Abstract
The successful immunotherapy of acute myeloid leukemia (AML) has been hampered because most potential antigenic targets are shared with normal hematopoietic stem cells (HSCs), increasing the risk of sustained and severe hematopoietic toxicity following treatment. C-type lectin-like molecule 1 (CLL-1) is a membrane glycoprotein expressed by >80% of AML but is absent on normal HSCs. Here we describe the development and evaluation of CLL-1-specific chimeric antigen receptor T cells (CLL-1.CAR-Ts) and we demonstrate their specific activity against CLL-1+ AML cell lines as well as primary AML patient samples in vitro. CLL-1.CAR-Ts selectively reduced leukemic colony formation in primary AML patient peripheral blood mononuclear cells compared to control T cells. In a human xenograft mouse model, CLL-1.CAR-Ts mediated anti-leukemic activity against disseminated AML and significantly extended survival. By contrast, the colony formation of normal progenitor cells remained intact following CLL-1.CAR-T treatment. Although CLL-1.CAR-Ts are cytotoxic to mature normal myeloid cells, the selective sparing of normal hematopoietic progenitor cells should allow full myeloid recovery once CLL-1.CAR-T activity terminates. To enable elective ablation of the CAR-T, we therefore introduced the inducible caspase-9 suicide gene system and we show that exposure to the activating drug rapidly induced a controlled decrease of unwanted CLL-1.CAR-T activity against mature normal myeloid cells.
Keywords: AML, CAR, CLL-1
Tashiro et al. demonstrate that T cells expressing a C-type lectin like molecule-1-specific chimeric antigen receptor (CLL-1 CAR) exert potent anti-leukemic activity against CLL-1+ AML cell lines as well as AML patient samples. Normal hematopoietic stem cells (HSCs) do not express CLL-1 and are spared by this CAR-T treatment.
Introduction
Treatment for acute myeloid leukemia (AML) has advanced only modestly over the past 30 years. Although chemotherapy can induce complete remission, it is toxic and has a high rate of failure. Moreover, standard chemotherapy often fails to eliminate leukemic stem cells (LSCs)—a small population of cells that are quiescent, are resistant to chemotherapy, and are likely responsible for AML initiation and subsequent relapse.1 Allogeneic hematopoietic stem cell transplantation (HSCT) may benefit some patients but toxicities and failure rates still remain high, excluding many elderly patients with significant morbidities in whom the disease is most common. Therefore, there has been great interest in targeting AML by less toxic immunotherapies with activity against LSCs.
The striking success of CD19-specific chimeric antigen receptor T cell (CAR-T) therapies against acute lymphoblastic leukemia (ALL) has not yet been matched in AML.2, 3, 4 One major obstacle to targeting AML with CAR-Ts is that many myeloid antigens are expressed at similar levels on normal and malignant cells. Eliminating leukemic cells therefore may occur at the expense of normal myeloid tissue, including myeloid progenitor cells, resulting in an unacceptable “on target, off tumor” effect. Several preclinical studies have reported CARs targeting AML-associated antigens such as Lewis Y,5 CD33,6, 7 CD44v6,8 CD123,7, 9, 10 and folate receptor β (FRβ).11, 12 Among these, Lewis Y, CD33, and CD123 have been used clinically but sustained complete responses have not yet been reported.5, 6, 13 Toxicities toward normal hematopoietic progenitor cells (HPCs) associated with the CD33 and CD123 CAR-T cell treatments have also been of particular concern.
C-type lectin-like molecule-1 (CLL-1) may be an effective alternative target for AML with specificity against leukemic progenitor cells and their progeny, while sparing normal myeloid precursor cells.14, 15 The antigen is a type II transmembrane protein and its expression is limited to myeloid lineage cells.16 CLL-1 is present on 85%–92% of AML of all French-American-British (FAB) classes (M0–M6).16, 17, 18 CLL-1 is also expressed on CD34+CD38− AML LSCs.15 When CD34+/CLL-1+ leukemic cells engraft in non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) mice, they outgrow to CLL-1+ blasts, suggesting that these cells have the functional properties of LSCs.19, 20 Additionally, CLL-1 is expressed on differentiated myeloid cells but not on normal hematopoietic stem cells (HSCs), indicating that a CLL-1-targeted therapy would spare these cells.15, 19
Here we generated CLL-1-specific CAR-Ts (CLL-1.CAR-Ts) and demonstrated selective killing of leukemic progenitor cells and their progeny. Although CLL-1.CAR-Ts killed mature normal myeloid cells, normal myeloid precursor cells were spared, judging by in vitro cord blood (CB) colony-forming assays. Since we also show that CLL-1.CAR-T activity can be electively terminated by inducible apoptosis following elimination of AML cells and LSCs, myeloid reconstitution in treated patients should occur via the unharmed normal precursor cells.
Results
CLL-1 Is Expressed by AML Cell Lines and Primary AML Blasts
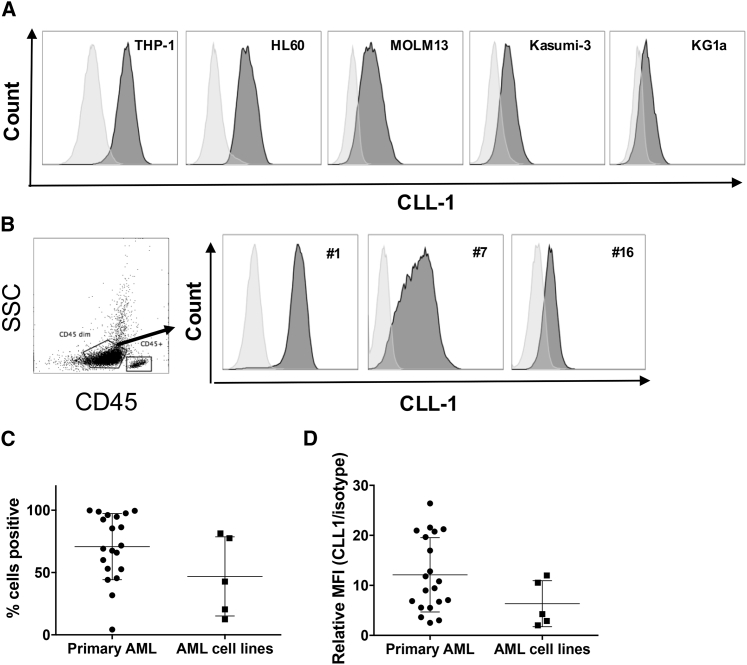
To validate CLL-1 as a target antigen for CAR-T cell therapy against AML, we first evaluated CLL-1 expression in AML cell lines and primary AML blasts. The chronic myeloid leukemia cell line K562 does not express CLL-1 (Figure S1A) and we used it as a negative control. Consistent with previous reports,17 CLL-1 was expressed by several AML cell lines at different intensities (Figure 1A). Next, we analyzed CLL-1 expression on peripheral blood samples from 19 patients with AML whose disease subtypes are summarized in Table 1. CLL-1 was detected in 95% of AML cases (18 of 19) with a range of positivity between 31.7% and 99.8% when gated on CD45dim/side scatter (SSC)low populations enriched for AML blasts (Figures 1B and 1C). Relative CLL-1 mean fluorescence intensities (MFIs) (normalized to isotype control) are summarized in Figure 1D. We also measured CLL-1 expression on peripheral blood from six healthy donors. As previously reported,21 CLL-1 expression was restricted to myeloid cells (i.e., granulocytes, mature/precursor dendritic cells [DCs], and monocytes); T and B lymphocytes and natural killer (NK) cells did not express CLL-1 (Figures S1B and S1C).
Figure 1.
CLL-1 Is Expressed in Several AML Cell Lines and Primary AML
(A) Surface expression of CLL-1 on AML cell lines THP-1, HL60, MOLM13, Kasumi-3, and KG1a was determined by flow cytometry using CLL-1-AF647 antibody (clone: 50C1) (dark gray) and isotype IgG2ak antibody (light gray). (B–D) Primary patient AML blasts from a diverse range of disease subtypes express CLL-1 (n = 19; shown in Table 1). (B) CLL-1 expression levels vary among leukemias, as reviewed by gating on a SSClow/CD45dim blast population using the same antibodies as in (A). (Left) One representative gating strategy of 19 AML patient samples. (Right) Data from three representative patients (patients 1, 7, and 16) are shown (right). (C) Combined data on the percentage of CLL-1-positive cells from primary AML (n = 19) and AML cell lines (n = 5). (D) Combined data on relative CLL-1 MFI to isotype MFI (CLL-1/isotype control) from primary AML (n = 19) and AML cell lines (n = 5). SSC, side scatter.
Table 1.
Patient Characteristics
| AML Sample ID | Age (Years) | Sex | Cytogenetics | CLL-1 Positivity | CLL-1 Relative MFI |
|---|---|---|---|---|---|
| 1 | 12 | M | 46, XY | 96.1 | 21.2 |
| 2 | 44 | F | Inv(16)(p13.1q22) | 69.1 | 12.8 |
| 3 | 67 | M | 47, XY, +21 | 86.3 | 17.0 |
| 4 | 80 | M | 46, XY | 67.5 | 26.4 |
| 5 | 27 | F | 46, XX | 31.7 | 7.1 |
| 6 | 78 | M | 46, XY, i(17)(q10) | 53.0 | 6.7 |
| 7 | 76 | M | 46, XY | 71.6 | 10.8 |
| 8 | 74 | M | trisomy 8 | 45.4 | 6.8 |
| 9 | 53 | M | t(15;17)(q24;q21) | 99.8 | 21.0 |
| 10 | 16 months | M | trisomy 8, MLL-R | 98.8 | 9.4 |
| 11 | 13 | M | t(6:11), MLL-R | 99.5 | 11.8 |
| 12 | 8 | M | t(9:11), MLL-R | 85.4 | 5.6 |
| 13 | 13 | M | 46, XY | 65.9 | 9.0 |
| 14 | 15 | M | unavailable | 60.0 | 5.5 |
| 15 | 16 | F | trisomy 8 | 92.5 | 20.8 |
| 16 | 14 | M | t(8:21) | 4.22 | 2.5 |
| 17 | 11 | F | t(16:21) | 52.6 | 3.6 |
| 18 | 84 | M | 46, XY | 94.7 | 19.7 |
| 19 | 69 | F | complex abnormalities, inv(16)(p13.1q22) | 44.1 | 3.0 |
F, female; ID, identification number; M, male; MLL-R, MLL gene rearrangement.
Generation and Evaluation of CLL-1-Specific CAR-Ts
We utilized a CLL-1-specific single-chain fragment variable (scFv) to create a panel of CLL-1.CARs with various costimulatory domains consisting of a CLL-1 scFv fused with a CD8α stalk and transmembrane domains (Figure S2A). We used the CD3ζ signaling domain (CLL-1.ζ) alone or in combination with one or two complementary costimulatory endodomains: CD28 (CLL-1.28ζ) or 4-1BB (CLL-1.BBζ), CD28 and 4-1BB (CLL-1.28.BBζ), or CD28 and OX40 (CLL-1.28.OX40ζ).22 A truncated version of CLL-1.CAR (CLL-1.Δ) was created by deleting intracellular signaling domains and was used as a control. To determine the functionally optimal construct, we compared the memory phenotype, cytokine production, and cytolytic ability of T cells expressing the five CLL-1.CARs. As summarized in Figures S3 and S4, the CLL-1.BBζ construct showed a trend of the greatest specific cytokine release and the most sustained cytolytic activity and was therefore used in all further studies.
CLL-1.BBζ CAR-Ts Produce Pro-inflammatory Cytokines in Response to CLL-1-Expressing Target Cells
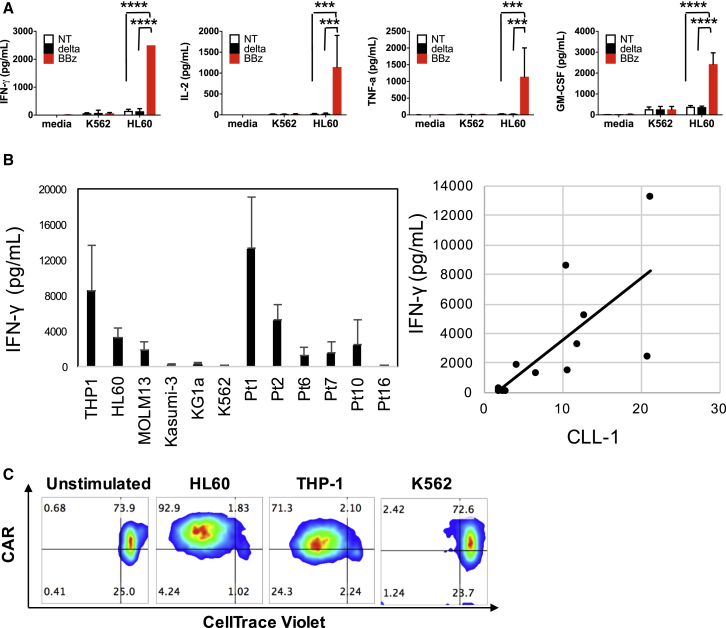
We used a multiplex assay to evaluate the cytokine production of CLL-1.CAR-Ts. When compared with non-transduced activated T cells (NT-ATCs) or CLL-1.Δ-Ts, CLL-1.BBζ CAR-Ts secreted significantly greater amounts of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-2, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Figure 2A) in response to the CLL-1-expressing AML cell line HL60. Background production of the cytokines by CLL-1.BBζ CAR-T was minimal and comparable to that of the NT-ATC and CLL-1.Δ-T controls. Moreover, CLL-1.BBζ CAR-Ts responded to a wide range of CLL-1-expressing target cells (Figure 2B, left) indicating that the CLL-1.CAR-T can target a broad span of antigen expression on AML blasts. The magnitude of IFN-γ production correlated with the relative CLL-1 MFI (normalized to isotype control) (Figure 2B, right; r(10) = 0.70, p < 0.01).
Figure 2.
CLL-1.BBζ CAR-Ts Exhibit Robust In Vitro Effector Function in Response to CLL-1+ Target Cells
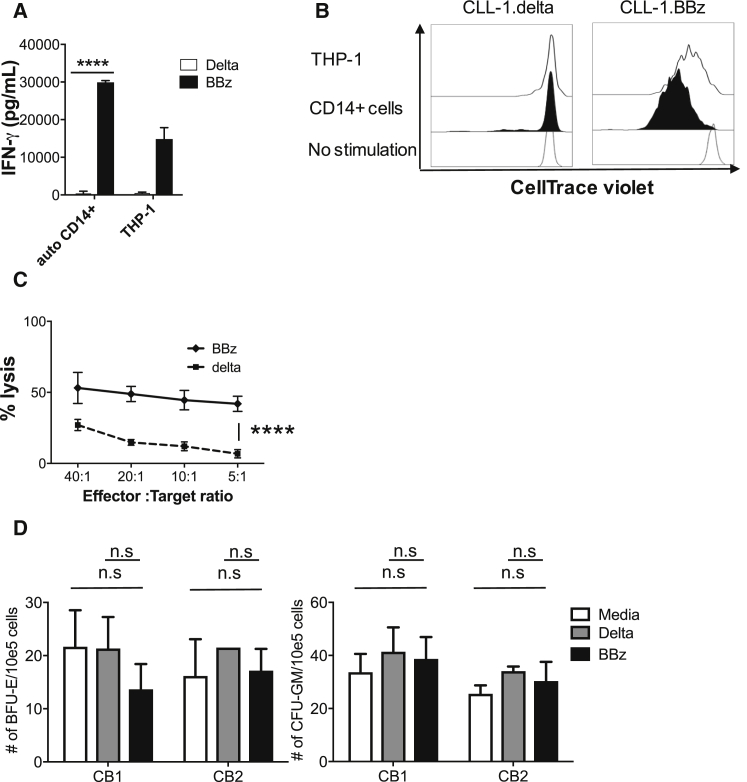
(A) CLL-1.BBζ CAR-Ts produce multiple cytokines in response to HL60 (CLL-1-positive) but not K562 (CLL-1-negative) cells. CLL-1.Δ-Ts, CLL-1.BBζ CAR-Ts, and non-transduced activated T cells (NT-ATCs) were incubated with HL60 and K562 at an E:T ratio of 1:1 or without any target cells for 24 hr. The supernatant was harvested and a 13-plex Luminex assay was performed. Data denote means ± SD from three donors. p values represent significant increases compared to CLL-1.Δ-T or NT-ATC. ***p < 0.001; ****p < 0.0001. (B) CLL-1.BBζ CAR-Ts respond with IFN-γ production in response to target cells with wide-ranging levels of CLL-1 expression. CLL-1.BBζ CAR-Ts were co-cultured with 12 different targets, including six primary AML patient PBMCs. (Left) IFN-γ concentrations were measured after 24 hr of co-culture. Data denote means ± SD from five donors after 24 hr of culture. (Right) There was a significant positive relationship between IFN-γ production and relative CLL-1 expression (CLL-1 MFI/isotype control MFI) of targets. r(10) = 0.7, p < 0.001. (C) CLL-1.CAR-Ts undergo specific proliferation in response to CLL-1-positive AML cell lines HL60 and THP-1 but not CLL-1-negative K562. T cells were labeled with CellTrace Violet at 5 μM and incubated with or without HL60, THP-1, or K562 at a 1:1 ratio. After 5 days of incubation, divided T cells were detected by dilution of CellTrace Violet. One representative dot plot is shown from four donors’ results. BBz, CLL-1.BBζ CAR-T; delta, CLL-1.Δ-T; NT, non-transduced activated T cell.
Antigen-dependent cytokine release was accompanied by proliferation. CLL-1.BBζ CAR-Ts were stained with CellTrace Violet and stimulated by CLL-1-expressing AML cell lines HL60 and THP-1, or the CLL-1-negative cell line K562. After 5 days of stimulation, CLL-1.BBζ CAR-Ts showed substantial proliferation (detected by CellTrace Violet dilution) only when stimulated with CLL-1+ cell lines HL60 or THP-1 (Figure 2C), indicating that the activation of CLL-1.CAR-Ts is CLL-1 specific. We also tested whether CLL-1.BBζ CAR-Ts could proliferate in response to primary AML samples. After stimulation by CLL-1-expressing primary AML samples (patients 1, 2, and 4), they showed robust proliferation (Figure S5). CLL-1.Δ-Ts also showed low-level proliferation, likely due to alloreactivity.
CLL-1.BBζ CAR-Ts Are Cytolytic against CLL-1-Expressing Targets
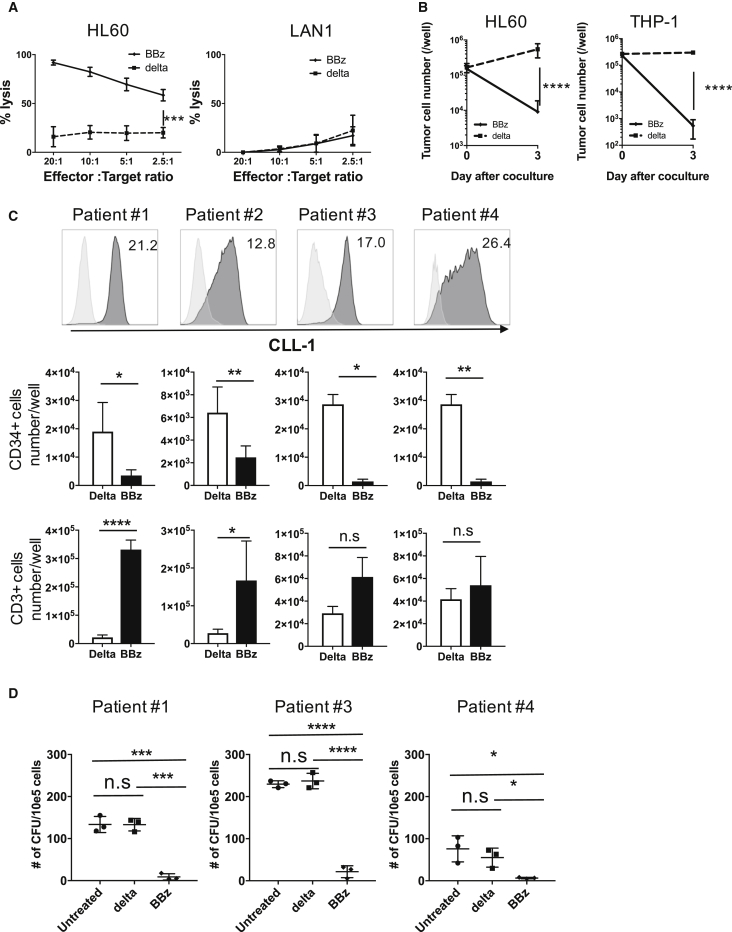
We used a luciferase-based cytotoxicity assay to verify the specific cytotoxicity of CLL-1.BBζ CAR-Ts. Compared to CLL-1.Δ-Ts, CLL-1.BBζ CAR-Ts exhibited significant cytotoxicity against HL60 cells, while the reactivity against a CLL-1-negative cell line (LAN1) was minimal (Figure 3A). We also assessed longer-term cytotoxicity against the CLL-1+ AML cell lines HL60 or THP-1 cells upon co-culture with CLL-1.BBζ CAR-Ts at an effector-to-target (E:T) ratio of 1:2 for 3 days. We observed a significant reduction in live tumor cells by flow at the end of culture with CLL-1.BBζ CAR-Ts compared to CLL-1.Δ-Ts, indicating robust and specific cytotoxicity of CAR-Ts against CLL-1+ targets (Figure 3B; HL60, p < 0.0001; THP-1, p < 0.0001).
Figure 3.
CLL-1.CAR-Ts Effectively Lyse CLL-1+ Target Cells and Inhibit Leukemic Colony Formation
(A) CLL-1.BBζ CAR-Ts exhibit specific killing of CLL-1-expressing target cells. GFPffluc-expressing HL60 cells were co-cultured with CLL-1.BBζ CAR-Ts for 5 hr at the indicated effector-to-target ratios. CLL-1.Δ-Ts were used as a control. CLL-1.BBζ CAR-Ts did not kill CLL-1 negative GFPffluc-expressing LAN1 cells. Data illustrate the means ± SEM of six donors for HL60 and three donors for LAN1. (B) CLL-1.BBζ CAR-Ts kill HL60 and THP-1 in 3 days co-culture assay. CLL-1.BBζ CAR-Ts were co-cultured with HL60-GFPffluc or THP-1 in the absence of exogenous cytokines at an E:T ratio of 1:2. Tumors were enumerated before and 3 days after co-culture using flow cytometry. Absolute cell numbers were calculated using counting beads. Data denote means ± SD from six donors for HL60 and three donors for THP-1. CLL-1.Δ-T was used as a control. p values represent a significant decrease in tumor cells compared to CLL-1.Δ-Ts. (C) CLL-1.BBζ CAR-Ts kill primary AML blasts in co-culture assay. CLL-1.Δ-Ts or CLL-1.BBζ CAR-Ts were co-cultured with primary AML patients PBMCs (relative CLL-1 MFI: patient 1, 21.2; patient 2, 12.8; patient 3, 17.0; patient 4, 26.4) in the absence of exogenous cytokine at an E:T ratio of 1:1. Tumor cells and T cell numbers were analyzed 3 days after co-culture by flow cytometry. Absolute cell numbers were calculated using counting beads. CD34+ (AML, middle panel) and CD3+ (T cells, bottom panel). Cell numbers on day 3 from six donors (patients 1 and 2) or three donors (patients 3 and 4) are shown. CLL-1 expression is shown in the upper panel. Against PBMCs from patients 1, 2, 4, and 8, CLL-1.BBζ CAR-Ts had significantly lower numbers of CD34+ cells (patient 1, p = 0.0197; patient 2, p = 0.0083; patient 3, p = 0.0067; patient 4, p = 0.0418). Against PBMCs from patients 1 and 2, CLL-1.BBζ CAR-Ts had significant T cell expansion (patient 1, p < 0.0001; patient 2, p = 0.0267) compared to CLL-1.Δ-Ts. No significant T cell expansion was observed against patients 3 and 4. Data denote means ± SD from six donors for patients 1 and 2 and three donors for patients 3 and 4. (D) Primary AML patient PBMCs (from patients 1, 3, and 4) were co-incubated with CLL-1.Δ-Ts or CLL-1.BBζ CAR-Ts for 5 hr at an E:T ratio of 10:1. The cells were then plated in semisolid methylcellulose progenitor culture for 12 days and scored for the presence of leukemic colony-forming units (CFUs). Total CFU numbers are shown. A consistent and significant decrease in leukemic colony numbers was observed when PBMCs from patients with AML were co-incubated with CLL-1.BBζ CAR-Ts. Data represent the mean ± SD of three independent experiments performed in duplicate.*p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001 (two-tailed paired t-test). BBz, CLL-1.BBζ CAR-T; delta, CLL-1.Δ-T; n.s., not significant.
Next, we tested whether CLL-1.BBζ CAR-Ts had anti-leukemic activity against primary AML samples. We co-cultured CLL-1.Δ-Ts or CLL-1.BBζ CAR-Ts with peripheral blood mononuclear cells (PBMCs) from four patients with AML at an E:T ratio of 1:1 in the absence of exogenous cytokines. Three days later, we enumerated CD34+ (AML) and CD3+ (CAR-T) cells. T cells expressing the CLL-1.BBζ CAR-T demonstrated potent cytotoxicity compared to CLL-1.Δ-Ts (patient 1: p = 0.0197, patient 2: p = 0.0083, patient 3: p = 0.0067, patient 4: p = 0.0418). Moreover, primary tumor cells induced expansion of CLL-1.BBζ CAR-Ts but not control CLL-1.Δ-Ts (patient 1, p < 0.0001; patient 2, p = 0.0267) (Figure 3C).
To confirm that CLL-1.BBζ CAR-Ts are cytotoxic against leukemic progenitor cells, we measured their ability to inhibit leukemic colony formation using T cells from three different healthy donors against leukemic cells from three patients with AML. In all combinations, leukemic colony formation was consistently and significantly inhibited upon incubation with CLL-1.BBζ CAR-Ts compared to CLL-1.Δ-Ts (Figure 3D). We conclude that CLL-1.CAR-Ts are cytotoxic against AML cell lines and primary AML cells.
CLL-1.BBζ CAR-Ts Exhibit Potent Anti-leukemic Activity In Vivo and Prolong Animal Survival
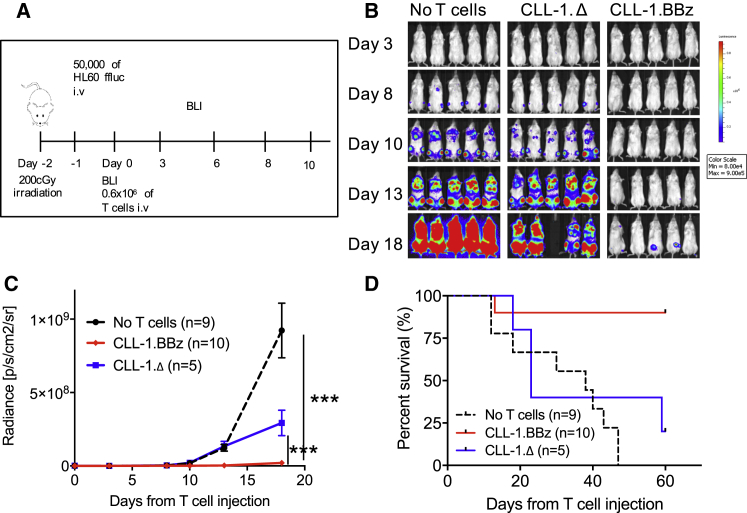
To confirm the in vivo anti-leukemic activity of CLL-1.BBζ CAR-Ts, we used a human xenograft mouse model of AML in which NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NSG) mice were systemically engrafted with HL60-GFP-firefly luciferase (GFPffluc) cells by intravenous injection. To mimic treatment of residual leukemia, mice received 0.6 × 106 of either CLL-1.Δ-Ts or CLL-1.BBζ CAR-Ts 24 hr after infusion of 50,000 HL60-GFPffluc (Figure 4A). We used bioluminescent imaging (BLI) to monitor tumor growth. Control groups receiving CLL-1.Δ-Ts demonstrated rapid leukemia progression, with a median survival of < 40 days (Figures 4B–4D). By contrast, 8 of 10 mice receiving CLL-1.BBζ CAR-Ts showed a consistently lower leukemia burden associated with significantly improved survival (Figures 4B–4D).
Figure 4.
CLL-1.CAR-T Inhibits HL60 Engraftment in Xenograft Models
(A) Schematic outline of the HL60 xenograft model. NSG mice were sub-lethally irradiated (200 cGy) on day −2 and then injected via the tail vein with 50,000 HL60-GFPffluc on day −1. Mice received 0.6 × 106 CLL-1.Δ-Ts or CLL-1.BBζ CAR-Ts and were followed with serial bioluminescent imaging (BLI). (B) Delayed leukemia engraftment was observed only in xenograft mice treated with CLL-1.BBζ CAR-T. (C) Summary BLI data from three independent experiments. (D) Survival analysis of HL60 xenograft mice revealed a survival advantage for CLL-1.BBζ CAR-T-treated mice compared to CLL-1.Δ-T-treated mice or untreated mice. ***p < 0.001. i.v., intravenous.
CLL-1.BBζ CAR-Ts Are Cytotoxic to Normal Mature Myeloid Cells but Not to Normal Myeloid Progenitor Cells
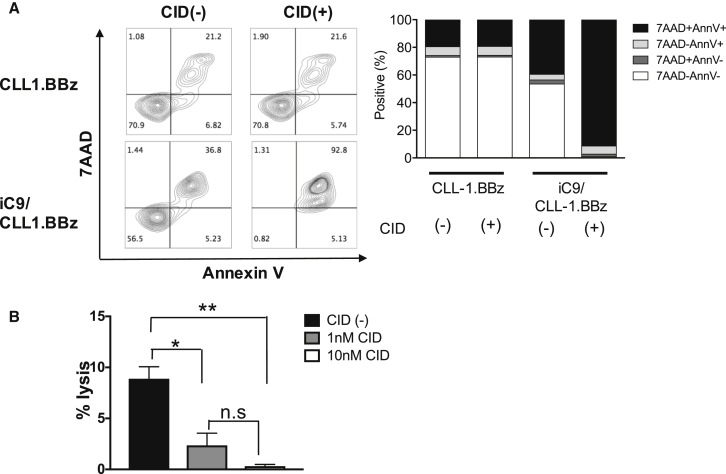
The CLL-1 antigen is expressed by normal differentiated myeloid cells, including granulocytes, DCs, and monocytes (Figure S1C). We therefore assessed the cytotoxic activity of CLL-1.CAR-Ts against normal autologous CD14+ myeloid cells. CLL-1.BBζ CAR-Ts were reactive against normal myeloid cells, leading to IFN-γ production (Figure 5A), proliferation (Figure 5B), and cytotoxicity (Figure 5C). Since CLL-1 is absent on HSCs and primitive myeloid precursors,19 however, these critical cell populations should be spared by CLL-1.CAR-Ts. As anticipated, co-culturing CLL-1.BBζ CAR-Ts with CB samples containing HSCs and myeloid precursors at an E:T ratio of 10:1 did not inhibit myeloid and erythroid colony formation (Figure 5D), indicating that the toxicity of CLL-1.CAR-Ts should be confined to mature myeloid cells and that myeloid progenitor cells should be spared. We determined whether the extent of toxicity to mature myeloid cells could be controlled by terminating the activity of CLL-1.CAR-Ts after leukemia elimination, thereby allowing post-treatment myeloid reconstitution via the unharmed normal precursor cells. We therefore introduced a clinically validated safety switch based on inducible caspase 9 (iC9).23, 24, 25 We double-transduced T cells with both CLL-1.BBζ CAR and ΔCD34-iC9 constructs (iC9/CLL-1.BBζ) and selected iC9-expressing cells using CD34 MACS beads. iC9/CLL-1.BBζ CAR-Ts killed HL60 target cells as effectively as T cells expressing CLL-1.BBζ alone (Figure S6A). Activation with the chemical inducer of dimerization (CID) dimerizer triggered apoptosis in > 90% of iC9/CLL-1.BBζ CAR-Ts (Figure 6A) and reversed the cytotoxic activity of iC9/CLL-1.BBζ CAR-Ts against CLL-1-expressing CD14+ autologous cells (Figure 6B). We also controlled iC9/CLL-1.BBζ CAR-T expansion in vivo using wild-type (WT)-HL60-bearing mice treated with 2 × 106 iC9/CLL-1.BBζ expressing GFPffluc. We tested low and high doses of CID to model efforts to produce titratable (limited and then more complete) control of potential toxicities. We administered the low-dose dimerizer (3 μg/mouse) to mice on days 14 and 16, which transiently decreased T cell signals and was followed by subsequent rebound. We administered CID (50 μg/mouse) on days 23, 25, and 27, which further decreased the T cell signal (Figure S6B).
Figure 5.
CLL-1.BBζ CAR-Ts Were Cytotoxic against Mature Monocytes But Did Not Inhibit Cord Blood Colony Formation In Vitro
(A) CLL-1.BBζ CAR-Ts produce IFN-γ in response to autologous CD14+ cells. CLL-1.Δ-Ts and CLL-1.BBζ CAR-Ts were incubated with autologous CD14+ cells or THP-1 at an E:T ratio of 1:1 for 24 hr. The supernatant was harvested and IFN-γ was assayed. THP-1 cells were used as a positive control. Data denote means ± SD from replicate experiments in tree donors. (B) CLL-1.BBζ CAR-Ts expand in response to autologous CD14+ cells. T cells were labeled with CellTrace Violet at 5 μM and incubated with or without autologous CD14+ cells at a 1:1 ratio. After 5 days of incubation, we measured T cell division by CellTrace Violet dilution. One representative histogram of results from three donors is shown. THP-1 cells were used as the positive control. (C) Cytotoxicity of CLL-1.BBζ CAR-Ts against autologous CD14+ cells was assessed in three donors, each in triplicate in 5-hr chromium release assays. CLL-1.Δ-T was used as a negative control. Data are means ± SD. (D) Mononuclear cells from two different cord blood units (CB1 and CB2) were co-incubated with CLL-1.BBζ for 5 hr at an E:T ratio of 10:1 and then plated in semisolid methylcellulose progenitor culture for 14 days and scored for the presence of burst-forming unit erythroid (BFU-E) and granulocyte-macrophage colony-forming units (GM-CFU). Total colony numbers are shown. CLL-1.Δ-T was used as a negative control. Data represent the mean ± SD of three independent experiments performed in duplicate. ****p < 0.0001. BBz, CLL-1.BBζ CAR-T; delta, CLL-1.Δ-T; n.s., not significant.
Figure 6.
CLL-1.BBζ CAR-Ts Expressing an Inducible Caspase-9 Gene Are Eliminated by a Chemical Inducer of Dimerization
(A) CLL-1.BBζ CAR-Ts or iC9/CLL-1.BBζ CAR-Ts were exposed to CID at 10 nM for 24 hr. Cells were harvested and stained with Annexin-V and 7-AAD. A dot plot from one donor is shown on the left and the summary from three donors is shown on the right. (B) iC9/CLL-1.BBζ CAR-Ts were exposed to CID at the indicated concentrations for 20 hr. 51Cr-labeled autologous CD14+ cells were added to the effector cells at a E:T ratio of 10:1. After 5 hr of incubation, 51Cr release was analyzed. Data represent the means ± SD of three replicates (n = 2 T cell donors). *p < 0.05; **p < 0.001. CID, chemical inducer of dimerization.
Discussion
We aimed to develop CAR-Ts that would target AML blasts and LSCs while sparing normal HSCs. We show that CAR-Ts specific for CLL-1 exhibit potent cytokine production, proliferation, and cytotoxicity against CLL-1-expressing AML cell lines and primary AML samples without disrupting normal HSCs. CLL-1.CAR-Ts also had anti-leukemic activity against human xenografts. Although CLL-1 is also expressed on normal differentiated myeloid cells and CLL-1.CAR-Ts are cytolytic against autologous CD14+ monocytes, normal precursor cells are unharmed by CLL-1.CAR-T treatment in colony-forming assays. Hence, deletion of the CAR-T cells either by natural attrition or exhaustion may allow full recovery from spared precursor cells. Alternatively, the iC-9 suicide gene system may allow for rapid and elective elimination of iC9/CLL-1.CAR-Ts in vitro and in vivo.23, 24, 25
Multiple immunotherapeutic approaches against AML have been explored, including vaccination, monoclonal antibodies (with or without toxins, cytotoxic small molecules, or radionuclides), and adoptive cell therapies, with only modest benefit shown thus far.26 The remarkable success of CAR-T cell therapy for B cell malignancies has obvious implications for the treatment of AML but, at a minimum, requires identification of AML-specific target(s) that can be detected by a single-chain antibody and are broadly expressed on malignant cells but not normal precursor cells. Such a CAR-T cell therapy would allow disease control without the need to rescue the patient from marrow aplasia with an allogeneic stem cell transplant. We chose to target CLL-1 with CAR-T for several reasons. First, CLL-1 is expressed by many AML subtypes.18, 19 In our cohort, CLL-1 was expressed in 95% of AML blasts, with a range of 31.7%–99.8% positivity in primary AML blasts, which is similar to previous reports.16, 17 Equally importantly, CLL-1 is not expressed by normal HSCs19; when we cultured mononuclear cells from CB with CLL-1.CAR-Ts for 5 hr at a 10:1 E:T ratio, colony formation was not inhibited. Additionally, CLL-1 is not expressed on non-hematopoietic tissues.16 These three characteristics make CLL-1 particularly attractive as a therapeutic target compared to other AML antigens that lack such circumscribed expression. Although CLL-1-specific monoclonal antibodies have not yet been tested clinically, two preclinical studies have shown that both a CLL-1 monoclonal antibody17 and a CLL-1-CD3 bispecific antibody27, 28 exhibited anti-leukemic efficacy in vitro and in xenograft models.
A major drawback of targeting CLL-1 is that this antigen is also variably expressed in mature myeloid cells. However, as normal progenitor cells are not targeted by CLL-1.CAR-Ts, the decline or active elimination of this effector population after therapy should allow mature myeloid cell regeneration. To facilitate T cell ablation, we introduced the iC9 suicide gene system into the CLL-1.CAR-T. The iC9-transduced activated T cells or CAR-Ts can be rapidly and effectively eliminated by administration of the activating dimerizer drug (CID) in vitro as well as in vivo in a range of pre-clinical models29, 30, 31 and in the clinic.23, 25 Alternatives to elective elimination of a CLL-1.CAR-T by a suicide system include the use of transient expression32 or small-molecule inducible systems33; however, these approaches have yet to be functionally validated in the clinic. AML is generally considered as a stem cell disease34, 35; since LSCs express CLL-1,15 the CAR-T we describe could in principle eradicate the disease. Our ultimate goal is to provide a CAR for AML that does not require HSCT. Although CLL-1 is absent on HSCs, and no effect of CLL-1 CAR T cells was observed in the short-term colony-forming assay, until we have direct clinical evidence of the selective sparing of human precursor cells in vivo, initial clinical studies will likely use these CAR-T cells to induce remission and act as a bridge to stem cell transplantation. If progenitor cells are indeed spared in these initial studies, we propose that iC9-CLL-1.CAR-Ts may be useful for induction failure or chemotherapy refractory relapses.
Targeting a single antigen, however, may not be sufficient for any CAR-T cell therapy. Relapse from epitope-loss variants or lineage switch after CD19 CAR-T cell therapy against ALL have already been reported,2, 36 and there are numerous other examples of tumor-antigen editing in response to immunotherapy.37, 38, 39 Moreover, activity against sub-populations of tumor cells expressing low levels of the target antigen will likely be suboptimal—a problem shared by all CAR-T cell approaches, even the successful CD19 CAR.40 Both of these limitations may require targeting of two or more tumor antigens, either by dual CARs41 or by adopting a tandem CAR exodomain that contains two differently targeted scFvs in a single CAR.42 Similar combination systems to attack CLL-1 and other AML-LSC antigens could be a practical method to broaden the range of targetable leukemias to include those that dimly express CLL-1 but highly express a second target antigen. As a second target for dual or tandem CAR, other LSC antigens, such as Tim-3, CD96, and CD123,15 may broaden the susceptible cell population. To avoid mature myeloid cell killing, it may be possible to use a split CAR strategy43 with the co-stimulatory CAR targeting an antigen that is expressed by AML blasts or LSCs but not mature myeloid cells. Conversely, combination of the CLL-1 CAR with an inhibitory CAR (iCAR)44 may also be possible, using an antigen that is expressed by mature myeloid cells but not by AML blasts or LSCs.
In conclusion, we have generated CLL-1.CAR-Ts that specifically target AML blasts and progenitor cells while sparing normal HSCs, and whose activity can be electively terminated by a suicide system.
Materials and Methods
CAR and iC9 Construction
To generate SFG.CLL-1ζ-internal ribosome entry site (IRES)-ΔCD19, we synthesized (Bio Basic) cDNA containing the VH and VL chains from the single-chain variable regions (scFv) of the CLL-1 monoclonal antibody.45 We then PCR amplified this fragment and used In-Fusion Cloning (Takara/Clontech) to insert the CLL-1.ζ CAR fragment into a linearized SFG vector that contained IRES and a truncated CD19 construct downstream of the ligation site. These PCR products were cloned into a backbone gamma retrovirus SFG vector 14g2a.zeta546 using XhoI and MluI sites. CLL-1.Δ, CLL-1.CD28ζ, CLL-1.41BBζ, CLL-1.CD28.41BBζ, and CLL-1.CD28.OX40ζ were also created by In-Fusion cloning. The construction of the iC9 suicide gene was previously reported.47 CD34 was used as a selectable marker of iC9 transduced cells.48
Retroviral Vector Production and T Cell Transduction
Retroviral vector production and T cell transduction were performed largely as previously described,49, 50 with substitution of 10 ng/mL IL-7 (Peprotech) and 5 ng/mL IL-15 (Peprotech) for IL-2.51 T cells were expanded in complete medium (CM) (45% RPMI 1640; HyClone), 45% Click’s media (Irvine Scientific), 2 mM L-glutamine (Gibco), and 10% fetal bovine serum (FBS) (HyClone). IL-7 and IL-15 were added to the culture during T cell expansion.
Cell Lines
We obtained the cell lines THP-1, HL60, MOLM13, Kasumi-3, KG1a, K562, LAN1 and 293T from ATCC. We maintained HL60, MOLM13, KG1a, and 293T in Iscove’s modified Dulbecco’s medium (IMDM; Gibco) and THP-1, Kasumi-3, K562, and LAN1 in RPMI. Media were supplemented with 2 mM L-glutamine, 10% or 20% FBS according to the manufacturer’s recommendations, as well as 1% penicillin-streptomycin (Invitrogen). Cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C. All cell lines were routinely tested for mycoplasma using the Mycoalert detection kit (Lonza). We transduced HL60 and LAN1 with a gamma retroviral vector encoding enhanced GFPffluc.
Samples from Healthy Donors and Patients with Leukemia
We obtained PBMCs from healthy donors and patients with AML who gave written informed consent to be entered on protocols approved by the Baylor College of Medicine Institutional Review Board, in accordance with the Declaration of Helsinki.
CD14+ monocytes were isolated from PBMCs with CD14 magnetic beads according to the manufacturer’s instruction (Miltenyi Biotech). We obtained de-identified cord blood units from the MD Anderson Cord Blood Bank (University of Texas, Houston).
Flow Cytometry
Fluorochrome conjugated isotype controls, anti-human CD45, CD4, CD8, CD3, CD45RA, CD33, CD34, CD19, CCR7, CD70, PD-L1, CD80, CD86, and CD40L were purchased from BD Biosciences, Beckman Coulter, Life Technologies, or Biolegend. CLL-1, IgG2ak, and CD45RO were obtained from BD Pharmingen. AF647-conjugated goat anti-mouse IgG antigen binding fragments (Fabs) were purchased from Jackson Immunoresearch. For primary AML samples, the CD45dim/SSClow populations were gated as the AML blast population. We acquired flow cytometric data by Gallios (Beckman Coulter) or BD FACSCanto II (BD Biosciences) and analyzed it using FlowJo (version 10; Tree Star).
Cytokine Release Assays
We cultured CAR-T cells (1 × 105) with or without 1 × 105 target cells in 200 μL CM. After 24 hr, supernatants were collected and analyzed directly or frozen at −80°C. We analyzed supernatants for the production of IFN-γ and IL-2 using the enzyme-linked immunosorbent assay (ELISA) (R&D Systems). We analyzed samples using the Milliplex kit according to the manufacturer’s instructions (Millipore).
Cytotoxicity Assay
We measured cytotoxicity against target cells using in vitro luciferase assays as previously described.52 Briefly, CLL-1+ HL60-GFPffluc cells or CLL-1− LAN1 GFPffluc cells were plated in 96-well black plates at 20,000 cells/well. T cells were added at multiple E:T ratios. After 5 hr of co-culture, D-luciferin (PerkinElmer) was added to each well and luminescence was quantified by a plate reader (Infinite M200; Tecan). The number of viable HL60-GFPffluc cells in each well was calculated based on a standard curve generated from serial dilutions of the target cells. We calculated T cell cytotoxicity using the following formula: percent cytotoxicity = (cell number in control well − cell number in assay well) × 100/cell number in control well (target cells alone). Cytotoxicity of CLL-1.CAR-Ts against autologous CD14+ monocytes was assessed by standard 51Cr release assays as previously described.53 For evaluation of iC9/CLL-1.CAR-T activity, we used a fixed E:T ratio (10:1) and added 1 μM or 10 μM of the CID (B/B homodimerizer, catalog no. 635058) from Clontech. After overnight incubation with CID, we added 51Cr-labeled autologous CD14+ target cells to the effector population and measured isotope release after 5 hr of incubation. Target cells were incubated in medium alone or in 1% Triton X-100 (Sigma-Aldrich) to determine spontaneous and maximum 51Cr release. Specific release was calculated as follows: percent-specific release = (test counts − spontaneous counts)/(maximum counts − spontaneous counts) × 100%.
Co-culture Assay
Transduced or non-transduced T cells (1 × 105/well) were co-cultured with tumor cell lines (2 × 105 /well) at an E:T ratio of 1:2 or with PBMCs from primary AML (1 × 105/well) at an E:T ratio of 1:1 in 48-well plates, in the absence of exogenous cytokines. For HL60-GFPffluc co-culture, cells were harvested and stained for CD3 after 3 days. We identified tumor cells by GFP expression. For serial co-culture assays, CD3+ T cells were collected every 3 days and counted by flow cytometry using CountBright beads (Thermo Fisher Scientific). We then replated and rechallenged T cells with fresh HL60-GFPffluc cells at the same E:T ratio. For THP-1 co-cultures, cells were harvested and stained for CD3 and CD33 to detect THP-1. For co-cultures of primary AML samples, cells were harvested and stained for CD3 and CD34 to differentiate between T cells and AML blasts. After assigning dead cells by measuring the population positive for 7-amino actinomycin D (7-AAD) (Thermo Fisher Scientific), residual tumor cells and T cells in cultures were enumerated by fluorescence-activated cell sorting (FACS) using CountBright beads.
Proliferation Assay
T cells were washed and resuspended at 1 × 106/mL in CM. CellTrace Violet (Thermo Fisher Scientific) was added at 5 μM to T cells. T cells were incubated at 37°C for 20 min and washed. T cells were plated at 0.5 × 106/well in 24-well plates with or without 0.5 × 106/well stimulator cells.
Colony-Forming Assay with Leukemic or Normal Hematopoietic Progenitors
Mononuclear cells from the CB of healthy donors or PBMCs from patients with AML were co-incubated with CLL-1.BBζ CAR-Ts or CLL-1.Δ-Ts at an E:T ratio of 10:1 for 5 hr and then plated in duplicate in methylcellulose-based medium supplemented with recombinant cytokines (MethoCult H4434 Classic; STEMCELL Technologies) as previously described.54 After 12–14 days of culture, we scored granulocyte-macrophage CFU and erythrocyte burst-forming unit erythroid (BFU) or leukemic colonies using an inverted microscope.
Xenograft Model of AML and BLI
NSG mice were purchased from the Jackson Laboratory and maintained at the Baylor College of Medicine Animal Facility. We sublethally irradiated (200 cGy) NSG mice (6–10 weeks of age) and injected them with 50,000 HL60-GFPffluc cells via their tail vein. Leukemia burden was monitored by BLI (in photons/s/cm2/steradian [sr]) using the Xenogen in vivo imaging system (IVIS) (Caliper Life Sciences). We injected 0.6 × 106 CLL-1.CAR-Ts or control CAR-Ts (CLL-1.Δ) on day 1 after tumor injection. All procedures complied with the requirements of the Institutional Animal Care and Usage Committee of Baylor College of Medicine. For the in vivo iC9/CLL-1.BBζ experiment, we injected NSG mice with 50,000 WT-HL60 cells via their tail vein day 1 after sublethal irradiation (200 cGy). We then injected 2 × 106 iC9/CLL-1.BBζ CAR-Ts labeled with GFPffluc on day 7 after tumor injection. T cell signals were monitored by BLI (in photons/s/cm2/sr). Mice were treated with either CID or vehicle on days 14, 16, 23, 25, and 27 after T cell injection. Mice in the CID group were given 3 μg CID on days 14 and 16 and then subsequently 50 μg CID on days 23, 25, and 27.
In Vitro Apoptosis Study
We incubated T cells in the presence of 10 nM CID for 24 hr. The cells were then harvested and stained with annexin V-allophycocyanin and 7-AAD. Flow cytometric data were acquired by Gallios (Beckman Coulter) and analyzed using FlowJo (version 10; Tree Star).
Statistical Analysis
We used GraphPad Prism 5 software (GraphPad Software) for statistical analysis and data are presented as means ± SE. For comparisons between two groups, we used the two-tailed Student’s t test. We compared three or more groups using one-way ANOVA with Bonferroni’s post-test. For the mouse experiments, we analyzed survival from the time of T cell injection by constructing Kaplan-Meier curves and using log-rank (Mantel-Cox) tests.
Author Contributions
H.T. designed and performed the research, analyzed the data, and wrote the manuscript; T. Shum designed and performed the research, analyzed the data, and edited the manuscript; T. Sauer. designed and performed the research; K.P. performed the research; M.M. designed the research and edited the manuscript; B.O. designed the research; R.H.R. and P.L. provided primary AML samples and collected patients’ data; C.M.R. and S.G. designed the research and analyzed the data; and M.K.B. directed the study, designed the research, and worked with the authors to develop the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Dr. Caroline Arber for helpful discussion and technical support with the colony-forming assay, the Texas Children’s Cancer and Hematology Centers Flow Cytometry Core for technical support with cell sorting, and Catherine Gillespie for critical review of the manuscript. This study was supported in part by grants from the National Cancer Institute (NCI) (P01CA094237 and NCI Cancer Center support P30CA125123), the Leukemia Lymphoma Society (6483-16), and the Cancer Prevention Research Institute of Texas (CPRIT) (RP160693). T. Shum is supported by NIH/NHBLI (T32HL092332), NIH/NIDDK (T32DK060445), and, in part, by the Howard Hughes Medical Institute Med into Grad Initiative. P.L. is supported by a Leukemia Texas grant and an American Society for Blood and Marrow Transplantation (ASBMT) Young Investigator Award.
Footnotes
Supplemental Information includes Supplemental Materials and Methods and six figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.05.024.
Supplemental Information
References
- 1.Ishikawa F., Yoshida S., Saito Y., Hijikata A., Kitamura H., Tanaka S., Nakamura R., Tanaka T., Tomiyama H., Saito N. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat. Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 2.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude S.L., Fitzgerald J.C., Fisher B.T., Li Y., Huang Y.S., Torp K., Seif A.E., Kavcic M., Walker D.M., Leckerman K.H. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr. Crit. Care Med. 2014;15:112–120. doi: 10.1097/PCC.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchie D.S., Neeson P.J., Khot A., Peinert S., Tai T., Tainton K., Chen K., Shin M., Wall D.M., Hönemann D. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol. Ther. 2013;21:2122–2129. doi: 10.1038/mt.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q.S., Wang Y., Lv H.Y., Han Q.W., Fan H., Guo B., Wang L.L., Han W.D. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol. Ther. 2015;23:184–191. doi: 10.1038/mt.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizzitola I., Anjos-Afonso F., Rouault-Pierre K., Lassailly F., Tettamanti S., Spinelli O., Biondi A., Biagi E., Bonnet D. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014;28:1596–1605. doi: 10.1038/leu.2014.62. [DOI] [PubMed] [Google Scholar]
- 8.Casucci M., Nicolis di Robilant B., Falcone L., Camisa B., Norelli M., Genovese P., Gentner B., Gullotta F., Ponzoni M., Bernardi M. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 9.Mardiros A., Dos Santos C., McDonald T., Brown C.E., Wang X., Budde L.E., Hoffman L., Aguilar B., Chang W.C., Bretzlaff W. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill S., Tasian S.K., Ruella M., Shestova O., Li Y., Porter D.L., Carroll M., Danet-Desnoyers G., Scholler J., Grupp S.A. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynn R.C., Poussin M., Kalota A., Feng Y., Low P.S., Dimitrov D.S., Powell D.J., Jr. Targeting of folate receptor β on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood. 2015;125:3466–3476. doi: 10.1182/blood-2014-11-612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynn R.C., Feng Y., Schutsky K., Poussin M., Kalota A., Dimitrov D.S., Powell D.J., Jr. High-affinity FRβ-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30:1355–1364. doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mardiros A., Forman S.J., Budde L.E. T cells expressing CD123 chimeric antigen receptors for treatment of acute myeloid leukemia. Curr. Opin. Hematol. 2015;22:484–488. doi: 10.1097/MOH.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rhenen A., Moshaver B., Kelder A., Feller N., Nieuwint A.W., Zweegman S., Ossenkoppele G.J., Schuurhuis G.J. Aberrant marker expression patterns on the CD34+CD38- stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia. 2007;21:1700–1707. doi: 10.1038/sj.leu.2404754. [DOI] [PubMed] [Google Scholar]
- 15.Kikushige Y., Shima T., Takayanagi S., Urata S., Miyamoto T., Iwasaki H., Takenaka K., Teshima T., Tanaka T., Inagaki Y., Akashi K. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7:708–717. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Bakker A.B., van den Oudenrijn S., Bakker A.Q., Feller N., van Meijer M., Bia J.A., Jongeneelen M.A., Visser T.J., Bijl N., Geuijen C.A. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64:8443–8450. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X., Singh S., Pardoux C., Zhao J., Hsi E.D., Abo A., Korver W. Targeting C-type lectin-like molecule-1 for antibody-mediated immunotherapy in acute myeloid leukemia. Haematologica. 2010;95:71–78. doi: 10.3324/haematol.2009.009811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darwish N.H., Sudha T., Godugu K., Elbaz O., Abdelghaffar H.A., Hassan E.E., Mousa S.A. Acute myeloid leukemia stem cell markers in prognosis and targeted therapy: potential impact of BMI-1, TIM-3 and CLL-1. Oncotarget. 2016;7:57811–57820. doi: 10.18632/oncotarget.11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rhenen A., van Dongen G.A., Kelder A., Rombouts E.J., Feller N., Moshaver B., Stigter-van Walsum M., Zweegman S., Ossenkoppele G.J., Jan Schuurhuis G. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 20.Pelosi E., Castelli G., Testa U. Targeting LSCs through membrane antigens selectively or preferentially expressed on these cells. Blood Cells Mol. Dis. 2015;55:336–346. doi: 10.1016/j.bcmd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X.W., Xu W.T., Wang X.W., Mu Y., Zhao X.F., Yu X.Q., Wang J.X. A novel C-type lectin with two CRD domains from Chinese shrimp Fenneropenaeus chinensis functions as a pattern recognition protein. Mol. Immunol. 2009;46:1626–1637. doi: 10.1016/j.molimm.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Pulè M.A., Straathof K.C., Dotti G., Heslop H.E., Rooney C.M., Brenner M.K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X., Dotti G., Krance R.A., Martinez C.A., Naik S., Kamble R.T., Durett A.G., Dakhova O., Savoldo B., Di Stasi A. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125:4103–4113. doi: 10.1182/blood-2015-02-628354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X., Naik S., Dakhova O., Dotti G., Heslop H.E., Brenner M.K. Serial activation of the inducible caspase 9 safety switch after human stem cell transplantation. Mol. Ther. 2016;24:823–831. doi: 10.1038/mt.2015.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., Straathof K., Liu E., Durett A.G., Grilley B. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckley S.A., Walter R.B. Antigen-specific immunotherapies for acute myeloid leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2015;2015:584–595. doi: 10.1182/asheducation-2015.1.584. [DOI] [PubMed] [Google Scholar]
- 27.Lu H., Zhou Q., Deshmukh V., Phull H., Ma J., Tardif V., Naik R.R., Bouvard C., Zhang Y., Choi S. Targeting human C-type lectin-like molecule-1 (CLL1) with a bispecific antibody for immunotherapy of acute myeloid leukemia. Angew. Chem. Int. Ed. Engl. 2014;53:9841–9845. doi: 10.1002/anie.201405353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leong S.R., Sukumaran S., Hristopoulos M., Totpal K., Stainton S., Lu E., Wong A., Tam L., Newman R., Vuillemenot B.R. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood. 2017;129:609–618. doi: 10.1182/blood-2016-08-735365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyos V., Savoldo B., Quintarelli C., Mahendravada A., Zhang M., Vera J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thokala R., Olivares S., Mi T., Maiti S., Deniger D., Huls H., Torikai H., Singh H., Champlin R.E., Laskowski T. Redirecting specificity of T cells using the Sleeping Beauty system to express chimeric antigen receptors by mix-and-matching of VL and VH domains targeting CD123+ tumors. PLoS ONE. 2016;11:e0159477. doi: 10.1371/journal.pone.0159477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budde L.E., Berger C., Lin Y., Wang J., Lin X., Frayo S.E., Brouns S.A., Spencer D.M., Till B.G., Jensen M.C. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE. 2013;8:e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y., Zheng Z., Cohen C.J., Gattinoni L., Palmer D.C., Restifo N.P., Rosenberg S.A., Morgan R.A. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol. Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C.Y., Roybal K.T., Puchner E.M., Onuffer J., Lim W.A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 35.Dick J.E. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 36.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottschalk S., Ng C.Y., Perez M., Smith C.A., Sample C., Brenner M.K., Heslop H.E., Rooney C.M. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001;97:835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 38.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulikakos P.I., Persaud Y., Janakiraman M., Kong X., Ng C., Moriceau G., Shi H., Atefi M., Titz B., Gabay M.T. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruella M., Barrett D.M., Kenderian S.S., Shestova O., Hofmann T.J., Perazzelli J., Klichinsky M., Aikawa V., Nazimuddin F., Kozlowski M. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fedorov V.D., Themeli M., Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang, P.K., Karsunky, H., and Tressler, R. November 2013. Antibodies specific for cll-1. U.S. patent US20130295118 A1.
- 46.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G., Huls M.H., Liu E., Gee A.P., Mei Z. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Straathof K.C., Pulè M.A., Yotnda P., Dotti G., Vanin E.F., Brenner M.K., Heslop H.E., Spencer D.M., Rooney C.M. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quintarelli C., Vera J.F., Savoldo B., Giordano Attianese G.M., Pule M., Foster A.E., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vera J., Savoldo B., Vigouroux S., Biagi E., Pule M., Rossig C., Wu J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savoldo B., Ramos C.A., Liu E., Mims M.P., Keating M.J., Carrum G., Kamble R.T., Bollard C.M., Gee A.P., Mei Z. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y., Zhang M., Ramos C.A., Durett A., Liu E., Dakhova O., Liu H., Creighton C.J., Gee A.P., Heslop H.E. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu D., Song L., Brawley V.S., Robison N., Wei J., Gao X., Tian G., Margol A., Ahmed N., Asgharzadeh S., Metelitsa L.S. Medulloblastoma expresses CD1d and can be targeted for immunotherapy with NKT cells. Clin. Immunol. 2013;149:55–64. doi: 10.1016/j.clim.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonifant C.L., Szoor A., Torres D., Joseph N., Velasquez M.P., Iwahori K., Gaikwad A., Nguyen P., Arber C., Song X.T. CD123-engager T cells as a novel immunotherapeutic for acute myeloid leukemia. Mol. Ther. 2016;24:1615–1626. doi: 10.1038/mt.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quintarelli C., Dotti G., De Angelis B., Hoyos V., Mims M., Luciano L., Heslop H.E., Rooney C.M., Pane F., Savoldo B. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood. 2008;112:1876–1885. doi: 10.1182/blood-2008-04-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.