Abstract
Patient-derived induced pluripotent stem cells (iPSCs) hold great promise for autologous cell replacement. However, for many inherited diseases, treatment will likely require genetic repair pre-transplantation. Genome editing technologies are useful for this application. The purpose of this study was to develop CRISPR-Cas9-mediated genome editing strategies to target and correct the three most common types of disease-causing variants in patient-derived iPSCs: (1) exonic, (2) deep intronic, and (3) dominant gain of function. We developed a homology-directed repair strategy targeting a homozygous Alu insertion in exon 9 of male germ cell-associated kinase (MAK) and demonstrated restoration of the retinal transcript and protein in patient cells. We generated a CRISPR-Cas9-mediated non-homologous end joining (NHEJ) approach to excise a major contributor to Leber congenital amaurosis, the IVS26 cryptic-splice mutation in CEP290, and demonstrated correction of the transcript and protein in patient iPSCs. Lastly, we designed allele-specific CRISPR guides that selectively target the mutant Pro23His rhodopsin (RHO) allele, which, following delivery to both patient iPSCs in vitro and pig retina in vivo, created a frameshift and premature stop that would prevent transcription of the disease-causing variant. The strategies developed in this study will prove useful for correcting a wide range of genetic variants in genes that cause inherited retinal degeneration.
Keywords: CRISPR, iPSCs, retinal degeneration
Using CRISPR-Cas9 to generate gene-corrected autologous iPSCs for the treatment of inherited retinal degeneration, induced pluripotent stem cells, and genome editing hold great promise for autologous cell replacement therapies for inherited diseases. Here, Burnight and colleagues developed CRISPR-Cas9-mediated genome editing strategies to target and repair the three most common types of retinal disease-causing variants in patient-derived iPSCs: exonic, deep intronic, and dominant gain of function.
Introduction
Inherited retinal dystrophies are an important cause of irreversible blindness in the Western world,1, 2, 3 and many individuals affected by one of these conditions experience extensive photoreceptor cell death during the course of their disease. Fortunately, for the majority of inherited retinal diseases, the inner retina and intracranial visual pathways remain intact years after the photoreceptor cells have been lost.4, 5, 6, 7 The preservation of the inner retina raises the possibility that stem-cell-based photoreceptor cell replacement could restore some vision to these individuals.
To date, embryonic stem cells,8, 9, 10, 11 fetal retinal progenitor cells,12, 13 post-mitotic photoreceptor precursor cells,14, 15 and induced pluripotent stem cells16, 17, 18, 19 have all been used with varying degrees of success to replace lost photoreceptor cells in a variety of animal models. Of these approaches, patient-specific induced pluripotent stem cells (iPSCs) have two major advantages: (1) they avoid the controversial use of embryonic or fetal tissue; and (2) they offer the best possible immunologic match to the patient.
In recent years, multiple groups have successfully generated iPSC-derived photoreceptor precursor cells from patients with molecularly confirmed inherited retinal blindness.16, 20, 21, 22, 23 These cells have been shown to accurately recapitulate disease phenotypes and as a result have been very valuable for elucidating disease pathophysiology ex vivo.16, 20, 21 The fact that patient-specific photoreceptor cells accurately recapitulate disease in vitro suggests that if iPSCs are to be used for autologous cell replacement, the cells’ disease-causing mutations will need to be corrected prior to differentiation and transplantation.
Recent advances in genome editing offer several options for targeting and correction of disease-causing mutations, including components of the CRISPR-Cas9 adaptive immune system.24, 25, 26, 27, 28, 29, 30, 31, 32 Using pre-designed single guide RNAs (sgRNAs) coupled with a human codon-optimized Cas9 nuclease, the CRISPR-Cas9 system can specifically target and edit human gene loci by inducing double-strand breaks (DSBs) followed by repair via either non-homologous end joining (NHEJ) or template-mediated homology-directed repair (HDR).33 Unlike gene augmentation, CRISPR-Cas9-mediated gene correction enables repaired genes to remain under the control of their endogenous regulatory elements, thus avoiding the risk of overexpression toxicity.34, 35 Moreover, CRISPR-Cas9 can correct genes of any size, which overcomes the obstacles associated with viral vector packaging limits.36 In addition, the recently characterized Staphylococcus aureus Cas9 cDNA is small enough that clinically proven adeno-associated virus (AAV) vectors can accommodate the CRISPR-Cas9 machinery, making in vivo genome editing possible.30, 37
Here, we report successful development of a CRISPR-based genome editing strategy for correction of three classes of disease-causing mutations: (1) exonic mutations, (2) deep intronic cryptic splice site mutations, and (3) dominant gain-of-function mutations. To correct mutations within protein-coding regions, homology-directed repair of CRISPR-Cas9-mediated double-stranded DNA breaks, via a wild-type donor template, is the most logical approach. To demonstrate the utility of this strategy, cells from patients with retinitis pigmentosa (RP) caused by a homozygous Alu insertion in exon 9 of the gene male germ cell-associated kinase (MAK),38 were used. This Alu insertion in MAK is the leading cause of RP in people of Jewish ethnicity.38 To correct deep intronic cryptic splice site mutations, CRISPR-Cas9 excision of the mutant intronic sequence and repair via NHEJ should be sufficient in most cases. To demonstrate this approach, the IVS26 mutation in the gene CEP290 was targeted. Mutations in CEP290 are the leading cause of Leber congenital amaurosis (LCA), and IVS26 is the most commonly seen mutation in this gene.39, 40 Finally, for dominant gain-of-function mutations, one can design mutation-specific CRISPR guides that selectively inactivate the mutant allele by creating a frameshift and causing premature arrest of translation. To test this approach, the dominant gain-of-function Pro23His rhodopsin (RHO) mutation was targeted. Mutations in rhodopsin are the most common cause of dominant RP (accounting for 25%–30% of RP families with this inheritance pattern41), and the Pro23His mutation is the most common RP-causing variant overall in North America, causing approximately 3% of all cases of the disease.42
This study was performed to devise and test a strategy for the correction of disease-causing genetic mutations, regardless of patient genotype. With this approach, we can now generate genetically corrected patient-specific stem cells for autologous transplantation from the majority of patients with an inherited retinal degeneration.
Results
CRISPR-Cas9 Specifically and Efficiently Modifies the MAK Locus
Using the CRISPR Design Tool (crispr.mit.edu), we designed three plasmids, each encoding an sgRNA targeting the Alu insertion in MAK (Figure 1A). Each sgRNA was cloned into a bicistronic vector containing the sgRNA driven by the human Pol III U6 promoter and a human codon-optimized Cas9 nuclease24, 43 driven by the chicken β-actin promoter. The T7E1 nuclease assay was employed to evaluate the ability of each sgRNA-Cas9 plasmid to create DSBs in HEK293T cells. A previously published sgRNA targeting the EMX1 locus was included as a positive control.24 In the absence of a homologous repair DNA template, cells endogenously repair DSBs via the NHEJ pathway, which results in the creation of insertions or deletions (indels).33 Each of the three MAK-specific sgRNAs successfully targeted the portion of the MAK gene containing the Alu insertion (Figure 1B). To quantify the efficiency of DSB formation, 80 clones from each guide were sequenced. Of the guides tested, sg1MAK was determined to have the highest cutting efficiency (31.2% ± 1.0% clones modified compared to sg2MAK and sg3MAK, with 21.6% ± 5.7% and 11.6% ± 5.2% clones modified, respectively; Figures S1A and S1B). No deleterious off-target cutting events were identified for sg1MAK (Figures S1D and S1E), and therefore this guide was used in the subsequent patient-specific iPSC experiments described below.
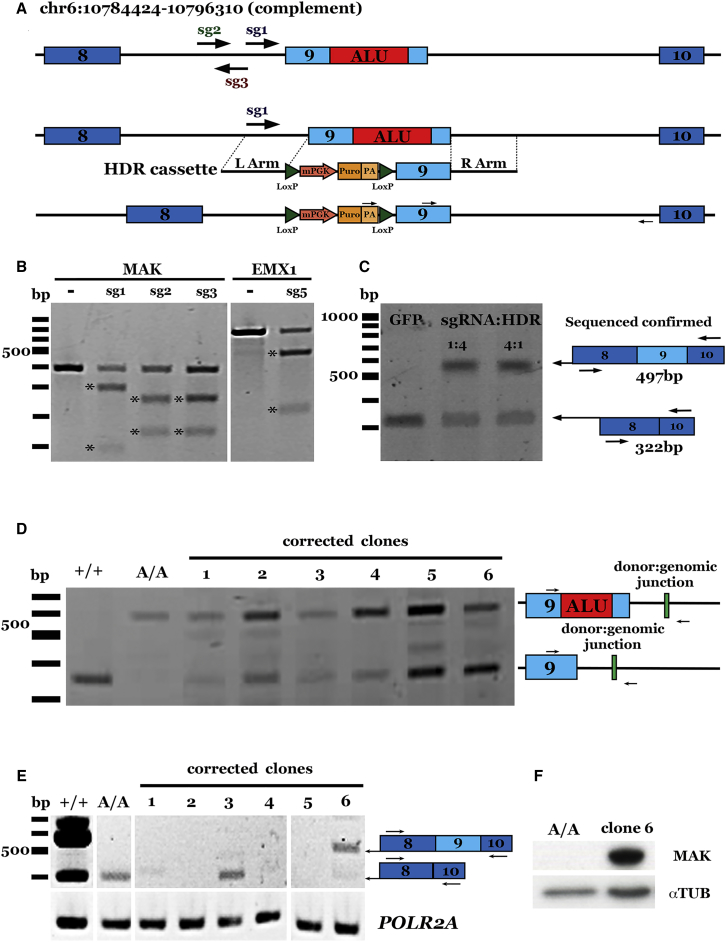
Figure 1.
CRISPR-Based Correction of an Alu Insertion in MAK
(A) Schematic representation of our CRISPR-Cas9/HDR strategy for the correction of the Alu insertion (top; red box) in MAK. sgRNAs tested are indicated by arrows. A donor plasmid (middle; HDR cassette) containing 500 bp of homology flanking a floxed puromycin selection cassette and the wild-type exon 9 sequence was co-transfected using various ratios of sg1-SpCas9-expressing plasmid, resulting in the repaired allele (bottom). (B) Representative gel image of T7E1 assays in HEK293T cells for each MAK sgRNA developed; a previously reported sgRNA targeting the EMX1 locus was included as a control.24 (C) Representative gel image demonstrating restoration of wild-type MAK transcript in sg1-SpCas9-treated, puromycin-selected, iPSC-derived photoreceptor precursor cells from a patient with molecularly confirmed MAK-associated retinitis pigmentosa. (D) Representative gel image confirming HDR in selected iPSC clones. +/+ indicates unaffected control cells; A/A indicates uncorrected patient iPSCs. (E) Representative gel image demonstrating restoration of wild-type MAK transcript in one puromycin-selected iPSC clone (clone 6) from a patient with molecularly confirmed MAK-associated retinitis pigmentosa. (F) Representative gel image confirming restoration of MAK protein expression in corrected iPSC clone 6.
Homologous Recombination-Mediated Genome Editing Restores MAK Expression in Patient Cells
To determine the optimal ratio of sgRNA-Cas9 plasmid to HDR plasmid for correcting the Alu insertion in exon 9, we co-delivered the sg1MAK-Cas9 plasmid and an HDR donor plasmid carrying 500 bp of homologous sequence flanking MAK exon 9 and a puromycin resistance cassette (Figure 1A) to HEK293T cells at various ratios. The target locus was amplified using primers complementary to the donor plasmid (upstream arrow) and intron 9 sequence downstream of the cassette (downstream arrow; Figure 1A). Agarose gel electrophoresis showed that the expected size band (1,124 bp) was only present when both the donor and sg1MAK-Cas9-expressing plasmids were co-transfected (Figure S1C).
To demonstrate our ability to correct the Alu insertion in patient cells using this strategy, iPSCs from a patient with MAK-associated RP were targeted (Table 1, patient 1). As shown previously,38 retinal cells generated from patients with a homozygous Alu insertion in exon 9 of the MAK gene lose, via nonsense mediated decay, expression of the normal exon-9-containing retinal MAK transcript (Figure 1C, lane 1). The HDR donor plasmid and the sg1MAK-Cas9 plasmid were co-delivered to patient-specific iPSCs via nucleofection at 1:4 and 4:1 CRISPR to donor ratios (the ratios determined to give the most robust results in the HEK293T experiment described above). Patient-specific iPSCs were subsequently differentiated into photoreceptor precursor cells, and RT-PCR using primers flanking exon 9 was performed. Cells that received CRISPR-Cas9 and a homology repair template at either ratio underwent HDR of the targeted MAK locus and restoration of the retinal exon-9-containing transcript. (Figure 1C, lanes 2 and 3). iPSCs that received CRISPR-Cas9 and a control GFP reporter plasmid did not (Figure 1C, lane 1).
Table 1.
Patient Samples Used in This Study
To obtain clonal populations of corrected cells, we delivered HDR donor and sg1MAK-Cas9 plasmids to patient-specific iPSCs. Following puromycin selection, resistant colonies were picked and expanded to analyze genomic correction at the MAK locus. PCR analysis using primers upstream of the Alu insertion in exon 9 and downstream of the right homology arm indicated that one allele was corrected in each of the six clones assessed (Figure 1D). Restoration of the exon-9-containing transcript (Figure 1E, clone 6) and full-length MAK protein was detected (Figure 1F, clone 6). Although biallelic correction was not observed, we hypothesize that because MAK-associated RP is a recessive disorder and there are no cases of the disease demonstrating haploinsufficiency, monoallelic correction will be sufficient. Collectively, these data demonstrate that we are able to successfully target and correct a disease-causing exonic variant and restore MAK expression in patient-derived cells using CRISPR-Cas9 mediated genome editing.
CRISPR-Cas9-Mediated Removal of a Deep Intronic Mutation in Human Cells
Mutations in the large, centrosomal protein gene CEP290 are the largest contributor to the severe childhood blinding disease LCA. Patients with CEP290-associated LCA typically present with poor visual acuity, nystagmus, and a non-recordable electroretinogram (ERG), all of which are due to loss of light-sensing photoreceptor cells. The most common disease-causing variant in CEP290 is a deep intronic cryptic splice site mutation (IVS26), which causes inclusion of a cryptic exon (exon X) and creation of an in-frame premature stop codon.39 AAV, the most commonly employed vector for U.S. Food and Drug Administration (FDA)-approved clinical gene therapy, cannot accommodate the full-length CEP290 (∼8 kb) cDNA. Although lentiviral-mediated CEP290 gene augmentation has been used to restore ciliogenesis in cells from patients with CEP290-associated LCA, significant overexpression cytotoxicity was also observed in these experiments.34 A genome editing strategy would overcome the vector packaging limitations and also allow gene expression to remain under control of endogenous regulatory elements, thereby reducing the possibility of overexpression cytotoxicity. We hypothesized that removal of the mutation via CRISPR-Cas9-mediated NHEJ would restore CEP290 expression.
To this end, we designed two sgRNA plasmids that express single guide RNAs, sg1CEP290 or sg2CEP290, under the control of the human U6 promoter. The sgRNAs, identified using the Optimized CRISPR Design Tool (crispr.mit.edu), were designed to target S. pyogenes Cas9 to sites surrounding the CEP290 IVS26 A > G mutation (c.2991+1655A > G; Figures 2A and 2B). To maximize genome editing efficiencies, we used a human codon optimized version of Cas9 that was fused to a SV40 nuclear localization sequence and a T2A-GFP tag (plasmid pCas9_GFP, a gift from Kiran Musunuru, Addgene #44719). To induce indel formation, one or both sgRNA plasmids, along with pCas9_GFP, were nucleofected into human iPSCs derived from a patient homozygous for the CEP290 IVS26 mutation c.2991+1655A > G (Table 1, patient 2).
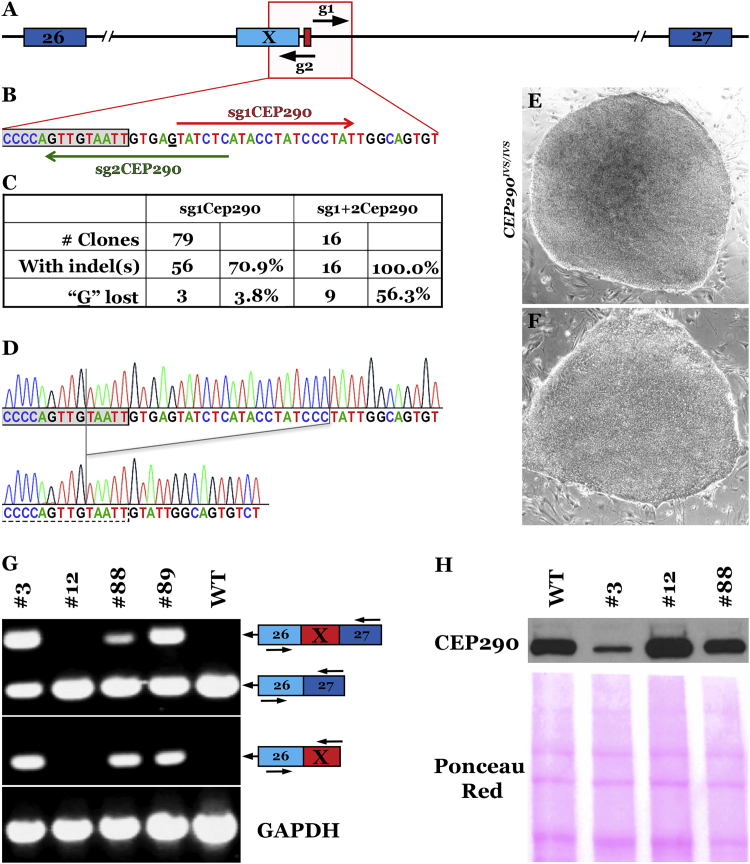
Figure 2.
CRISPR-Cas9 Correction of the Deep Intronic IVS26 Mutation in CEP290
(A) Location of the two single guide RNAs. (B) sgRNA1 and 2 were designed to generate S. pyogenes Cas9-induced deletions that remove the mutant nucleotide and normalize CEP290 pre-mRNA splicing in hiPSCs homozygous for the CEP290 c.2991+1655A > G mutation (nucleotide 1655 of intron 26 is underlined). The gray box represents the 3′ end of the cryptic exon X. (C) Quantification of indel formation frequency and loss of the CEP290 disease-causing mutation with the single- or dual-guide strategy. (D–F) Representative sequence alignments (D) and phase-contrast images (4×) of two CEP290 clones: clone #3 (E), unchanged, and clone #12 (F), homozygous deletion. (G) CEP290 transcript analysis by semiquantitative RT-PCR. (Top) Amplicons obtained using primers located in exons 26 and 27 (E26, E27; EX represents inclusion of the cryptic exon X). (Bottom) Amplicons obtained with primers located in exons 26 and X. GAPDH was amplified as a control. (H) Western blot demonstrating the increase in full-length CEP290 protein expression in LCA-iPSCs with no (clone #3), one (clone #88), or two (clone #12) repaired alleles. Nitrocellulose Ponceau S. staining is shown to demonstrate even sample processing and loading.
Human iPSC colonies that emerged after sorting and single-cell plating of GFP+ SSEA5+ live cells were picked, expanded, and analyzed for the presence of indels; in total, 95 clones were analyzed by sequencing of genomic PCR products. Using either a single- or a dual-guide strategy, we achieved very high indel formation efficiencies, with 70% (sg1CEP290) to 100% (sg1+2CEP290) of clones carrying an indel in at least one of the CEP290 alleles (Figure 2C). Importantly, loss of the disease-causing mutant nucleotide in at least one allele was observed in >50% of clones obtained using the dual-guide approach (Figure 2C).
Clones in which the mutation was lost in one (clone 88) or both alleles (clone 12, Figure 2D), along with unmodified control clones (clones 3 and 89), were selected for further analysis. Despite the very high on-target efficiency, no off-target activity was detected for either sg1CEP290 or sg2CEP290 (Figure S2), and all clones maintained a normal iPSC morphology (Figure 2E versus Figure 2F) and were euploid (data not shown). To test if proper splicing was restored in genome-edited clones, we performed RT-PCR using primers located in exons 26, X (cryptic exon), and 27. In wild-type control human iPSC (hiPSC) cDNA samples, no evidence of inclusion of the aberrant exon X was found. As previously reported for other cell types,44 homozygous mutant hiPSC clones 3 and 89 expressed both wild-type (exon 26 fused to exon 27) and mutant (exon-26-exon-X-exon-27 spliced together) CEP290 mRNAs (Figure 2G). Importantly, clone 12 (homozygous deletion) showed an increase in the wild-type CEP290 transcript and a complete lack of amplification of the cryptic exon, whereas heterozygous clone 88 showed an intermediate phenotype, indicating that loss of the mutant nucleotide does indeed restore proper CEP290 pre-mRNA splicing.
Lastly, we looked at expression of full-length CEP290 protein in these clones by western blot analysis (Figure 2H). We were able to show that CEP290 protein levels increased with the number of repaired alleles; repair of a single allele led to a marked increase in CEP290 protein expression that nevertheless failed to reach wild-type levels (clone 88). On the other hand, repair of both alleles (clone 12) fully restored CEP290 protein expression levels. Overall, these data provide a proof of principle that CRISPR-Cas9-mediated NHEJ can be used efficiently and safely to remove the disease-causing nucleotide and to normalize splicing and expression of full-length CEP290 protein in LCA patient-derived cells. However, in its current form, the CRISPR-Cas9 machinery from S. pyogenes is unsuitable for AAV-mediated in vivo genome editing due to cargo size limitations of the vector.45
Staphylococcus aureus Cas930, 37 is small enough to be packaged into AAV and could, in principle, permit both in vitro and in vivo correction. For this reason, we chose S. aureus CRISPR-Cas9 for the next experiments. However, there were no suitable S. aureus protospacer adjacent motif (PAM) sites near the IVS26 mutation to facilitate a single-cut strategy similar to those described above using S. pyogenes Cas9. We therefore designed pairs of guide RNAs that flank the IVS26 mutation (Figure 3A) and cloned them into a dual-expression plasmid carrying the cDNA for S. aureus Cas9 as well as AAV2 inverted terminal repeats (ITRs) for viral packaging.30 Transfection of this construct into HEK293T cells followed by PCR amplification of the targeted locus demonstrated successful removal of the expected fragment using guide pair sg4/5CEP290, with an efficiency of approximately 20% (Figure S3A). No deleterious off-target cutting events were identified for either sg4CEP290 or sg5CEP290 (Figure S4), and therefore this guide pair was used for the subsequent patient-specific iPSC experiments described below.
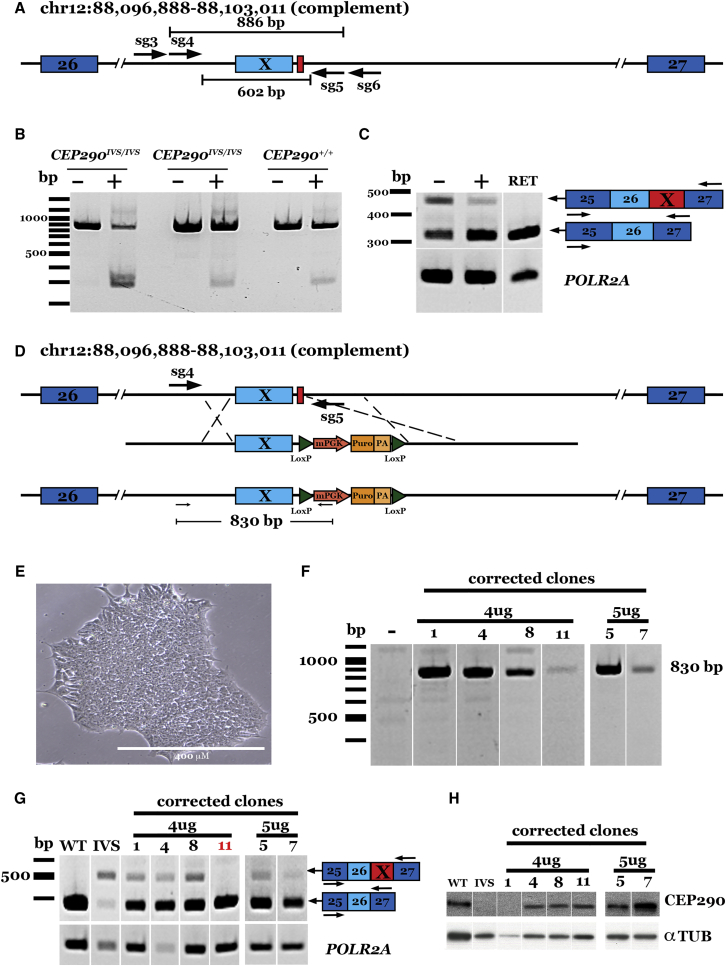
Figure 3.
CRISPR-Cas9 Correction of the Deep Intronic IVS26 Mutation in CEP290 Using S. aureus Cas9
(A) Schematic representation of our paired guide strategy for the correction of the deep intronic IVS26 cryptic splice site mutation in CEP290. Two guides targeting upstream (sg3 and sg4) of the IVS26 mutation (red box) were each paired with two guides (sg5 and sg6) targeting downstream of the mutation to delete the exon X sequence and IVS26 mutation. (B) Representative gel image showing sg4/5-SaCas9-mediated IVS26 deletion in patient-derived iPSCs. Two lines of patient-derived iPSCs carrying homozygous IVS26 genotypes and those from a control unaffected individual were transfected with sg4/5-SaCas9-expressing plasmid (+) or Cas9 only (−). (C) Representative gel image demonstrating restoration of CEP290 transcript in sg4/5-SaCas9-treated iPSCs. POLR2A was amplified as a loading control. RET, human control retina cDNA. (D) Schematic representation of our CRISPR-Cas9/HDR strategy for the correction of the IVS26 cryptic splice site mutation (top; red box) in CEP290. Sg4/5RNA pair-expressing plasmid was co-delivered with a donor plasmid (middle; HDR cassette) containing 500 bp of homology flanking a floxed puromycin selection cassette and the wild-type IVS26 sequence resulting in the repaired allele (bottom). (E) Bright field image of a homozygous IVS26 patient-derived iPSC clone post-puromycin selection (note normal feeder-free iPSC morphology). Scale bar, 400 μM. (F) Representative gel image of genomic DNA from iPSC clones co-transfected with sg4/5-expressing plasmid and donor plasmid and cultured under puromycin selection. (G) Representative gel image demonstrating restoration of CEP290 transcript in sg4/5-SaCas9/donor-plasmid-treated iPSC clones. POLR2A was amplified as a loading control. WT, unaffected control iPSC cDNA; IVS, uncorrected patient-derived iPSCs carrying homozygous IVS26 alleles. (H) Western blot demonstrating restoration of full-length CEP290 protein in CRISPR/HDR-treated iPSC clones. Anti-α-Tub was used as a loading control.
To demonstrate removal of the IVS26 mutation in patient-specific cells, the sg4/5-SaCas9-expressing plasmid was delivered to iPSCs derived from two separate patients with molecularly confirmed CEP290-associated LCA, each homozygous for the IVS26 variant. Plasmid electroporation followed by PCR amplification of the locus confirmed removal of the mutation-containing fragment in patient-derived cells (Figure 3B). Importantly, normal CEP290 transcript appeared to be increased in patient cells following the CRISPR-Cas9 correction, as demonstrated by semiquantitative RT-PCR, with primers that flank the cryptic exon X (Figure 3C).
Although we demonstrated successful removal of the cryptic splice mutation in patient cells using NHEJ-mediated repair with both our single- and dual-cut strategies, homology-dependent repair and positive drug selection is advantageous (i.e., does not rely on fluorescence activated cell (FAC) sorting or large-scale clonal expansion) when employing CRISPR-Cas9 correction ex vivo. Therefore, we designed an HDR donor template plasmid (carrying ∼500-bp homologous sequence flanking the wild-type (WT) sequence and a puromycin selection cassette [Figure 3D]) that was co-delivered with the sg4/5-SaCas9-expressing plasmid to patient-specific iPSCs. Following puromycin selection, resistant colonies (Figure 3E) were picked and expanded for genomic and expression analysis. 5 of 12 puromycin-resistant clones carried at least one corrected allele, as indicated by PCR amplification of the 5′ donor-genomic junction using primers complementary to the donor sequence and intronic sequence upstream of the donor homology arm (Figures 3F and S3B). When transcript expression was evaluated by RT-PCR using primers flanking the cryptic exon X, only WT CEP290 expression was detected in clone 11, indicating bi-allelic correction in this clone (Figure 3G). Importantly, CEP290 protein expression was restored in five of the clones (Figure 3H). Together, these experiments support the hypothesis that a CRISPR-Cas9-mediated correction can be used to remove the common deep intronic cryptic splice site mutation in CEP290 IVS26 and restore CEP290 transcript and protein in the cells of patients with CEP290-associated LCA.
Allele-Specific Targeting of a Dominant Mutation Causing RP in Human Cells
For dominant diseases caused by harmful gain-of-function mutations, it is unlikely that gene augmentation would be effective in mitigating the disease phenotype. Treatment of these diseases will usually require silencing the mutant allele, ideally while leaving the normal allele intact. To demonstrate the feasibility of using CRISPR-Cas9 to execute such an allele-silencing strategy, the common Pro23His mutation in RHO was targeted. The P23H locus was first surveyed to identify suitable guide and PAM sequences for specifically targeting the mutant allele. Both S. pyogenes (NGG) and S. aureus (NNGRRT) PAMs were considered, and two S. aureus guides specific for the His allele were designed such that the mutation fell within the seed region of the guide (Figure 4A). Control guides targeting the Pro allele were also designed and cloned into an expression construct carrying the U6-driven guide expression cassette as well as the SaCas9 cDNA sequence.30 To test allele specificity, constructs expressing both guides (sgH23-1 and sgH23-2) and their respective controls (sgP23-1 and sgP23-2) were transfected into HEK293T cells, which carry only the wild-type Pro allele. T7E1 assays and subsequent TA cloning and sequencing experiments for each guide demonstrated that the guides targeting the P allele had 19-fold (sgP23-1 versus sgH23-1) to 22-fold (sgP23-2 versus sgH23-2) greater activity than those targeting the His allele (14.9% ± 2.2% NHEJ versus 0.8% ± 0.8% NHEJ and 16.5% ± 1.4% NHEJ versus 0.8% ± 0.4% NHEJ, respectively; Figures 4B and 4C). Guide sgH23-2 was chosen over sgH23-1 because of its superior on-target cutting efficiency. No deleterious cutting events at other loci were detected for sgH23-2 (Figure S5), and therefore this guide was used for the subsequent patient-specific iPSC experiments described below.
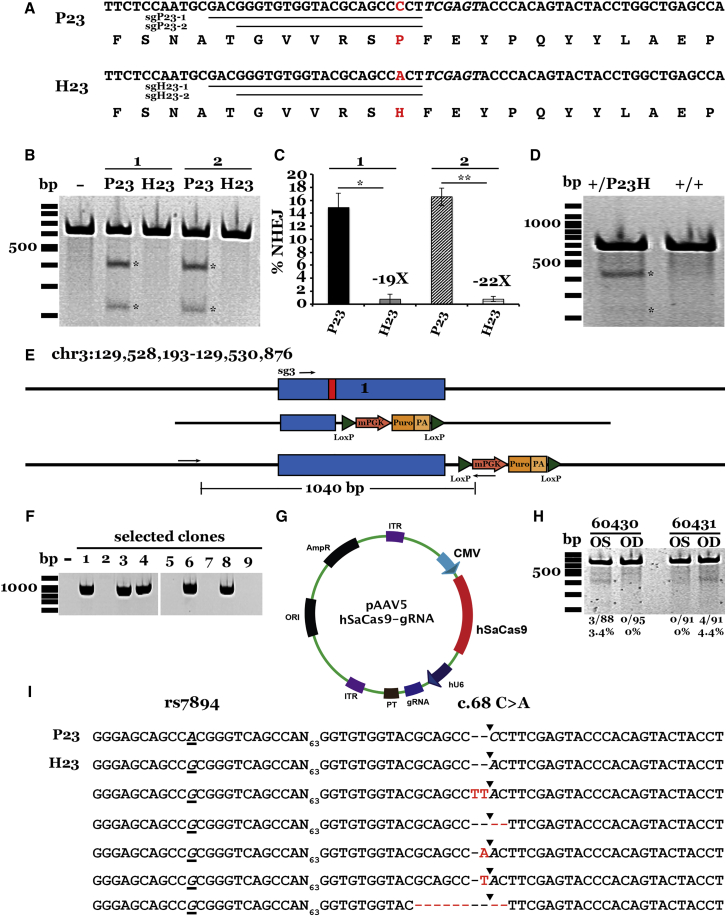
Figure 4.
Allele-Specific Targeting of the Dominant Gain-of-Function Pro23His Mutation in RHO
(A) Nucleotide and amino acid sequences of unaffected (P23) and affected (H23) RHO alleles. Underlined sequences indicate guides targeting each allele. Red text indicates mutation (c.68 C > A transversion at codon 23) lying in the seed region of each guide. (B) Representative gel image of T7E1 assays in HEK293T cells for each RHO sgRNA tested. (C) Histogram showing percent NHEJ in HEK293T cells transfected with each guide. Efficiency was quantified by subcloning and Sanger sequencing (please refer to Materials and Methods for further details). At lest 61 clones were sequenced per transfection. Data are represented as mean ± SEM. n = 3; *p < 0.01; **p < 0.001. (D) Representative gel image of T7E1 assays in patient-derived iPSCs (+/P23H) and iPSCs from an unaffected individual (+/+) transfected with plasmids expressing the sgH23-2 guide and SaCas9. (E) Schematic representation of our CRISPR-Cas9/HDR strategy for the correction of the Pro23His mutation (top; red box) in RHO. Sg3-hSpCas9-expressing plasmid was co-delivered with a donor plasmid (middle; HDR cassette) containing 500 bp of homology flanking a floxed puromycin selection cassette and the wild-type (Pro) sequence, resulting in the repaired allele (bottom). (F) Representative gel image of genomic DNA from iPSC clones co-transfected with sg3-expressing plasmid and donor plasmid and cultured under puromycin selection. (G) Schematic of transgene cassette plasmid carrying the allele-specific guide H23-2 and the humanized SaCas9 expression cassettes flanked by AAV2 ITRs packaged into the AAV5 serotype vector. (H) Representative gel image of T7E1 assays performed on genomic DNA isolated from the retinas of two animals treated unilaterally with AAV2/5-sgH23-2-SaCas9 vector. Amplicons were gel purified, subcloned, and sequenced via Sanger sequencing. Sequences from recovered clones (I) were aligned using Lasergene DNA analysis software. The percentage of alleles demonstrating NHEJ is indicated at the bottom of each lane. The rs7984 A/G SNP occurs 93 bp upstream of the Pro23His mutation. The P23 allele carries the rs7984 A nucleotide (top; underlined and italicized), whereas the H23 allele carries the rs7984 G nucleotide (second from top; underlined and italicized). Sequenced amplicon clones from sgH23-2-treated animals revealed formation of indel in the H23 allele only.
To test allele-specific targeting in patient cells, the sgH23-2-expression construct was delivered to iPSCs derived from a patient with the Pro23His genotype. As a control, the sgH23-2-expressing construct was also delivered to iPSCs from an unaffected individual (i.e., Pro alleles only). T7E1 nuclease assays indicated that modification had occurred in iPSCs from the patient harboring the His allele but not in those of the unaffected control (Figure 4D). To confirm this specificity, amplicons from the patient sample were TA cloned and Sanger sequenced. A single nucleotide polymorphism that occurs 93 bp upstream of the Pro23His mutation (rs7984; Figure 3I) was used for phase determination. Of the 86 clones sequenced, indel formation was detected in the His allele only (data not shown).
In addition to our allele-specific strategy, as above, we also employed homology-dependent repair. We designed a donor template carrying a ∼500 bp homologous sequence flanking the WT Pro23 sequence and a puromycin selection cassette (Figure 4E). Using the CRISPR Design Tool as previously described, we selected three guides to clone into the bicistronic vector expressing S. pyogenes Cas9 and tested cleavage efficiency via T7E1 assays in HEK293T cells (Figure S5C). The guide with the highest efficiency (sg3; Figure S5C) was used in subsequent experiments. The donor HDR plasmid was co-delivered with the sg3-Cas9-expressing plasmid to patient-specific iPSCs. Following puromycin selection, resistant clones were expanded. Five of nine puromycin-resistant clones carried the correction cassette, as demonstrated by PCR, with primers amplifying the 5′ donor-genomic junction (Figure 4F).
One of the advantages of using the S. aureus Cas9 (as employed in our allele-specific strategy) is the size of the cDNA is within the packaging limit of the clinically approved AAV viral vector. Thus, we extended our studies by packaging the sgH23-2-SaCas9 transgene cassette into AAV5 (Figure 4G). To test allele-specific CRISPR-Cas9 correction in vivo, we employed a transgenic pig model that carries the human Pro23His mutation46 (see Supplemental Materials and Methods). 2.5 × 109 TU AAV5-sgH23-2-SaCas9 were injected sub-retinally into five transgenic pigs unilaterally at 7 1/2 weeks of age. At 3 weeks post-injection, animals were sacrificed and retinas dissected. To assess NHEJ at the Pro23His locus in injected pigs, we performed T7E1 assays on DNA isolated from injected retinas. Uninjected contralateral retinas were used as controls. Two of five animals injected demonstrated successful modification of the Pro23His locus when compared to control sham-injected retinas (Figure 4H). We quantified NHEJ in these animals via subcloning and Sanger sequencing, as described above. We observed 3.4% (3/88) and 4.4% (4/91) NHEJ in animals 60430 and 60431, respectively (Figures 4H and 4I). Collectively, these experiments demonstrate the feasibility of using CRISPR-based genome editing in vitro for allele-specific targeting and correction of iPSCs from patients with autosomal dominant disease.
Discussion
In this study, we present data to demonstrate the utility of the CRISPR-Cas9 genome editing system for genetically correcting iPSCs generated from patients with inherited retinal degenerative blindness. Specifically, we have devised a strategy that can be used to correct disease-causing variants, regardless of genomic location and mode of inheritance (coding sequence mutations requiring homology-dependent repair; deep intronic cryptic splice site mutations that can be corrected via NHEJ; and dominant gain-of-function mutations that can be corrected via allele-specific indel formation).
To demonstrate repair of coding sequence variants, we targeted a 354-bp Alu insertion in the MAK gene, the leading cause of recessive RP in people of Jewish ancestry. By successfully removing this large repetitive element and subsequently correcting the MAK locus in patient-specific iPSCs, we demonstrated that this repair strategy could be used to correct virtually any disease-causing mutation. In this experiment, we used a plasmid-based homology-dependent repair approach because of the size and location of the MAK variant. However, a common alternative to plasmid-based homology directed repair is use of single-stranded oligodeoxynucleotides (ssODNs),47 which have demonstrated efficient correction of small insertions, deletions, and single-point mutations. Unlike plasmids, ssODNs can be rapidly and inexpensively synthesized without the need for cloning. In fact, when coupled with Cas9-guide RNA ribonucleoprotein (RNP) complexes, the entire system can be easily designed and purchased as ready-to-use reagents. In conjunction with the recent report by Yoshimi and colleagues,48 who used a 1-kb ssODN to knock-in GFP at the rat Thy1 locus in zygotes, this strategy may now be useful for correction of large insertions or deletions, such as the one targeted in this study. That said, the plasmid-based approach used in our study has several major advantages. For example, selection elements, such as antibiotic resistance cassettes, can be included and flanked by loxP sites for easy Cre-mediated excision following correction. In the absence of selection, HDR efficiencies in stem cells are typically less than 2%.49 Thus, selection approaches like this can be very useful. A second major advantage of the plasmid-based system is that in-house production can be readily adapted for GLP and later cGMP applications. cGMP-grade Cas9 proteins and ssODNs are not currently available as off-the-shelf reagents nor is it currently known at which rate ssODNs randomly integrate into the genome when delivered at the dose required to facilitate HDR. For these reasons, the plasmid-based system is most appropriate for clinical applications at the present time.
Although coding sequence variants are the most common cause of recessive retinal degenerative blindness, many patients harbor mutations in introns that disrupt gene function. For instance, many deep intronic variants that disrupt transcript splicing have been reported in the genes ABCA4,50 USH2A,17 and CEP290,39 the leading causes of Stargardt disease, RP, and LCA, respectively. Unlike coding sequence variants that require HDR, disease phenotypes that result from cryptic splice site mutations can often be corrected by mutation-specific deletions and NHEJ. In this study, we corrected the most common deep intronic variant in the gene CEP290, a cryptic splice activator in IVS26. By co-delivering either the S. pyogenes or S. aureus Cas9 cDNA and one/two single guide RNAs with sequences that flank the IVS26 mutation, we were able to delete the cryptic splice site and restore the wild-type CEP290 in the cells of patients affected with CEP290-associated LCA. Recently, Young and colleagues51 employed a similar strategy for correction of the Duchenne muscular dystrophy gene dystrophin (DMD). By taking advantage of CRISPR-Cas9-mediated NHEJ, the authors demonstrated deletion of up to 725 kb of DMD sequence and restoration of functional dystrophin protein.51 This approach has the potential to be used for in vivo correction. In fact, S. aureus Cas9 is small enough to be efficiently packaged into the clinically relevant AAV.30, 37, 52 AAV-mediated delivery of CRISPR-Cas9 both locally and systemically in a mouse model of Duchenne muscular dystrophy was shown to restore the Dmd reading frame in myofibers, cardiomyocytes, and muscle stem cells.52 Moreover, genetically corrected animals showed a partial recovery in muscle function.52 Although patients with CEP290-associated LCA are often born with very poor vision, many retain a small zone of non-functioning foveal cone photoreceptor cells that can be readily detected with in vivo imaging.53, 54 It is possible that by restoring CEP290 function in these remaining photoreceptor cells, one could restore some vision to these patients. To be most effective, it is likely that such a treatment would need to be administered in the first few years of life while the retino-cortical pathways are still plastic enough to respond to visual stimulation. Individuals who have not experienced formed vision in the first decade of life would be much less likely to benefit from restoration of CEP290 function in their remaining foveal photoreceptors.
Unlike recessive diseases, which are typically caused by a loss of gene function, dominant disorders often result from the action of a harmful mutant gene product. Many dominant retinal disease-causing mutations have been identified to date and one of the most common is the Pro23His variant in rhodopsin (RHO).55 The Pro23His RHO mutation causes mis-folding of the protein product, which leads to endoplasmic reticulum (ER) stress and subsequent photoreceptor cell death.56, 57 Loss-of-function variants in RHO do not cause disease, and carriers of recessive mutations do not develop a photoreceptor cell phenotype.58 These data raise the possibility of treating Pro23His-associated RP by selectively silencing the mutant allele and leaving the normal allele intact. By identifying an S. aureus Cas9 PAM site adjacent to the Pro23His variant, we were able to build sgRNAs that target the disease allele. These reagents are selective and should be useful for the generation of genetically corrected patient-specific iPSCs. Using a similar approach, Courtney and colleagues59 recently demonstrated allele-specific targeting of heterozygous SNPs in the gene KRT12, which causes autosomal dominant Meesmann’s epithelial corneal dystrophy (MECD). Efficient reduction of mutant KRT12 transcript and protein was demonstrated via transfection into HEK293T AD293 cells in vitro and via direct injection into the corneal stroma of a humanized MECD mouse model in vivo.59 Similarly, Yoshimi et al.60 recently reported production of an sgRNA construct capable of discriminating an SNP in rat embryonic fibroblasts, thereby correcting a dominant phenotype in embryos. Collectively, these data support the development of CRISPR-based allele-specific targeting and deletion of dominant disease-causing variants.
The ultimate goal of this study was to develop a comprehensive CRISPR-based genome editing strategy that would enable the generation of genetically corrected iPSCs for autologous retinal cell replacement. One of the major concerns when using any genome editing approach is the potential for off-target modification. Of the 217 predicted off-target sites identified in this study, only nine were located in protein coding regions of the genome, and none of these sites were modified using any of the guides that were developed during this study. As promising as these findings are, for clinical applications of CRISPR-based genome editing, empirical assessment of the integrity of the whole genome may be necessary after the editing has been performed.30, 61, 62, 63, 64 Genome-wide unbiased identification of DSBs enabled by sequencing (GUIDE-seq) can detect Cas9 off-target cleavage in living cells. This method tags Cas9-induced DSBs by integrating blunt, double-stranded oligodeoxynucleotides. These tagged DSBs can be precisely mapped to the genome using next-generation sequencing and bioinformatic analysis. Using this method, off-target modification can be detected at frequencies as low as 0.1% or less.65 Alternatively, perhaps an even more comprehensive approach would be to employ whole genome sequencing to evaluate genomic integrity following correction in a thorough and unbiased manner.66
The ability to package the smaller S. aureus Cas9 into an AAV vector raises the exciting possibility of in vivo CRISPR-based therapies. Diseases that result from deep intronic and dominant gain-of-function variants, in which NHEJ may be sufficient to mitigate the disease phenotype, are particularly attractive targets. That said, concerns regarding off-target modification in vivo are more difficult to address. Unlike autologous iPSCs, which can be thoroughly screened prior to transplantation, it is difficult to guarantee that in vitro off-target results will directly translate to the in vivo condition. For instance, although in vivo off-target analysis can be performed in animal models after treatment using BLESS (breaks labeling, enrichment on streptavidin, and next-generation sequencing – an approach that detects DSBs in situ through ligating biotinylated adaptors in post-mortem fixed tissues30, 64), there is no guarantee that animal data will directly translate to the patients for whom the treatment is intended. There will always be some degree of uncertainty associated with the genomic differences between the test subjects and the eventual patients. However, it is important to keep in mind that all medical interventions have some risk of an untoward outcome, and this risk is usually somewhat proportional to the potency of the treatment. Physicians simply balance the risk of harm to the probability of benefit when choosing whether to employ the treatment in a given patient. Although this risk-benefit calculation will be different for every disease, and perhaps for every patient, the recent approval of a phase 1 clinical trial of the safety of in vivo zinc finger nuclease genome editing for the treatment of Hemophilia B illustrates that the FDA is now willing to consider the utility of these cutting-edge approaches.
Patients with severe photoreceptor cell loss from inherited retinal disease often have significant preservation of the retinal vasculature, inner retinal neurons, retinocortical connectivity, and retinotopic mapping, and the existence of this relatively healthy inner retina provides hope that stem-cell-based photoreceptor cell replacement will one day be useful for restoring vision to these patients. However, in most diseases of the outer retina, the blood retinal barrier is compromised, providing the immune system with unfettered access to the injured tissue. If one could use autologous cells for therapeutic transplants, it would reduce the risks of immunologic injury to the transplanted cells and collateral immunologic injury to the still viable inner retina. For this reason, the advent of methods for generating autologous iPSC-derived photoreceptor precursor cells17, 20, 38, 67 was arguably the most significant advance toward an effective photoreceptor replacement therapy to date. As promising as autologous iPSCs are for photoreceptor cell replacement, the means by which iPSC-derived photoreceptor cells are delivered to the dystrophic retina is nearly as important as the cell source. Without a remaining retinal outer nuclear layer for donor cells to integrate into, cells injected into the subretinal space as a bolus cell suspension are often poorly oriented and subject to significant cell loss due to efflux and apoptosis.68, 69, 70, 71 Therefore, in addition to using an autologous cell source, it will likely be important to employ some form of cell delivery scaffold designed to promote proper cellular alignment and post-transplant survival.72 This will be especially critical for diseases such as late-stage AMD, in which co-delivery of multiple cell types, including photoreceptors and RPE, may be required in order to achieve successful therapeutic outcomes.
The one remaining hurdle for using such methods to treat patients with Mendelian diseases of the retina was the availability of a robust technology that could be used to correct the cells’ disease-causing mutations prior to transplantation. In this study, we showed that the CRISPR-Cas9 system can be used to correct disease-causing mutations in patient-specific cells, regardless of their genomic location and mode of inheritance. We employed three methods: HDR, single- and dual-guide NHEJ approaches for removing intronic mutations, and allele-specific NHEJ. As promising as the NHEJ approaches are for in vivo correction, the ability to include a drug selection cassette in the homology-dependent repair construct makes HDR a desirable approach for genetic modification of iPSCs in vitro. Specifically, the ability to select via induced drug resistance reduces the amount of manual isolation and clonal expansion required before identifying correctly modified iPSC clones (i.e., a 1% correction efficiency in the absence of a selection cassette would require on average that the experimenter expand and screen 100 iPSC clones before identifying a single positive). Given that the efficiency of genome editing in iPSCs is often significantly lower than that of commonly used experimental cell lines (HEK293Ts, HeLas, etc.),49 this imparts a significant time and cost advantage. On the other hand, as shown in Figure 4, because the NHEJ approaches do not rely on homologous recombination, which will undoubtedly be extraordinarily inefficient in post-mitotic cells, it may be more amenable to in vivo genome editing. Regardless of the approach used, the findings presented in this study bring us one step closer to the goal of providing an autologous cell-based treatment for patients with advanced inherited retinal degenerative blindness.
Materials and Methods
Patients
All patients provided written, informed consent for this study, which was approved by the Institutional Review Board of the University of Iowa (project approval #200202022) and adhered to the tenets set forth in the Declaration of Helsinki. Patient 1 is homozygous for a molecularly confirmed 353-bp Alu insertion in exon 9 of MAK, which we previously reported to cause a loss of transcript and an inability to produce functional MAK protein.38 Patients 2–4 are each homozygous for a c.2991 + 1655 A > G deep intronic cryptic splice site mutation in CEP290. Patient 5 is heterozygous for a c.163 C > A (Pro23His) mutation in the gene encoding rhodopsin (RHO) (Table 1).
Screening of Gene-Specific sgRNAs
Determination of On-Target Cutting Efficiency of Single Guides
sgRNAs and CRISPR-Cas9 expression constructs were first tested in HEK293T cells via lipofection (Table 2). HEK293T cells were maintained in standard culture medium (minimal essential medium alpha [MEMα; Thermo Fisher Scientific], 10% FBS, and 0.2% Primocin [InvivoGen]) and passaged using standard culture conditions as described previously.73 500 ng of each plasmid were transfected into 4 × 105 cells with Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. 48 hr post-transfection, genomic DNA was isolated (Machery-Nagel Nucleospin Tissue Kit, Clontech) and loci of interest were PCR amplified using the AccuPrime Taq DNA Polymerase High Fidelity Kit (Thermo Fisher Scientific) and locus-specific primers (Table S1). PCR reactions from sgMAK and sgP23H-transfected cells were column purified using the MinElute PCR Purification Kit according to the manufacturer’s instructions (QIAGEN). 200 ng of each purified PCR reaction were heteroduplexed in 20 μL reactions with 1X Buffer 2 (New England Biolabs) as follows: 95°C for 10 min, 95°C–85°C at −2°C/s, 85°C for 1 min, 85°C–75°C at −0.3°C/s, 75°C for 1 min, 75°C–65°C at −0.3°C/s, 65°C for 1 min, 65°C–55°C at −0.3°C/s, 55°C for 1 min, 55°C–45°C at −0.3°C/s, 45°C for 1 min, 45°C–35°C at −0.3°C/s, 35°C for 1 min, 35°C–25°C at −0.3°C/s, 25°C for 1 min, 25°C–4°C at −0.3°C/s, and 4°C hold. Heteroduplexes were treated with 1 μL of T7E1 Nuclease (New England Biolabs) for 30 min at 37°C and subsequently resolved via gel electrophoresis using 2% pre-cast agarose E-gels (Thermo Fisher Scientific). To assess the target efficiency of individual sgRNAs, the amplified target locus was TA cloned using the TOPO PCR2.1 TA cloning kit according to the manufacturer’s instructions (Thermo Fisher Scientific). Colonies were picked and Sanger sequenced with M13(−20)F primer (Functional Biosciences). Sequences were aligned and read using LaserGene SeqMan Pro alignment software (DNASTAR). For each sgRNA, at least 80 clones were evaluated from each of the three independent transfection assays.
Table 2.
Guide Sequences Tested
| Guide | Sequence | PAM | Locus | Figure |
|---|---|---|---|---|
| sg1MAK | GATAGTAGCTTGTGCATGAA | AGG | chr6:+10792160 | Figure 1 |
| sg2MAK | TATGGAACGCTCCTTGAGAA | TGG | chr6:+10792116 | Figure 1 |
| sg3MAK | ACTAACAGTCCCCATTCTCA | AGG | chr6:−10792116 | Figure 1 |
| sg1CEP290 | TATCTCATACCTATCCCTAT | TGG | chr12:-88102776 | Figure 2 |
| sg2CEP290 | GAGATACTCACAATTACAAC | TGG | chr12:+88102748 | Figure 2 |
| sg3CEP290 | TTAACGTTATCATTTTCCCA | AAGAGT | chr12:−88102763 | Figure 3 |
| sg4CEP290 | GTTTCATTCTGTCACCCAGG | CTGGAT | chr12:−88102837 | Figure 3 |
| sg5CEP290 | GTAAGACTGGAGATAGAGAC | AGGAAT | chr12:+88101105 | Figure 3 |
| sg6CEP290 | TTTTGACAGTTTTTAAGGCG | GGGAGT | chr12:+88101195 | Figure 3 |
| sgH23-1 | GACGGGTGTGGTACGCAGCCACT | TCGAGT | chr3:+129528801 | Figure 4 |
| sgH23-2 | GGGTGTGGTACGCAGCCACT | TCGAGT | chr3:+129528801 | Figure 4 |
Determination of On-Target Cutting Efficiency at IVS26 Locus
To avoid size-based cloning bias from the disparate PCR products amplified in the double-guide assay, TaqMan-based qPCR assays were used to assess the targeting efficiency of guide pairs at the IVS26 locus. Relative copy number was determined using a plasmid standard curve. To generate the plasmid used in the standard curve, a 200-bp region surrounding the IVS26 mutation was amplified from the HEK293T template DNA using the primers ExonXF: CCATCATGCCCGGCTAATTT and ExonXR: CTAAGACACTGCCAATAGGGATAG and TA cloned into pCR2.1-TOPO TA. The plasmid was serially diluted in 10-fold increments to generate standard concentrations of 109–103 genomes/μL. 100 ng of genomic DNA from IVS26-treated HEK293Ts or untreated control cultures were used as template in TaqMan qPCR reactions to determine IVS26 copy number. The primer/probes were designed using the Custom TaqMan Assay Design Tool (http://www.thermofisher.com/us/en/home.html). Forward – CTCCTAAAGTGCTGGGATTACAGAT, Reverse – ACTGCCAATAGGGATAGGTATGAGAT, Probe – 5′-FAM-CAGGTGCGGTGGCTCA-NFQ-3′. Reactions were carried out on the StepOne Plus Real-Time PCR System (Applied Biosystems) according to the manufacturer’s instructions.
Delivery of CRISPR-Cas9 Machinery to CEP290- and RHO-Patient-Specific iPSCs
Large-scale endonuclease-free CRISPR-Cas9 plasmids were prepared using either the QIAGEN Plasmid Midi kit (Takara Clontech; Figure 2) or the Macherey-Nagel NucleoBond Xtra Midi kit (Takara Clontech; Figure 3) according to the manufacturer’s instructions. Patient 2 (Figure 2): hiPSCs were subjected to one round of single-cell passaging on MEFs (using Accutase [Thermo Fisher Scientific] and 10 μM Y27632) prior to transfection. 5 μg per sgRNA plasmid was combined with 5 μg of pCas9_GFP and nucleofected into 500,000 single-cell dissociated CEP290-patient iPSCs using the Lonza 2D Nucleofector Technology and the Amaxa Stem Cell Kit 1 (VPH-5012) with program B-016 according to the manufacturer’s instructions. Nucleofected cells were plated onto MEFs in a 24-well plate using a 50:50 mix of fresh and MEF-conditioned hESC medium with 10 μM Y27632. 48 hr after transfection, the hiPSCs were again harvested with Accutase in the presence of 10 μM Y-27632. Cells were stained with SSEA5-APC (1:60, BioLegend, #355210) and 7AAD (1:60, Life Technologies, #A1310) on ice for 5 min prior to sorting with the BD FACS Aria II (BSL2). Approximately 3,500 GFP+APC+7AAD- cells were re-plated in replicates onto MEFs (six-well plate) in a 50:50 mix of fresh and MEF-conditioned hESC medium with 10 μM Y27632 and grown in hESC-conditioned medium until colonies formed, after which the medium was switched to regular hESC medium. Patients 3–5 (Figures 3 and 4): 5 μg of pX601-AAV-sg4/5CEP290 or 6 μg of pX601-AAV-sgH23-2 plasmids were transfected into 2 × 106 CEP290- or RHO-patient-specific iPSCs, respectively, via NEON transfection using Buffer R according to the manufacturer’s instructions (Thermo Fisher Scientific). For clonal selection experiments in iPSCs from patients 1, 3, 4, and 5, EditPro Stem transfection reagent (MTI-GlobalStem) was used according to the manufacturer’s instructions. Guide-expressing and HDR plasmids were delivered in 1:1 molar ratios to iPSCs cultured at ∼50% confluency in six-well laminin-coated tissue culture dishes. 2 days post transfection, cultures were expanded onto 100-mm laminin-coated tissue culture dishes and placed under puromycin (0.05 μg/mL) selection. Puromycin was increased to 0.1 μg/mL and 0.5 μg/mL on each of 2 successive days following initial selection. Puromycin-resistant clones were selected after 1 week for further analysis.
Statistical Analysis
For transfection experiments in HEK239T cells, NHEJ was analyzed in three separate transfections. Two-tailed Student’s t tests were performed. Significance was determined at p < 0.05.
Author Contributions
E.R.B. designed, performed, and analyzed cloning and screening of MAK, CEP290, and RHO CRISPR reagents, designed and performed off-target analyses for MAK, CEP290, and RHO guides, and wrote and edited the manuscript; M.G. performed CEP290 hiPSC CRISPR experiments, designed and performed the genotyping, off-target analyses, and RT-PCR analyses, and wrote and edited the manuscript; M.G., A.T., and R.T. performed CEP290 western blot analyses; L.A.W. wrote and edited the manuscript; K.R.A. performed MAK, CEP290, and RHO reprogramming and gene transfer experiments; J.M.H. and J.L.A. performed sequencing on MAK, CEP290, and RHO patients; C.J. and E.H.S. performed pig subretinal injections and helped analyze the in vivo CRISPR genome editing data; J.W.R and M.K.A. produced the P23H pigs for the in vivo genome editing experiments; D.L.K. performed cloning and screening of CEP290 and RHO CRISPR reagents; R.F.M. wrote and edited the manuscript; G.Q.D. and T.M.S. supervised the CEP290 reprogramming and CRISPR project; T.M.S. designed the sgRNAs and CRISPR experiments; E.M.S. performed clinical diagnosis, provided the patient cells, performed patient genotyping, and wrote and edited the manuscript; B.A.T. supervised design, performance, and analysis of MAK, CEP290, and RHO CRISPR experiments and wrote and edited the manuscript.
Conflicts of Interest
G.Q.D. is a member of the Scientific Advisory Boards of MPM Capital, Inc., 28-7 Therapeutics, and RAZE Therapeutics. All other authors have no conflict of interest.
Acknowledgments
The authors are appreciative of reagents and helpful discussions from Dr. Feng Zhang and Dr. Patrick Hsu. The authors would like to thank the NSRRC for providing the Pro23His transgenic sperm that was used to re-derive the Pro23His pig model of retinal degenerative blindness. This work was supported by the Stephen A. Wynn Foundation (to R.F.M., E.M.S., and B.A.T.), the National Eye Institute (R01EY024588 to R.F.M., E.M.S., and B.A.T.; R01EY026008 to E.M.S. and B.A.T.), and the Grousbeck Family Foundation to (G.Q.D., R.F.M., E.M.S., and B.A.T.).
Footnotes
Supplemental Information includes Supplemental Materials and Methods, five figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.05.015.
Supplemental Information
References
- 1.Tucker B.A., Mullins R.F., Stone E.M. Stem cells for investigation and treatment of inherited retinal disease. Hum. Mol. Genet. 2014;23(R1):R9–R16. doi: 10.1093/hmg/ddu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory-Evans K., Bhattacharya S.S. Genetic blindness: current concepts in the pathogenesis of human outer retinal dystrophies. Trends Genet. 1998;14:103–108. doi: 10.1016/s0168-9525(98)01402-4. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C., Foster A. Blindness in children: control priorities and research opportunities. Br. J. Ophthalmol. 2001;85:1025–1027. doi: 10.1136/bjo.85.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush R.A., Hawks K.W., Sieving P.A. Preservation of inner retinal responses in the aged Royal College of Surgeons rat. Evidence against glutamate excitotoxicity in photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 1995;36:2054–2062. [PubMed] [Google Scholar]
- 5.Santos A., Humayun M.S., de Juan E., Jr., Greenburg R.J., Marsh M.J., Klock I.B., Milam A.H. Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch. Ophthalmol. 1997;115:511–515. doi: 10.1001/archopht.1997.01100150513011. [DOI] [PubMed] [Google Scholar]
- 6.Light J.G., Fransen J.W., Adekunle A.N., Adkins A., Pangeni G., Loudin J., Mathieson K., Palanker D.V., McCall M.A., Pardue M.T. Inner retinal preservation in rat models of retinal degeneration implanted with subretinal photovoltaic arrays. Exp. Eye Res. 2014;128:34–42. doi: 10.1016/j.exer.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullins R.F., Kuehn M.H., Radu R.A., Enriquez G.S., East J.S., Schindler E.I., Travis G.H., Stone E.M. Autosomal recessive retinitis pigmentosa due to ABCA4 mutations: clinical, pathologic, and molecular characterization. Invest. Ophthalmol. Vis. Sci. 2012;53:1883–1894. doi: 10.1167/iovs.12-9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirai H., Mandai M., Matsushita K., Kuwahara A., Yonemura S., Nakano T., Assawachananont J., Kimura T., Saito K., Terasaki H. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc. Natl. Acad. Sci. USA. 2016;113:E81–E90. doi: 10.1073/pnas.1512590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakowski J., Gonzalez-Cordero A., West E.L., Han Y.-T., Welby E., Naeem A., Blackford S.J., Bainbridge J.W., Pearson R.A., Ali R.R. Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell-derived self-forming retina. Stem Cells. 2015;33:2469–2482. doi: 10.1002/stem.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamba D.A., Gust J., Reh T.A. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Cordero A., West E.L., Pearson R.A., Duran Y., Carvalho L.S., Chu C.J., Naeem A., Blackford S.J., Georgiadis A., Lakowski J. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 2013;31:741–747. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou P.Y., Peng G.H., Xu H., Yin Z.Q. c-Kit+ cells isolated from human fetal retinas represent a new population of retinal progenitor cells. J. Cell Sci. 2015;128:2169–2178. doi: 10.1242/jcs.169086. [DOI] [PubMed] [Google Scholar]
- 13.Luo J., Baranov P., Patel S., Ouyang H., Quach J., Wu F., Qiu A., Luo H., Hicks C., Zeng J. Human retinal progenitor cell transplantation preserves vision. J. Biol. Chem. 2014;289:6362–6371. doi: 10.1074/jbc.M113.513713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gust J., Reh T.A. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest. Ophthalmol. Vis. Sci. 2011;52:5266–5272. doi: 10.1167/iovs.10-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLaren R.E., Pearson R.A., MacNeil A., Douglas R.H., Salt T.E., Akimoto M., Swaroop A., Sowden J.C., Ali R.R. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 16.Tucker B.A., Park I.-H., Qi S.D., Klassen H.J., Jiang C., Yao J., Redenti S., Daley G.Q., Young M.J. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker B.A., Mullins R.F., Streb L.M., Anfinson K., Eyestone M.E., Kaalberg E., Riker M.J., Drack A.V., Braun T.A., Stone E.M. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. eLife. 2013;2:e00824. doi: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamba D.A., McUsic A., Hirata R.K., Wang P.-R., Russell D., Reh T.A. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao T., Zhang Z.-N., Westenskow P.D., Todorova D., Hu Z., Lin T., Rong Z., Kim J., He J., Wang M. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell. 2015;17:353–359. doi: 10.1016/j.stem.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker B.A., Anfinson K.R., Mullins R.F., Stone E.M., Young M.J. Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Transl. Med. 2013;2:16–24. doi: 10.5966/sctm.2012-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamm D.M., Meyer J.S. Directed differentiation of human induced pluripotent stem cells: a retina perspective. Regen. Med. 2010;5:315–317. doi: 10.2217/rme.10.28. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz S.D., Hubschman J.-P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., Mickunas E., Gay R., Klimanskaya I., Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 23.Lamba D.A., Karl M.O., Ware C.B., Reh T.A. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinek M., East A., Cheng A., Lin S., Ma E., Doudna J. RNA-programmed genome editing in human cells. eLife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mali P., Yang L., Esvelt K.M., Aach J., Güell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeder M.L., Linder S.J., Cascio V.M., Fu Y., Ho Q.H., Joung J.K. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods. 2013;10:977–979. doi: 10.1038/nmeth.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ran F.A., Hsu P.D., Lin C.-Y., Gootenberg J.S., Konermann S., Trevino A.E., Scott D.A., Inoue A., Matoba S., Zhang Y. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran F.A., Cong L., Yan W.X., Scott D.A., Gootenberg J.S., Kriz A.J., Zetsche B., Shalem O., Wu X., Makarova K.S. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinstiver B.P., Prew M.S., Tsai S.Q., Nguyen N.T., Topkar V.V., Zheng Z., Joung J.K. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat. Biotechnol. 2015;33:1293–1298. doi: 10.1038/nbt.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinstiver B.P., Pattanayak V., Prew M.S., Tsai S.Q., Nguyen N.T., Zheng Z., Joung J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun N., Abil Z., Zhao H. Recent advances in targeted genome engineering in mammalian systems. Biotechnol. J. 2012;7:1074–1087. doi: 10.1002/biot.201200038. [DOI] [PubMed] [Google Scholar]
- 34.Burnight E.R., Wiley L.A., Drack A.V., Braun T.A., Anfinson K.R., Kaalberg E.E., Halder J.A., Affatigato L.M., Mullins R.F., Stone E.M. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014;21:662–672. doi: 10.1038/gt.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo S., Mullins R.F., Dumitrescu A.V., Bhattarai S., Gratie D., Wang K., Stone E.M., Sheffield V., Drack A.V. Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1. Invest. Ophthalmol. Vis. Sci. 2013;54:6118–6132. doi: 10.1167/iovs.13-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colella P., Cotugno G., Auricchio A. Ocular gene therapy: current progress and future prospects. Trends Mol. Med. 2009;15:23–31. doi: 10.1016/j.molmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Friedland A.E., Baral R., Singhal P., Loveluck K., Shen S., Sanchez M., Marco E., Gotta G.M., Maeder M.L., Kennedy E.M. Characterization of Staphylococcus aureus Cas9: a smaller Cas9 for all-in-one adeno-associated virus delivery and paired nickase applications. Genome Biol. 2015;16:257. doi: 10.1186/s13059-015-0817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tucker B.A., Scheetz T.E., Mullins R.F., DeLuca A.P., Hoffmann J.M., Johnston R.M., Jacobson S.G., Sheffield V.C., Stone E.M. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 2011;108:E569–E576. doi: 10.1073/pnas.1108918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Hollander A.I., Koenekoop R.K., Yzer S., Lopez I., Arends M.L., Voesenek K.E., Zonneveld M.N., Strom T.M., Meitinger T., Brunner H.G. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perrault I., Delphin N., Hanein S., Gerber S., Dufier J.-L., Roche O., Defoort-Dhellemmes S., Dollfus H., Fazzi E., Munnich A. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum. Mutat. 2007;28:416. doi: 10.1002/humu.9485. [DOI] [PubMed] [Google Scholar]
- 41.Inglehearn C.F., Tarttelin E.E., Plant C., Peacock R.E., al-Maghtheh M., Vithana E., Bird A.C., Bhattacharya S.S. A linkage survey of 20 dominant retinitis pigmentosa families: frequencies of the nine known loci and evidence for further heterogeneity. J. Med. Genet. 1998;35:1–5. doi: 10.1136/jmg.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 43.Hsu P.D., Scott D.A., Weinstein J.A., Ran F.A., Konermann S., Agarwala V., Li Y., Fine E.J., Wu X., Shalem O. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parfitt D.A., Lane A., Ramsden C.M., Carr A.-J.F., Munro P.M., Jovanovic K., Schwarz N., Kanuga N., Muthiah M.N., Hull S. Identification and correction of mechanisms underlying inherited blindness in human iPSC-derived optic cups. Cell Stem Cell. 2016;18:769–781. doi: 10.1016/j.stem.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z., Yang H., Colosi P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross J.W., Fernandez de Castro J.P., Zhao J., Samuel M., Walters E., Rios C., Bray-Ward P., Jones B.W., Marc R.E., Wang W. Generation of an inbred miniature pig model of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2012;53:501–507. doi: 10.1167/iovs.11-8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X., Wang Y., Huang H., Chen B., Chen X., Hu J., Chang T., Lin R.J., Yee J.K. Precise gene modification mediated by TALEN and single-stranded oligodeoxynucleotides in human cells. PLoS ONE. 2014;9:e93575. doi: 10.1371/journal.pone.0093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshimi K., Kunihiro Y., Kaneko T., Nagahora H., Voigt B., Mashimo T. ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat. Commun. 2016;7:10431. doi: 10.1038/ncomms10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin S., Staahl B.T., Alla R.K., Doudna J.A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife. 2014;3:e04766. doi: 10.7554/eLife.04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braun T.A., Mullins R.F., Wagner A.H., Andorf J.L., Johnston R.M., Bakall B.B., Deluca A.P., Fishman G.A., Lam B.L., Weleber R.G. Non-exomic and synonymous variants in ABCA4 are an important cause of Stargardt disease. Hum. Mol. Genet. 2013;22:5136–5145. doi: 10.1093/hmg/ddt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young C.S., Hicks M.R., Ermolova N.V., Nakano H., Jan M., Younesi S., Karumbayaram S., Kumagai-Cresse C., Wang D., Zack J.A. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell. 2016;18:533–540. doi: 10.1016/j.stem.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabebordbar M., Zhu K., Cheng J.K.W., Chew W.L., Widrick J.J., Yan W.X., Maesner C., Wu E.Y., Xiao R., Ran F.A. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351:407–411. doi: 10.1126/science.aad5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cideciyan A.V., Aleman T.S., Jacobson S.G., Khanna H., Sumaroka A., Aguirre G.K., Schwartz S.B., Windsor E.A., He S., Chang B. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum. Mutat. 2007;28:1074–1083. doi: 10.1002/humu.20565. [DOI] [PubMed] [Google Scholar]
- 54.Pasadhika S., Fishman G.A., Stone E.M., Lindeman M., Zelkha R., Lopez I., Koenekoop R.K., Shahidi M. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2010;51:2608–2614. doi: 10.1167/iovs.09-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartong D.T., McGee T.L., Sandberg M.A., Berson E.L., Asselbergs F.W., van der Harst P., De Vivo I., Dryja T.P. Search for a correlation between telomere length and severity of retinitis pigmentosa due to the dominant rhodopsin Pro23His mutation. Mol. Vis. 2009;15:592–597. [PMC free article] [PubMed] [Google Scholar]
- 56.Saliba R.S., Munro P.M.G., Luthert P.J., Cheetham M.E. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J. Cell Sci. 2002;115:2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- 57.Mendes H.F., van der Spuy J., Chapple J.P., Cheetham M.E. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol. Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Wilson J.H., Wensel T.G. The nature of dominant mutations of rhodopsin and implications for gene therapy. Mol. Neurobiol. 2003;28:149–158. doi: 10.1385/MN:28:2:149. [DOI] [PubMed] [Google Scholar]
- 59.Courtney D.G., Moore J.E., Atkinson S.D., Maurizi E., Allen E.H.A., Pedrioli D.M.L., McLean W.H., Nesbit M.A., Moore C.B. CRISPR/Cas9 DNA cleavage at SNP-derived PAM enables both in vitro and in vivo KRT12 mutation-specific targeting. Gene Ther. 2016;23:108–112. doi: 10.1038/gt.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshimi K., Kaneko T., Voigt B., Mashimo T. Allele-specific genome editing and correction of disease-associated phenotypes in rats using the CRISPR-Cas platform. Nat. Commun. 2014;5:4240. doi: 10.1038/ncomms5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X., Wang Y., Wu X., Wang J., Wang Y., Qiu Z., Chang T., Huang H., Lin R.J., Yee J.K. Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat. Biotechnol. 2015;33:175–178. doi: 10.1038/nbt.3127. [DOI] [PubMed] [Google Scholar]
- 62.Osborn M.J., Webber B.R., Knipping F., Lonetree C.-L., Tennis N., DeFeo A.P., McElroy A.N., Starker C.G., Lee C., Merkel S. Evaluation of TCR gene editing achieved by TALENs, CRISPR/Cas9, and megaTAL nucleases. Mol. Ther. 2016;24:570–581. doi: 10.1038/mt.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiarle R., Zhang Y., Frock R.L., Lewis S.M., Molinie B., Ho Y.-J., Myers D.R., Choi V.W., Compagno M., Malkin D.J. Genome-wide translocation sequencing reveals mechanisms of chromosome breaks and rearrangements in B cells. Cell. 2011;147:107–119. doi: 10.1016/j.cell.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crosetto N., Mitra A., Silva M.J., Bienko M., Dojer N., Wang Q., Karaca E., Chiarle R., Skrzypczak M., Ginalski K. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat. Methods. 2013;10:361–365. doi: 10.1038/nmeth.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsai S.Q., Zheng Z., Nguyen N.T., Liebers M., Topkar V.V., Thapar V., Wyvekens N., Khayter C., Iafrate A.J., Le L.P. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2015;33:187–197. doi: 10.1038/nbt.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiley L.A., Burnight E.R., DeLuca A.P., Anfinson K.R., Cranston C.M., Kaalberg E.E., Penticoff J.A., Affatigato L.M., Mullins R.F., Stone E.M. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci. Rep. 2016;6:30742. doi: 10.1038/srep30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Small K.W., DeLuca A.P., Whitmore S.S., Rosenberg T., Silva-Garcia R., Udar N., Puech B., Garcia C.A., Rice T.A., Fishman G.A. North Carolina macular dystrophy is caused by dysregulation of the retinal transcription factor PRDM13. Ophthalmology. 2016;123:9–18. doi: 10.1016/j.ophtha.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomita M., Mori T., Maruyama K., Zahir T., Ward M., Umezawa A., Young M.J. A comparison of neural differentiation and retinal transplantation with bone marrow-derived cells and retinal progenitor cells. Stem Cells. 2006;24:2270–2278. doi: 10.1634/stemcells.2005-0507. [DOI] [PubMed] [Google Scholar]
- 69.Yao J., Ko C.W., Baranov P.Y., Regatieri C.V., Redenti S., Tucker B.A., Mighty J., Tao S.L., Young M.J. Enhanced differentiation and delivery of mouse retinal progenitor cells using a micropatterned biodegradable thin-film polycaprolactone scaffold. Tissue Eng. Part A. 2015;21:1247–1260. doi: 10.1089/ten.tea.2013.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kundu J., Michaelson A., Baranov P., Young M.J., Carrier R.L. Approaches to cell delivery: substrates and scaffolds for cell therapy. Dev. Ophthalmol. 2014;53:143–154. doi: 10.1159/000357369. [DOI] [PubMed] [Google Scholar]
- 71.Diniz B., Thomas P., Thomas B., Ribeiro R., Hu Y., Brant R., Ahuja A., Zhu D., Liu L., Koss M. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest. Ophthalmol. Vis. Sci. 2013;54:5087–5096. doi: 10.1167/iovs.12-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Worthington K.S., Wiley L.A., Kaalberg E.E., Collins M.M., Mullins R.F., Stone E.M., Tucker B.A. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater. 2017;55:385–395. doi: 10.1016/j.actbio.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnston J.C., Gasmi M., Lim L.E., Elder J.H., Yee J.K., Jolly D.J., Campbell K.P., Davidson B.L., Sauter S.L. Minimum requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectors. J. Virol. 1999;73:4991–5000. doi: 10.1128/jvi.73.6.4991-5000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.