments on the tooth surface. This structure contains a variety of bacteria that form a complex ecological environment in which streptococci and other caries-related microorganisms produce acid, which is the direct cause of tooth decay [21]. In order to be effective at preventing caries, probiotics must have an inhibitory effect on Streptococcus species. Studies on intestinal microbes have confirmed that some lactic acid bacteria such as L. rhamnosus GG are able to have significant inhibition effects on pathogens [22]. In our experiment, the numbers of S. mutans, S. spp and S. sanguinis cells in the mixed plaque biofilm decreased significantly when co-cultured with lactobacilli, indicating that probiotic lactobacilli may play a positive role in the prevention of caries.
It would be interesting to observe the antibacterial effect of probiotic lactobacilli on the growth of MS and multispecies biofilms isolated from children with active caries, however, the oral cavity is inhabited by a highly diverse and complex bacterial ecosystem that is difficult to simulate in vitro [23]. Our results are in vitro results, therefore, to study in vivo the changes that occur in the oral flora after taking probiotics is still necessary for an understanding of the role of probiotic lactobacilli in oral health care. In future experiments, we plan to administer probiotic products to children and detect changes in the oral micro-ecology by using high-throughput sequencing technologies.
Conclusions
The four probiotic strains tested here were able to inhibit the growth of MS and multispecies biofilms in vitro, had effects on the composition bacterial biofilms in vitro, and may have potential for use in the prevention of dental caries.
Footnotes
Source of support: This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81371142), Grants for Medical Technology of Zhejiang Province (Grant No. 2012ZDA0320), the Provincial and Ministerial Project of Zhejiang Province (Grant No. 2015120726 ) and the Education Department of Zhejiang Province (Grant No. 2012C34011)
References
- 1.Lara-Villoslada F, Olivares M, Sierra S, et al. Beneficial effects of probiotic bacteria isolated from breast milk. Br J Nutr. 2015;98(Suppl 1):S96–100. doi: 10.1017/S0007114507832910. [DOI] [PubMed] [Google Scholar]
- 2.Toiviainen A. Probiotics and oral health: In vitro and clinical studies. Eur Neurol. 2015;21:392–95. [Google Scholar]
- 3.Wang, Chang MJ, Lee WJ, et al. The effects of funoran-containing xylitol chewing gum on dental plaque. J Polyr Engin. 2016;34:203–8. [Google Scholar]
- 4.Sievers K, Silk H. Fluoride varnish for preventing dental caries in children and adolescents. Am Fam Physician. 2016;93(9):743–44. [Google Scholar]
- 5.Banerjee G, Sengupta A, Roy T, et al. Isolation and characterization of two fluoride resistant bacterial strains from fluoride endemic areas of west Bengal, India: Assessment of their fluoride absorption efficiency. Fluoride. 2016;49(4) [Google Scholar]
- 6.Hashiba T, Takeuchi K, Shimazaki Y, et al. Chewing xylitol gum improves self-rated and objective indicators of oral health status under conditions interrupting regular oral hygiene. Tohoku J Exp Med. 2015;235:39–46. doi: 10.1620/tjem.235.39. [DOI] [PubMed] [Google Scholar]
- 7.Peres MA, Sheiham A, Liu P, et al. Sugar consumption and changes in dental caries from childhood to adolescence. J Dent Res. 2016;95:388–94. doi: 10.1177/0022034515625907. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard DS, Seridan B, Saraoui T, et al. Lactic acid bacteria isolated from bovine mammary microbiota: Potential allies against bovine mastitis. PLoS One. 2015;10:e0144831. doi: 10.1371/journal.pone.0144831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine DH. Lactoferrin: A roadmap to the borderland between caries and periodontal disease. J Dent Res. 2015;94(6):768–76. doi: 10.1177/0022034515577413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández CE, Giacaman RA, Tenuta LM, Cury JA. Effect of the probiotic lactobacillus rhamnosus LB21 on the cariogenicity of Streptococcus mutans UA159 in a dual-species biofilm model. Caries Res. 2015;49:583–90. doi: 10.1159/000439315. [DOI] [PubMed] [Google Scholar]
- 11.Lin X, Chen X, Chen Y, et al. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2014;21:128–34. doi: 10.1111/odi.12257. [DOI] [PubMed] [Google Scholar]
- 12.Buenosilva B, Koo H, Falsetta ML, et al. Effect of neovestitol-vestitol containing Brazilian red propolis on accumulation of biofilm and development of dental caries. Biofouling. 2013;29:1233–42. doi: 10.1080/08927014.2013.834050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Ji J, Chen X, et al. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr Polym. 2014;102:351–59. doi: 10.1016/j.carbpol.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 14.Nozari A, Motamedifar M, Seifi N, et al. The effect of Iranian customary used probiotic yogurt on the children’s salivary cariogenic microflora. J Dent. 2015;16:81–86. [PMC free article] [PubMed] [Google Scholar]
- 15.Ashwin D, Vijayaprasad KE, Nara A, Sarpangala M. Effect of probiotic containing ice-cream on Salivary Mutans Streptococci (SMS) levels in children of 6–12 years of age: A randomized controlled double blind study with six-months follow up. J Clin Diagn Res. 2015;9(2):ZC06–9. doi: 10.7860/JCDR/2015/10942.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Hu D, Liu J, et al. [Selection and identification of ssDNA aptamers specific to clinical isolates of Streptococcus mutans strains with different cariogenicity]. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33(5):738–41. [in Chinese] [PubMed] [Google Scholar]
- 17.Chuang L-C, Huang C-S, Ou-Yang L-W, Lin S-Y. Probiotic Lactobacillus paracasei effect on cariogenic bacterial flora. Clin Oral Investig. 2011;15:471–76. doi: 10.1007/s00784-010-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed A, Dachang W, Lei Z, et al. Effect of Lactobacillus species on Streptococcus mutans biofilm formation. Pak J Pharm Sci. 2014;27:1523–28. [PubMed] [Google Scholar]
- 19.Lopez-Medina E, Fan D, Coughlin LA, et al. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathol. 2015;11:e1005129. doi: 10.1371/journal.ppat.1005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasslöf P, Hedberg M, Twetman S, Stecksén-Blicks C. Growth inhibition of oral mutans streptococci and candida by commercial probiotic lactobacilli-an in vitro study. BMC Oral Health. 2010;10:18. doi: 10.1186/1472-6831-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh PD, Head DA, Devine DA. Dental plaque as a biofilm and a microbial community – implications for treatment. J Oral Biosci. 2015;6(Suppl 1):S14. doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee S, Ramesh A. Bacteriocin producing probiotic Lactobacillus plantarum inhibit adhesion of Staphylococcus aureus to extracellular matrix (ECM): Quantitative insight and implications in antibacterial therapy. J Med Microbiol. 2015;64(12):1514–26. doi: 10.1099/jmm.0.000181. [DOI] [PubMed] [Google Scholar]
- 23.Shoji M, Takeshita T, Maruyama F, et al. [Recent advances in the field of oral bacteriology]. Nihon Saikingaku Zasshi. 2015;70(2):333–38. doi: 10.3412/jsb.70.333. [in Japanese] [DOI] [PubMed] [Google Scholar]