Abstract
Introduction
The FDA issued a warning following 24 cases of HBV reactivation during DAA therapy for HCV, including individuals with inactive, occult and past HBV infection. Clinical presentations ranged from asymptomatic fluctuations in HBV DNA to fulminant hepatic failure, liver transplantation and death. The mechanism is unknown.
Areas covered
HCV/HBV coinfection is common, particularly in regions endemic for HBV. HCV and HBV utilize host factors to support replication; both viruses evade/impair host immunity. Clinical presentations of HBV reactivation during DAAs are summarized. Other causes of HBV reactivation are revisited and recent data regarding HBV reactivation are presented.
Expert opinion
HBV reactivation during DAAs for HCV occurs, with life-threatening consequences in some individuals. The risk of HBV reactivation is observed in all HBV stages. The rapid removal of HCV likely alters and liberates host-viral +/− viral-viral interactions that lead to increased HBV replication. As immune reconstitution occurs with HCV removal, host recognition of HBV DNA likely ensues followed by vigorous host immune responses leading to liver injury (HBV flare). These cases highlight the importance of HBV testing prior to initiating DAA therapy, the need for close monitoring of HBV during therapy and timely administration of anti-HBV therapy to prevent serious sequelae.
Keywords: Direct acting antiviral (DAA) therapy, HBV flare, HBV reactivation, Hepatitis B virus (HBV), Hepatitis C virus (HCV) coinfection
1. Introduction
The development of all oral interferon-free combination direct-acting antivirals (DAAs) has revolutionized the treatment of chronic hepatitis C virus infection (HCV), affording extremely high cure rates (>95%) with few adverse events. Since their approval by many drug regulatory agencies worldwide, these agents are now widely available in many countries as first line therapy for HCV. The registration studies for these agents did not include individuals with a history of hepatitis B virus infection (HBV), another hepatotropic virus that is often found in patients with HCV owing to their shared transmission routes. Recently, at least 24 cases of hepatitis B virus (HBV) reactivation have been reported to the United States Food and Drug Administration (FDA) Event Reporting System or published in the literature in individuals undergoing DAA therapy for HCV infection who also have a past or current history of HBV infection (1–8). This resulted in the FDA issuing a safety alert for risk of HBV reactivation during DAA therapy in these patients, requiring a boxed warning to be added to the drug label, the most prominent warning. It has long been recognized that immunosuppressive therapy may reactive HBV, and hence prophylactic therapy is recommended in patients undergoing immunosuppressive therapy. However, HBV reactivation in the context of DAAs for HCV has been an unexpected sequelae, as DAA were not known to be directly immunomodulatory with their mechanism of action specifically targeting HCV non-structural proteins that are critical in the replication cycle of HCV.
2. HCV virology
HCV, a member of the Flaviviridae family, is a single-stranded positive sense enveloped RNA virus. The ~9,600 nucleotide genome encodes 3 structural proteins (core and 2 envelope) and 7 non-structural proteins (p7, NS2, NS3, NS4a, NS4b, NS5a and NS5b) (9). Owing to the very high replication rate and the error prone RNA-dependent RNA polymerase, HCV exists as a quasispecies of genetically different (mutated) but related viruses (9–11). HCV is divided into 6 major genotypes that have distinct geographic distributions (12). HCV is a hepatotropic blood borne virus, and therefore transmission occurs following parenteral exposure to HCV-infected blood or blood product derivatives. Vertical and sexual transmission rates are very low and therefore are not considered major routes of transmission, with the notable exception of sexual transmission in men who have sex with men or individuals with high risk sexual practices (13).
Following exposure, HCV binds to a receptor complex on hepatocytes that includes CD81, low-density lipoprotein receptors, scavenger receptor type B1 and tight junction proteins in hepatocytes. After receptor-mediated endocytosis, the virus uncoats and the genome is released into the cytoplasm, where translation into a single polyprotein occurs. Host and viral proteases (notably the NS3/4a protease) then cleave this polyprotein into the 10 mature structural/non-structural proteins. A membrane-associated replication complex forms and transcription takes place, dependent on the RNA-dependent RNA polymerase (NS5b). Assembly of new progeny virions occurs and mature virions are then released from hepatocytes.
3. HBV virology
HBV is a small DNA virus that belongs to the Hepadnaviridae family. The ~3.2 kilobase genome is a partially double stranded relaxed circular DNA (rcDNA) and is contained within a nucleocapsid core surrounded by a lipid bilayer containing viral glycoproteins (14). The viral genome contains 4 overlapping open reading frames, the S, C, P and X genes. The S gene encodes the HBV surface antigen (HBsAg), the C gene encodes the HBV core protein or the HBV e antigen (HBeAg) depending on whether the translation is initiated from the core or precore region, the P (Pol) gene encodes the polymerase and the X gene encodes the HBV X protein (HBxAg). HBxAg has been shown to have multiple functions related to signal transduction, transcriptional activation, inhibition of protein degradation, repair of DNA, apoptosis, cell cycle progression and host chromosome stability (15). Therefore, the X protein is thought to play an important role in the oncogenic potential of HBV.
The HBV lifecycle differs from HCV as it requires reverse transcription of an RNA copy of the genome, the pre-genomic RNA (pgRNA) (14). The HBV receptor remained unidentified until 2012 when it was reported that the bile acid transporter sodium taurocholate cotransporting polypeptide (NTCP) was the receptor or part of a receptor complex recognizing the pre-S1 region of HBV (16). Additional mechanisms and processes for viral entry have not been identified. After binding, HBV is internalized and uncoating occurs with subsequent release of the rcDNA, and the rcDNA is converted to covalently closed circular DNA (cccDNA). The minus rcDNA strand is a complete full-length genome, but the positive strand is variable in length, and requires positive strand synthesis completion followed by terminal end modification and covalent ligation to form cccDNA. cccDNA persists in the nucleus of the host as an episomal mini-chromosome and serves as the template for transcription of the pgRNA (polymerase, core proteins and viral progeny), the pre-core mRNA (HBeAg), and subgenomic RNA (long, middle and short S proteins, HBsAg and X protein) (17). The pgRNA is reverse transcribed into viral DNA, a pathway that is distinct and independent of HB protein production. HBV DNA integrates into the host genome, but this integration has no role in viral synthesis. Nucleocapsid assembly occurs when HBeAg, HBcAg and rcDNA are packaged within mature neocapsids containing HBsAg and the envelope, and secreted from the cell. Alternatively, the nucleocapsid can be recycled into the nucleus to replenish cccDNA. As HBsAg and the long, medium and short forms of the S proteins are produced in excess of viral progeny, these S proteins are secreted from the cell and can be detected in the serum.
4. Prevalence of HCV and HBV infection
Chronic HCV (CHC) infection and chronic HBV (CHB) infection remain significant global concerns. The majority of individuals (>75%) exposed to HCV do not spontaneously clear virus and develop chronic infection (18), and more than 185 million individuals are infected worldwide (12, 19). In addition there are more than 248 million individuals with chronic HBV infection globally, defined as positive HBV surface antigen (HBsAg) seroprevalence (20). In contrast to HCV, age of acquisition of HBV is a critical determinant of likelihood of the development of chronic HBV. Perinatally acquired HBV results in chronic HBV in over 90% of infants (21), and between 20–60% develop chronic HBV if acquired from 6 months to 5 years of age (22, 23), whereas the minority (5%) of adults who acquire HBV develop chronic HBV (23).
The majority of individuals are asymptomatic from HCV and/or HBV infection, with many experiencing non-specific symptoms such as intermittent vague right upper quadrant pain and fatigue, often only developing significant symptoms when end-stage liver disease has already been established. Therefore, many individuals with HCV or HBV may not seek medical attention resulting in under-diagnosis of CHC and CHB. The prevalence of CHB is also further under-recognized due to lack of diagnosis of occult HBV when using HBsAg as a marker of CHB. Occult HBV is a form of CHB characterized by positive HBV DNA levels but negative HBsAg serology. High prevalence areas of HCV and HBV are often found in low-income countries where the infrastructure may not exist or be possible for testing and/or reporting.
4.1 Prevalence of HCV infection
It has been well known that both HCV and HBV infection demonstrate distinct global geographic prevalence distributions (Table 1) (24), reflecting their differing modes of transmission. HCV prevalence is divided into low population prevalence (<1.5% anti-HCV positive), moderate prevalence (1.5–3.5% anti-HCV positive) and high prevalence (>3.5% anti-HCV positive). Data generated from a systematic review by the Global Burden of Disease study group (GBD) has estimated that low prevalence regions include high-income Asia Pacific nations, North America and tropical Latin America; moderate prevalence of HCV is found in Australasia, Southeast Asia, Caribbean, Eastern Europe, Western Europe, Andean Latin America, Central Latin America, Southern Latin America, Oceania and all regions of Sub-Saharan Africa; and high prevalence regions include Central Asia, East Asia and North Africa/Middle East (Table 1) (24). The country with the highest prevalence of HCV is Egypt, where more than 10% of the population is infected owing to iatrogenic spread of HCV during mass population inoculation for schistosomiasis (25).
Table 1.
Global prevalence of chronic hepatitis B virus (HBV) infection and chronic hepatitis C virus (HCV) infection. Chronic HBV infection was defined as HBsAg seroprevalence. Prevalence data for chronic HBV was generated from a systematic review for the GBD, representing 161 countries and study population of over 119 million individuals (20). HCV was defined as anti-HCV seroprevalence. Prevalence data for HCV infection was generated from a review for the GBD on changing HCV prevalence (24).
| HBsAg Prevalence | Anti-HCV Prevalence | ||||||
|---|---|---|---|---|---|---|---|
| Low <2.0% |
Lower intermediate 2.0–4.99% |
Higher intermediate 5.0–7.99% |
High ≥8.0% |
Low <1.5% |
Intermediate 1.5–3.5% |
High >3.5% |
|
| High-income | |||||||
| High-income Australasia | 2.2 (1.4–4.1) | 2.7 (2.2–3.2) | |||||
| High-income Asia Pacific | 3.4 (1.8–5.0) | 1.4 (1.2–1.5) | |||||
| High-income North America | 0.5 (0.3–0.8) | 1.3 (1.1–1.6) | |||||
| High-income Southern Latin America | 0.7 (0.6–0.8%) | 1.6 (1.1–2.2) | |||||
| Western Europe | 0.8 (0.4–1.1) | 2.4 (2.2–2.7) | |||||
| Central Europe, Eastern Europe, Central Asia | |||||||
| Central Asia | 6.4 (4.3–8.6) | 3.8 (3.0–4.5) | |||||
| Central Europe | 2.2 (0.7–3.7) | 2.4 (2.0–2.8) | |||||
| Eastern Europe | 3.6 (1.4–5.7) | 2.9 (2.3–3.5) | |||||
| South Asia | |||||||
| South Asia | 2.6 (1.2–4.0) | 3.4 (2.6–4.4) | |||||
| Southeast Asia, East Asia and Oceania | |||||||
| East Asia | 5.5 | 3.7 (3.1–4.5) | |||||
| Southeast Asia | 4.4 (2.2–6.5) | 2.0 (1.7–2.3) | |||||
| Oceania | 12.8 (7.8–18.7) | 2.3 (2.1–3.1) | |||||
| Latin America and Caribbean | |||||||
| Andean Latin America | 1.5 (0.4–2.6) | 2.0 (1.4–2.7) | |||||
| Caribbean | 4.7 (1.6–7.7) | 2.1 (1.6–2.6) | |||||
| Central Latin America | 0.9 (0.3–1.5) | 1.6 (1.3–1.9) | |||||
| Tropical Latin America | 0.7 | 1.2 (1.0–1.4) | |||||
| North Africa and Middle East | |||||||
| North Africa and Middle East | 2.6 (1.6–3.6) | 3.6 (3.2–4.1) | |||||
| Sub-Saharan Africa | |||||||
| Central Sub-Saharan Africa | 10.6 (8.4–12.8) | 2.3 (1.6–3.1) | |||||
| East Sub-Saharan Africa | 9.0 (6.5–11.4) | 2.0 (1.6–2.4) | |||||
| South Sub-Saharan Africa | 12.2 (6.7–17.7) | 2.1 (1.7–2.5) | |||||
| Western Sub-Saharan Africa | 12.4 (11.0–13.9) | 2.8 (2.4–3.3) | |||||
4.2 Prevalence of HBV infection
The GBD study group also performed a similar systematic review in HBV, including 1,800 reports of HBV surface antigen prevalence (HBsAg, the hallmark of CHB infection) from 161 countries. HBV prevalence was estimated by extrapolating the reported study population prevalence to the total population of each region/country. The same 21 defined global regions were then divided in to low (<2.0%), lower intermediate (2–4.99%), higher intermediate (5–7.99%) and high (>8%) HBV prevalence (Table 1) (20). Low prevalence regions include high-income North America, Latin America and Western Europe. Regions with lower intermediate prevalence include high-income Australasia, Central Europe, South and Southeast Asia, high-income Asia Pacific, North Africa/Middle East and the Caribbean, whereas Central Asia and East Asia have a higher intermediate prevalence of HBsAg. Regions with a >8% HBsAg prevalence included all regions of Sub-Saharan Africa (9.0–12.4%) and Oceania (12.8%) (Table 1) (20). The countries with the highest prevalence were South Sudan (22.4%) and Kiribati (22.7%). However, this study of HBV prevalence did not take in to consideration individuals with occult HBV infection, defined as negative HBsAg but with detectable serum HBV DNA levels, and therefore may underestimate the global burden of CHB.
4.3 Prevalence of HCV/HBV coinfection
Owing to the shared transmission routes of HCV and HBV, coinfection with HCV and HBV is reasonably common in endemic regions and in individuals with parental risk factors, such as persons who inject drugs, individuals undergoing hemodialysis and individuals with inherited blood disorders requiring blood products in regions where blood donor screening is either inadequate or not available. However, the true global and regional prevalence of HCV/HBV coinfection is not known owing to the absence of large population-based epidemiological studies, significant heterogeneity among study designs and definitions, and a lack of studies investigating the prevalence of occult HBV infection that would otherwise be missed in studies only reporting HBsAg seroprevalence.
However, there are a few several smaller population based studies that show great disparity in prevalence of HBV/HCV coinfection that may reflect the differing regional prevalence and transmission routes of HCV and HBV, and the populations being studied (Table 2). In general, the prevalence of HBV coinfection in HCV positive individuals ranges from 0.7–5.8% (26–29), and the prevalence of HCV coinfection in HBsAg positive individuals is between 3.4–23.0% (27, 30–32). In other specific populations, including patients with chronic liver disease, children and pregnant women, the prevalence of HCV/HBV coinfection ranged between 0.0% and 16.0% (27).
Table 2.
Prevalence of hepatitis C virus (HCV)/hepatitis B virus (HBV) coinfection according to global regions defined by the Global Burden of Diseases Study Group (20–32).
| Region | Country | Population studied | HBV/HCV coinfection prevalence | |
|---|---|---|---|---|
| High income North America | United States | Chronic HCV positive patients | n = 102,971 | 1.4% |
| n = 1,257 | 5.8% | |||
| n = 743 | 3.4% | |||
| Chronic HBV positive patients | n = 148 | 10.8% | ||
| Western Europe | Italy | Chronic HBV positive patients | n = 837 | 7.0% |
| n = 302 | 14.2% | |||
| n = 184 | 9.8% | |||
| Spain | Chronic HBV positive patients | n = 132 | 12.9% | |
| Central Europe | Romania | Routine medical check | n = 2,540 | 0.2% |
| North Africa/Middle East | Egypt | Chronic HCV positive patients | n = 3,300 | 0.7% |
| Iran | Chronic HBV positive patients | n = 139 | 12.3% | |
| Yemen | Antenatal screening | n = 400 | 0.0% | |
| High-income Asia Pacific | Japan | Chronic HBV positive patients | n = 82 | 22.0% |
| n = 156 | 12.8% | |||
| Central Asia | Mongolia | Pediatric population | n = 655 | 1.2% |
| Chronic liver disease population | n = 207 | 7.7% | ||
| Turkey | Chronic liver disease population | n = 1950 | 2.6% | |
| South Asia | India | Chronic liver disease population | n = 251 | 5.9% |
| n = 150 | 16.0% | |||
| n = 1,673 | 3.0% | |||
| Southeast Asia | Thailand | Chronic HBV positive patients | n = 296 | 2.7% |
| East Asia | China | Chronic HBV positive patients | n = 712 | 14.5% |
| n = 193 | 11.4% | |||
| n = 998 | 4.1% | |||
| Taiwan | Chronic HBV positive patients | n = 1,498 | 12.0% | |
| n = 100 | 18.0% | |||
| n = 323 | 3.4% |
4.3.1 Prevalence of HBV in HCV positive individuals
One of the largest studies from the United States interrogated the National Veterans Affairs HCV Clinical Case Registry to determine the prevalence of HBV coinfection in HCV positive individuals with confirmed HCV viremia (26). Of the 102,971 individuals with HCV, 1.4% were also found to have HBV coinfection, defined as a positive HBsAg, detectable HBV DNA or positive HBeAg, demonstrating a low prevalence of HBV coinfection in US veterans with CHC. This cohort was predominantly Caucasian or African American (53.8% and 31.5%, respectively) and only 0.2% were Asian. This low prevalence rate of HBV coinfection in HCV positive American Veterans may reflect that HBV is predominantly acquired as adults in the United States. Other smaller studies from the United States have reported the prevalence of HBV coinfection between 3.4–5.8%. Asian ethnicity, younger age, persons who inject drugs and higher number of lifetime sexual partners were all associated risk factors for HCV/HBV coinfection. In the Middle East, small studies from Egypt and Turkey have reported that the prevalence of HBV coinfection in HCV positive individuals was 0.7% and 2.6%, respectively (28, 29).
4.3.2 Prevalence of HCV in HBV positive individuals
The majority of studies that have investigated the prevalence of HCV infection in HBsAg positive individuals are from Asia and Europe. In the United States, a small study of 148 HBsAg positive individuals found that 10.8% were anti-HCV positive (33). In Asian regions the prevalence of HCV coinfection in HBsAg positive individuals ranged between 2.7 and 22.0%. The prevalence was 0.2–14.2% in Western and Central Europe, and 12.3% in North Africa/the Middle East (27).
5. Clinical presentations of HCV/HBV coinfection
There are several clinical presentations of HCV/HBC coinfection that again reflect the local transmission routes of each virus.
5.1 Simultaneous acute HCV and HBV coinfection
Acute infection with HCV and HBV simultaneously is exceedingly rare but can occur if the exposure source is dually infected with both viruses and transmission of both viruses occurs, for example after a needle stick injury (34) or following receipt of dually contaminated blood products (35). Limited data suggests that the long-term outcomes are unchanged in this context, although some patients demonstrated delayed appearance of HBsAg in the serum and a truncated period of HBsAg seroprevalence compared to HBV monoinfected individuals (36, 37).
5.2 Hepatitis C superinfection in CHB
HCV superinfection in individuals with pre-existing CHB is the most common presentation of HCV/HBV coinfection. It is particularly common in HBV endemic regions, such as the Asia Pacific (38, 39). Several studies have suggested that acute HCV infection in this context may be associated with more severe hepatitis and therefore should be considered in CHB patients who present with fulminant and sub-fulminant hepatitis, and fulminant hepatic failure (38, 40–42). HCV superinfection in the context of CHB is an important clinical entity as it has been associated with greater progression of liver disease to cirrhosis and a higher incidence of hepatocellular carcinoma (HCC) compared to HBV superinfection, HBV monoinfection or HCV monoinfection (38).
5.3 Hepatitis B superinfection in CHC
Occurring less commonly is HBV superinfection in CHC, and often presents as an acute deterioration in liver function in individuals with underlying CHC (43). The long-term clinical sequelae of HBV superinfection in CHC individuals are less clear owing to limited data, and contrasting studies have been published reporting disappearance of anti-HCV during HBV superinfection (43) while others report an increased risk of fulminant hepatic failure with hepatic encephalopathy and ascites (44).
5.4 Occult HBV in CHC
The final scenario of HCV/HBV coinfection is occult HBV (HBsAg negative but HBV DNA positive) in individuals with CHC. The prevalence of this type of coinfection is unknown but has been reported to range from 15.6 to 48.9% (45–47). Long-term natural history studies have suggested that there may be increased risk of cirrhosis and HCC in occult HBV infection also (48–50). This observation highlights the importance of performing complete HBV testing in all patients with CHC.
6. Host-viral interaction and immune response to HCV and HBV
The host immune response is critical in determining outcome of viral infection. Both HCV and HBV have developed mechanisms to evade normal host innate and adaptive immune responses to allow viral persistence and the development of chronic infection (summarized in Table 3).
Table 3.
Summary of innate and adaptive immune evasion by hepatitis C virus (HCV) infection and hepatitis B virus (HBV) infection.
| Innate immune evasion | Adaptive immune evasion | |
|---|---|---|
| HCV |
|
|
| HBV |
|
|
RIG-I = Retinoic acid-inducible gene I
TLR = Toll-like receptor
IFN = Interferon
NK = Natural killer
6.1 HCV and evasion of host immune responses
6.1.1 HCV and innate immunity
The host’s first line of defense against invading pathogens is the innate response. Following infection, HCV is recognized by viral sensing innate immune pattern recognition receptors (PRRs) such as retinoic acid-inducible gene I (RIG-I) and toll-like receptor 3 (TLR3), which recognize pathogen-associated molecular patterns. This leads to downstream activation of several innate immune response pathways, including interferon regulatory factor 3 and NF-κB, leading to induction of chemokines, cytokines and subsequent upregulation of antiviral interferon-stimulated genes (ISGs) (51). Normally, these innate immune responses help control and limit the spread of viral infection, and also help orchestrate the more specific adaptive immune response.
However, HCV has developed a number of mechanisms to evade the host innate immune response. The NS3/4a protease is able to disrupt RIG-I and TLR3 signaling through cleavage of the adaptor molecules that are required for signal transduction and the amplification loop that normally follows (52, 53). Other HCV proteins, such as the HCV core, NS5a and E2 proteins also have developed strategies to evade immune responses. HCV core protein overexpression has been shown to inhibit and degrade STAT1, which is required for JAK-STAT signal transduction and induction of ISGs (54, 55). In addition, the NS5a and E2 proteins may also attenuate the action of several ISGs (56, 57).
Although Natural Killer (NK) cells originate from lymphoid progenitors, they do not require antigen presentation and do not undergo clonal expansion like other lymphocytes in order to achieve their effector function. Therefore, they are considered part of the innate immune system. They have a critical role in surveillance of the cellular environment for the presence of infected or transformed cells. Upon recognition of such cells, they release cytotoxic granules that lead to cell death. This is an important first line defense against invading pathogens as the adaptive immune system is being recruited. Several studies have shown that NK cell function is altered by CHC whereby NK cells have divergent phenotypes characterized by increased expression of activation markers but impaired effector cytokine production (58).
6.1.2 HCV and adaptive immunity
The second arm of the immune system is the adaptive immune response, which generates specific immune responses directed towards the invading pathogen and generates immunologic memory. It is comprised of cellular (T cell) and humoral (B cell) immune responses. Humoral responses appear to play only a minor role in control of HCV; however cellular immune responses are critical in determining outcome. It should also be noted that CD4 and CD8 T cell effector function is not only important for viral control, but also in mediating and perpetuating liver injury that occurs in response to viral infection, and this is thought to mediate liver injury during CHC and CHB.
HCV-specific T cells play a critical role in determining the outcome of HCV infection, whereby potent and sustained HCV-specific CD4 and CD8 T cell responses against multiple HCV epitopes is associated with spontaneous clearance of HCV (59, 60). Strong and vigorous multi-epitope specific CD4 T cell responses producing T-helper 1 cytokines such as IFN-γ and TNF-α are detected early during acute HCV in individuals who spontaneously clear virus (61). Depletion of the CD4 T cells prior to infection leads to undetectable CD8 T cell responses and viral persistence, whereas depletion of theses CD4 T cells at the peak of infection when CD8 T cell responses are already detectable did not affect viral outcome (62). This highlights the importance of CD4 T cells in coordinating and maintaining CD8 T cell responses. As mentioned, vigorous and prolonged multi-specific CD8 T cells responses are also critical for spontaneous clearance of HCV, and the timing of the appearance of these CD8 T cells coincides closely with viral clearance (63). Dysfunction in many domains of T cell function has been demonstrated in individuals with CHC, including higher levels of markers of CD8 T cell exhaustion (64), reduced proliferation following stimulation with antigens including HCV (65, 66), insufficient priming of CD4 T cells by antigen presenting cells (67), suppression by regulatory T cells (68), lack of appropriate functional effector T cell responses (69) and production of cytokines that induce tolerogenic regulatory T cells that produce IL10 and TGF-β (70). As the cytotoxic CD8 T cells mediate the inflammatory and cytotoxic response that perpetuates liver injury in viral hepatitis, it has been proposed that this increase in tolerogenic regulatory T cells limits necroinflammation (71, 72). Despite the fact that memory T cells can be identified in these patients at very low frequencies, this immunologic memory is not protective against re-infection with HCV (73). Owing to the high mutation rate of HCV, some groups have identified viral escape mutations that confer a survival advantage in epitopes targeted by CD8 T cells in over 50% of individuals with CHC, and these escape mutants are likely selected for in the quasispecies (74).
Neutralizing antibodies are usually an important host antiviral response, both for clearance of infection and for protection against reinfection; however their role in HCV is limited. Only neutralizing antibodies directed at the viral envelope will prevent viral entry. Several groups have shown the target of these neutralizing antibodies is the hypervariable region 1 (HVR1) of the E2 viral envelope protein (75). Host selection pressure of this poorly conserved region leads to the development of escape mutants through rapid amino acid substitutions. Therefore, despite the high prevalence of neutralizing antibodies in CHC individuals, they fail to neutralize virus effectively. Cross-reactive neutralizing antibodies are also generated, but this occurs later in the course of CHC. As observed in T cell responses, several mechanisms lead to failure of binding to the cognate epitopes (75). This myriad of T cell and B cell dysfunction, evasion of specific T cell and B cell responses, plus the addition of innate immune dysfunction observed in CHC, leads to perpetuation of HCV persistence.
6.2 HBV and evasion of host immune responses
6.2.1 HBV and innate immunity
Similar to HCV, HBV has developed mechanisms to evade the host immune response to establish chronic infection. The innate immune response to HCV demonstrates induction of a type I IFN response within the liver, characterized by the upregulation of hundreds of ISGs. Interestingly, this response is insufficient to clear HCV in the majority and ISGs remained elevated throughout the course of the infection suggesting exhaustion or desensitization. In contrast, acute HBV infection elicits very little by way of type I IFN intrahepatic response and IFN-α levels are extremely low (76). This may be due to the differences in the HBV replication cycle where the genome is fully contained within the nucleocapsid in the cytoplasm during transport into the nucleus and also during replication. Therefore, the viral DNA/RNA intermediates may not be seen by the host innate sensing PRRs (77). Despite the lack of induction of a type I IFN response, HBV infection of primary human hepatocytes has shown induction of proinflammatory cytokines, particularly IL-6 (78). This may be important for coordination of the adaptive immune response.
Like HCV, HBV has also developed mechanisms to evade the host immune response. Firstly, the HBV X protein has been shown to bind to the adaptor molecule required for RIG-I, leading to abrogation of signal transduction of IRF3 and type I IFNs (79). In addition, the HBV polymerase was shown to inhibit IFN-β promoter activity upstream of IRF3, possibly by reducing the interaction of DDX3 with downstream targets in the RIG-I and TLR pathways, and inhibit IRF3 phosphorylation and dimerization. Incubation with other HBV proteins in mouse models, namely the HBsAg and HBeAg, has been shown to abrogate TLR signaling with pre-incubation of HBsAg or HBeAg prior to stimulation with the appropriate TLR ligand, leading to reduced type I IFN production and ISG induction (80). The role of HBeAg in disrupting TLR signaling was also observed in ex vivo liver tissue from patients with CHB, where TLR2 expression (which recognizes viral glycoproteins) was significantly reduced in HBeAg positive individuals compared to HBeAg negative individuals (81). This finding was confirmed in in vitro human models using human hepatic cell lines transduced with HBV baculovirus that expresses HBeAg or the G1896A pre-core mutant (HBeAg negative). Furthermore, TLR2 stimulation was shown to reduce HBV replication and reduce nucleocapsid formation (82). Most TLR ligands have been shown to have anti-HBV activity in transgenic mice (83) and a TLR7 agonist is currently in clinical development for HBV, confirming its role in disease pathogenesis (84). These data highlight the important of the role of the innate immune system despite the absence of a classical type I IFN signature.
As occurs in HCV, NK cell dysfunction is also observed in HBV with impaired production of IFN-γ, an important type II IFN that has antiviral and immunoregulatory properties (85).
Therefore, multiple HBV proteins interfere with the host innate immune response to reduce PRR signaling, leading to inhibition of cytokine and type I IFN production, and interference with NK function.
6.2.2 HBV and adaptive immunity
Virus-specific T cell responses are similarly critical in CHB. As observed in HCV, spontaneous clearance of acute HBV is associated with early vigorous and multi-specific CD4 T cell responses (77), and similarly strong, sustained and multi-epitope CD8 T cell responses are also important for HBV clearance (63). The role of both CD4 and CD8 T cells has been confirmed in depletion studies, where depletion of CD4 T cells prior to (but not at the peak of infection when CD8 T cells were detected) was associated with HBV persistence (62) and CD8 T cell depletion led to prolonged infection with delayed viral clearance. Both non-cytolytic mechanisms (through induction of cytokines such as IFN-γ and TNF-α) and cytolytic mechanisms are also important (86, 87). Cytotoxic functions of CD8 T cells also have a particular role in HBV eradication given the persistence of cccDNA within infected hepatocytes, as cell death is a key method to clear infected hepatocytes (88). Viral escape as a cause of T cell failure is uncommon in HBV compared to HCV, and the predominant causes for T cell failure are exhaustion and functional impairment that leads to T cell deletion (89).
B cells are activated by T-helper 2 CD4 T cells and produce immunoglobulins (Ig) or antibodies that can complex with and neutralize specific antigens. In contrast to HCV, humoral immune responses are extremely important in HBV, particular in infection or re-infection. This is demonstrated by the prevention of HBV following vaccination or in individuals who receive HBV immunoglobulin following exposure to HBV (90, 91). Anti-HBs has been shown to effectively neutralize HBsAg and clear HBV infection in a woodchuck model of human HBV (92). Furthermore, the timing and magnitude of anti-HBs production was an important determinant of viral inhibition and elimination from serum. Although anti-HBs is produced during acute and chronic HBV infection, it complexes with circulating HBsAg (which is produced 1000-fold in excess) and therefore free unbound anti-HBs is only detected after resolution of CHB (93). Other studies in patients with various stages of HBV, including those with resolved infection, have shown that individuals with CHB demonstrate down regulation of necessary co-stimulatory molecules on B cells and hyperactivation of the total B cell population, characterized by increased IgM and IgG production compared to healthy controls or those with past infection (94). Following stimulation, HBV-specific B cells from individuals with CHB were unable to further increase Ig production, suggesting functional impairment. Therefore, early and adequate B cell function, with the assistance of CD4 T helper cells is required for clearance of HBV.
7. Viral-viral interaction in the setting of HCV/HBV coinfection
7.1 Effect of HCV/HBV coinfection on viral replication and viral clearance
In addition to host-viral interactions, there may also be viral-viral interactions that take place in the context of HCV/HBV coinfection. There are conflicting data regarding the ability of each virus to interfere with viral replication of the other virus in HCV/HBV coinfection. In vitro data in cell culture models of coinfection have demonstrated that overexpression of the HCV core protein inhibits HBV replication (89, 95–97), most pronounced in HCV genotype 1 infection (89). Overexpression of the NS2 protein was also found to have an inhibitory effect on HBV gene expression and HBV replication. The NS5a protein has been shown to also interfere with HBV replication, although one group found that the NS5a protein enhanced HBV replication (98), whilst another group demonstrated inhibition of HBV replication by the NS5a protein (99).
As hepatotropic pathogens, both viruses infect hepatocytes and utilize host machinery to carry out viral replication. Accordingly, it has been suggested that part of the inhibitory effect of either virus on each other may be due to competition for the same host machinery. The number of hepatocytes infected during HCV or HBV infection varies considerably from <10–46% in HCV (100, 101) and <10–27% in HBV (102, 103). More recent studies have demonstrated that hepatocytes can be infected by both HCV and HBV simultaneously (104, 105). More recent studies using replicating virus cell culture models demonstrated that HCV and HBV can infect the same hepatocyte, and selective inhibition of each virus did not affect replication and gene expression of the other virus (105). Interestingly, coinfected hepatocytes were found to have lower HBV and HCV levels than hepatocytes monoinfected with either virus, using in situ hybridization (102, 106). This suggested that both viruses may directly have inhibitory effects on each other when coexisting in the same hepatocyte. A more recent study compared HBV infection in chimpanzees with and without CHC (107). In chimpanzees with CHC and classical upregulation of ISGs, onset of HBV infection was delayed and attenuated compared to HCV-naïve chimpanzees. Furthermore, the number of HBV-infected hepatocytes was lower in CHC chimpanzees, suggesting that establishment of HBV was impaired or replication space was limited by the circulating ISGs in CHC chimpanzees in contrast to cell culture based studies where there did not seem to be viral interference. Similarly, in a chimpanzee model of CHB, acute superinfection with HCV led to a reduction in HBsAg titers (108, 109), also suggesting the HCV modulates HBV replication. This also supports the clinical finding that occult HBV infection with HBsAg seroclearance but detectable HBV DNA and HBeAg seroconversion are more common in CHC (39, 102, 106, 110). Therefore, there appears to be some viral-viral interaction, although whether this is a direct or indirect effect has not been fully elucidated.
Several groups have presented data from large clinical studies to determine the interaction between the two viruses in human disease. Many studies report that HCV is the dominant virus with relative suppression of HBV replication (111–115). In contrast, other studies have found that HBV is more dominant, or that there is no dominant virus with reciprocal inhibition compared to either virus monoinfection (116–121), and one other study showed higher HCV RNA clearance rates in coinfection compared to HCV monoinfection (74% vs. 14%) (122). However many of these studies are largely cross-sectional with only one time point for HCV and HBV testing, and may explain the widely varying clinical presentations described. To address this, a large Italian multicenter study longitudinally followed anti-HCV and HBsAg positive individuals over 1 year with bimonthly viral load testing (123). A third of the cohort demonstrated widely varying patterns of virological profiles, mostly in HBV, confirming that there are important fluctuations in viral replication over time and a single time point is insufficient to determine dominance of the viruses.
7.2 Effect of HCV/HBV coinfection on natural history and disease outcome
Clinical studies consistently report that HCV/HBV coinfection is associated with more aggressive liver disease, with faster progression of fibrosis to cirrhosis, and greater rates of complications of cirrhosis such as hepatic decompensation and hepatocellular carcinoma (HCC) (30, 120). In a meta-analysis of studies reporting outcomes of HCV/HBV coinfected individuals, the relative risk of HCC was 165.0 in HCV/HBV coinfection (>7-fold increase), compared to 22.5 in HBV monoinfection and 17.3 in HCV monoinfection (124).
7.3 Effect of HCV/HBV coinfection on host immune responses
Host immunological responses in the context of dual HCV/HBV infection have only been examined in a small number of studies. Chen et al., demonstrated that levels of the proinflammatory cytokines IL-6, IL-8 and TNF-α were lower compared to monoinfection with either virus (116). These results were consistent with previous in vitro data demonstrating that dendritic cells increased production of anti-inflammatory cytokines such as IL-10 when co-cultured with HBV and HCV proteins, which in turn inhibits the production of profinflammatory cytokines (125).
In a study by Chu and colleagues, the frequency and intensity of antibody responses to NS4, but not to NS3, NS5, NS1 or E2, were found to be significantly lower in HCV/HBV coinfected patients (126). In contrast, anti-E2 antibody frequency and levels were found to be lower in the context of HCV/HBV coinfection compared to HCV monoinfection in two other studies (116).
8. Antiviral therapy for HCV and HBV
Both CHC and CHB cause progressive liver damage leading to cirrhosis, liver failure, hepatocellular carcinoma (HCC) and death (127, 128). However, differences in the replication cycle of each virus make the goals of HCV antiviral therapy and HBV antiviral therapy different. The primary goal of anti-HCV therapy is complete viral eradication, whereas owing to the persistence of covalently closed circular DNA (cccDNA) within the nucleus of infected hepatocytes (the template for transcription) (129), the main aim of anti-HBV therapy is viral suppression to reduce the long-term consequences of CHB.
For the past 2 decades, the mainstay of antiviral therapy for CHC was a combination of pegylated-interferon-α (peg-IFNα) plus ribavirin. This was associated with suboptimal responses (overall 54–56% (130–132)) and significant toxicity that limited widespread use of this therapy. Recent advances in antiviral drug discovery for CHC have led to the development of all oral IFN-free combinations of direct-acting antivirals (DAAs) that specifically target HCV proteins required for HCV replication. These regimens have revolutionized HCV therapy, allowing extremely high cure rates in most individuals (>95%) with minimal adverse events (133).
The most common form of anti-HBV therapy are the nucleos(t)ide analogues (NAs) that directly target the HBV polymerase (reverse transcriptase) leading to suppression of HBV DNA synthesis. These agents do not lead to eradication of cccDNA and therefore these therapies are not curative and generally considered to be life-long. Peg-IFNα also has antiviral activity against HBV, and a finite course of peg-IFNα is also approved as an alternative therapy for HBV in some individuals. While the precise mechanism for the anti-HBV effect of peg-IFNα is not known, peg-IFNα is an immunomodulatory agent that results in the upregulation of hundreds of interferon-stimulated genes (ISGs), leading to an antiviral state. Although serological clearance of CHB, defined as HBsAg seroconversion, occurs more frequently with peg-IFNα than with NAs, it is still infrequent, and sustained viral suppression after completion of peg-IFNα is only observed in 10–35% of individuals (134).
9. Immune reconstitution/restoration following HCV eradication with DAA therapy
It has been well demonstrated that cure of HCV with IFN-based therapy led to restoration of the impaired innate immune response, however T cell function did not fully recover despite viral eradication (135). In addition restoration of HBV-specific T cell function was not observed in CHB patients treated with peg-IFNα. In contrast to IFN, recent studies have demonstrated that DAA therapy not only improves innate immune function with restoration of type I IFN responses and normalization of NK cell function (136–139), however DAAs also improve the defective T cell function with reversal of the exhausted T cell phenotype, increased CD8 T cell proliferation, improved effector function, memory differentiation and improved virus specific T cell responses (140, 141). Therefore, DAAs are superior to IFN-based therapy in fully restoring defective host responses to HCV.
10. HBV reactivation
10.1 HBV reactivation during IFN-free DAA therapy
Owing to the dual action of peg-IFNα on both HCV and HBV replication, treatment of HCV in the context of HBV coinfection with IFN-based therapy was straightforward and it was common to observe improvement in HBV replication during anti-HCV therapy in those coinfected with CHB. In contrast, the newer DAAs target specific HCV proteins involved in the HCV replication cycle and therefore have no activity against HBV. The registration studies for the DAAs that led to FDA approval of these agents specifically excluded individuals with HBV coinfection. Although the precise impact of DAAs on HBV replication was unknown, it was not thought to be a clinically significant issue as there was no direct immune modulatory action ascribed to these agents.
In the period since their approval, individuals with HCV/HBV coinfection or CHC with a history of past HBV infection have been receiving DAA-based therapy. Somewhat unexpectedly, HBV reactivation has been observed in some of these patients, both in individuals with current HBV infection (inactive hepatitis: HBsAg positive with or without detectable HBV DNA levels), occult HBV (HBsAg negative but detectable HBV DNA levels) and in individuals with evidence of HBV past infection only (HBsAg and HBV DNA negative but anti-HBc positive). The latter was especially unexpected, as reactivation previously had only been seen in the context of significant immunosuppression (see section 10.2.1).
In total, the FDA has received notification of 24 cases of HBV reactivation in HCV patients receiving DAAs with concurrent HBV infection or past HBV infection, either through the Food and Drug Administration Adverse Event Reporting System (FAERS) or from the published literature (summarized in Table 4) (2–8, 142). The cases cover the spectrum of appropriately untreated HBV, including patients with inactive HBV, occult HBV and isolated anti-HBc positivity. Among the cases reported was an individual with past HBV infection who developed fulminant hepatic failure requiring liver transplantation attributed to HBV reactivation. In addition, the FDA reported that there were 2 deaths among the 24 patients with HBV reactivation, both with fulminant hepatic failure from HBV reactivation. Table 5 summarizes the available patient demographics and clinical characteristics of each of the 24 reported cases from the FAERS and the two case reports where individual clinical data are available. HBV reactivation was divided into 5 categories: isolated increase in HBV DNA, mild-moderate hepatitis (defined as ALT<500 with normal bilirubin, n=15), moderate-severe hepatitis (defined as ALT<1,500 with normal bilirubin, n=2), severe hepatitis with jaundice (defined as any ALT increase with accompanying jaundice, n=2), and fulminant hepatic failure (severe hepatitis with jaundice and evidence of liver synthetic dysfunction, n=3). Although statistical inference could not be made with small patients numbers in the majority of the groups, those who developed moderate-severe hepatitis were younger (median 48.5 years vs. >56.0 years for the other groups). Furthermore, the median baseline HBV DNA levels was higher in those who subsequently developed moderate-severe hepatitis with or without jaundice (355 and 1,350 IU/mL, respectively compared to 22.5 IU/mL with those with isolated HBV DNA increase, and 39.5 IU/mL for those with mild-moderate hepatitis). HBsAg status did not appear to predict HBV reactivation or severity of HBV reactivation, and there were no immediately obvious factors that favored the developed of fulminant hepatic failure over other HBV reactivation presentations.
Table 4.
Summary of the United States Food and Drug Administration Adverse Event Reporting System (FAERS) reported cases of hepatitis B virus (HBV) reactivation during direct-acting antiviral (DAA) therapy and cases of HBV reactivation published in the literature that are not covered by the FAERS report.
| Case | Study | Age | Sex | HCV genotype | Past HCV treatment | DAA regimen | HBV characteristics pre DAA | Timing of HBV reactivation from start of DAAs | HBV characteristics during DAA therapy | Type of HBV reactivation |
|---|---|---|---|---|---|---|---|---|---|---|
| Cases of HBV reactivation notified to the FDA | ||||||||||
| 1 | FAERS | 59 | Male | 1a | Not reported | Paritaorevir/r + ombitasvir + dasabuvir + RBV | HBsAg(+) HBeAg(−) HBV DNA 2,700 IU/mL (Inactive carrier) |
Week 4 | ALT 497 Bilirubin 1.3 mg/dL HBV DNA 1,700,000 IU/mL (treated with TDF) |
HBV flare with acute hepatitis and jaundice |
| Week 6 | ALT 2264 Bilirubin 6.8 mg/dL |
|||||||||
|
| ||||||||||
| 2 † | Ende et al. | 59 | Female | 1b | IFN+RBV | Sofosbuvir + Simeprevir + RBV |
HBsAg(−) Anti-HBc(+) HBV DNA not detected (Past infection) |
Week 11 |
HBV DNA 29,000,000 IU/mL HBsAg(+) ALT 2,263 Bili 9.1mg/dL INR 1.9 (required liver transplantation) |
Fulminant hepatic failure requiring liver transplantation |
|
| ||||||||||
| 3 | FAERS | 69 | Male | 1 | Not reported | Daclatasvir + Asunaprevir | HBsAg not reported HBeAg(−) HBV DNA 2.5 log copies/mL (Inactive carrier) |
Week 4 Week 8 |
Increased ALT ALT 237 HBV DNA 6.7 log copies/mL |
Mild hepatitis |
| 4 | De Monte et al. | 53 | Male | 4d | IFN+RBV | Sofosbuvir + Ledipasvir | HBsAg(−) Anti-HBc(+) HBV DNA not detected (Past infection) |
Week 6 | HBV DNA 8.9 log IU/mL ALT 1026 |
Moderate hepatitis without jaundice |
| 5 * | Kimura et al. | 65 | Female | 1 | Naïve | IFN + RBV + Simeprevir | HBsAg(+) HBeAg(−) HBV DNA 3.3 log IU/mL (Inactive carrier) |
Week 5 post-treatment (ceased treatment week 3 due to occular bleeding) | ALT 32 HBV DNA 4.3 log copies/mL (treated with ETV) |
Isolated increase in HBV DNA - Regimen included IFN + RBV |
| 6 | Collins et al. | 57 | Male | 1a | IFN+RBV | Sofosbuvir + Simeprevir | HBsAg(−) Anti-HBc(+) HBV DNA <20 IU/mL (+)(Occult HBV) |
Week 2 | HBV DNA peaked 11,255 IU/mL at week 4 (treated with TDF) | Isolated increase in HBV DNA |
| 7 | Collins et al. | 55 | Male | 1a | IFN+RBV | Sofosbuvir + Simeprevir | HBsAg(+) HBeAg(−) HBV DNA 2,300IU/mL (Inactive carrier) |
Week 8 | HBV DNA 22,000,000 IU/mL (treated with TDF) | Isolated increase in HBV DNA |
| 8 | FAERS | 44 | Female | 3a | Sofosbuvir + RBV | HBsAg(+) HBV DNA 244 IU/mL (Inactive carrier) |
Week 7 | ALT 64 HBV DNA 26,194 IU/mL |
Isolated increase in HBV DNA | |
| Week 11 | HBV DNA 132,712 IU/mL (treated with ETV) | |||||||||
| 9 | Balagopal et al. | 48 | Female | Not reported | Not reported | Sofosbuvir + Ledipasvir | HBsAg(+) HBV DNA not detected (Inactive carrier) |
Week 4 Week 8 Week 12 |
HBV DNA 96 IU/mL HBV DNA 303 IU/mL (peaked at week 8) HBV DNA undetectable by week 12 (no treatment required) |
Isolated increase in HBV DNA |
| 10 | FAERS | 58 | Male | 1a | Not reported | Daclatasvir + Sofosbuvir + RBV | HBsAg(+) HBeAg(−) HBV DNA 92 IU/mL (Inactive carrier) |
~Week 6–8 | ALT 57 HBV DNA 10,900 IU/mL (treated with TDF) |
Isolated increase in HBV DNA |
| 11 | FAERS | 79 | Female | 1b | Not reported | Daclatasvir + Asunaprevir | HBsAg(+) HBeAg(−) HBV DNA <2.1 log copies/mL (Inactive carrier) |
Not reported | HBV DNA 3.0 log copies/mL ALT normal |
Isolated increase in HBV DNA |
| 12 | FAERS | 57 | Female | 1b | IFN+RBV | Daclatasvir + Asunaprevir | HBsAg(+) HBeAg(−) HBV DNA not detected (Inactive carrier) |
Not reported | HBV DNA 2.4 log copies/mL | Isolated increase in HBV DNA |
| 13 | FAERS | 52 | Female | 1 | Not reported | Daclatasvir + Asunaprevir | HBsAg(+) HBeAg(−) HBV DNA <2log copies/mL (Inactive carrier) |
Week 4 | HBV DNA 3.9 log copies/mL | Isolated increase in HBV DNA |
|
| ||||||||||
| 14 † | FAERS | 57 | Female | Not reported | Not reported | Daclatasvir + Asunaprevir |
HBsAg (+) HBeAg(−) HBV DNA 3.9 log IU/mL (Inactive carrier) |
Week 8 |
ALT 2114 Bilirubin 13.2 mg/dL Prothrombin 24% HBV DNA 7.4 log copies/mL (Fulminant hepatic failure) (treated with ETV) |
Fulminant hepatic failure and death
|
|
| ||||||||||
| 15 | FAERS | 71 | Female | 1 | IFN+RBV | Daclatasvir + Asunaprevir | HBsAg(+) HBeAg not reported HBV DNA <2.1 log copies/mL (Inactive carrier) |
Week 12 | HBV DNA 4.1 log copies/mL | Isolated increase in HBV DNA |
|
| ||||||||||
| 16 † | FAERS | 73 | Female | Not reported | Naïve | Daclatasvir + Asunaprevir |
HBsAg(+) HBeAg not reported HBV DNA not detected on ETV (Treated HBV) |
Week 7 |
ALT 462 Bilirubin 2.5 mg/dL (DAAs ceased) |
Fulminant hepatic failure and death - Patient elected to ceased ETV prior to commencing DAA therapy against the advice of the treating physician |
| Week 7–8 |
ALT 2461 Bilirubin 19.5 mg/dL HBV DNA 8.4 log copies/mL (HBV DNA undetectable 17 days prior) Died from multiorgan failure |
|||||||||
|
| ||||||||||
| 17 | FAERS | 36 | Male | Not reported | Not reported | Sofosbuvir + Ledipasvir | HBsAg(−) Anti-HBc(+) HBV DNA <LLD (Inactive carrier) |
Week 3 | ALT 381 Fever 38°C with lung infiltrates on CT scan Diagnosed with influenza Ceased DAAs at week 4 Restarted DAAs 1 week later |
Moderate HBV flare without jaundice |
| Week 6 | HBV DNA 2.4 log copies/mL ALT normal |
|||||||||
| 18 | FAERS | 44 | Female | Not reported | IFN | Sofosbuvir + Ledipasvir | HBsAg(+) HNeAg not reported HBV DNA 3.6 log copies/mL (Inactive carrier) |
Week 8 Week 9 |
ALT 84 ALT 552 HBV DNA 9.0 log copies/mL ALT 1417 |
Moderate HBV flare without jaundice |
| 19 | FAERS | 53 | Female | 1 | Not reported | Sofosbuvir + RBV | HBsAg(+) HBeAg(−) HBV DNA <2.1 log copies/mL (Inactive carrier) |
Week 10 | HBV DNA 3.2 log copies/mL | Isolated increase in HBV DNA |
| 20 | FAERS | 49 | Female | 1b | Not reported | Daclatasvir + Asunaprevir | HBsAg(+) HBeAg(−) HBV DNA 2.7 log copies/mL (Inactive carrier) |
Week 2 Week 4 Week 8 Week 9–10 |
HBV DNA 4.1 log copies/mL HBV DNA 5.4 log copies/mL HBV DNA 7.9 log copies/mL HBV DNA 8.5 log copies/mL ALT 523 (treated with ETV) |
Mild HBV flare |
| 21 | FAERS | 78 | Female | Not reported | Not reported | Sofosbuvir + Ledipasvir | HBsAg(+) HBeAg(−) HBV DNA 2.1 log copies/mL (Inactive carrier) |
Week 9 | ALT 61 HBV DNA 3.5 log copies/mL (peak) |
Mild HBV flare |
| 22 | FAERS | 78 | Male | Not reported | Not reported | Sofosbuvir + Ledipasvir | HBsAg(+) HBV DNA Not reported (Unable to characterize) |
Week 4 Week 8 |
HBV DNA 3.3 log copies/mL HBV DNA 5.2 log copies/mL (peak) |
Isolated increase in HBV DNA |
| 23 | FAERS | 69 | Male | Not reported | IFN+RBV | Daclatasvir + Asunaprevir | HBsAg(+) HBeAg not reported HBV DNA not detected (Inactive carrier) |
Week 6 | HBV DNA 5.0 log copies/mL (peak) | Isolated increase in HBV DNA |
| 24 | FAERS | 63 | Male | Not reported | Not reported | Daclatasvir + Asunaprevir | HBsAg not reported HBeAg(−) HBV DNA 3.3 log copies/mL (Inactive carrier) |
Week 4–8 | HBV DNA 4.7 log copies/mL | Isolated increase in HBV DNA |
|
| ||||||||||
| Additional cases of HBV reactivation published in the literature | ||||||||||
|
| ||||||||||
| 1 | Takayama et al. | 69 | Male | 1b | Not reported | Daclatasvir + Asunaprevir | HBsAg(+) HBeAg(−) HBV DNA 2.5 log copies/mL (Inactive carrier) |
Week 6 | HBV DNA 7 log copies/mL (treated with ETV) | Isolated increase in HBV DNA |
| 2 | Ou et al. | 53 | Male | 1b | IFN+RBV | Sofosbuvir + Ledipasvir | HBsAg(+) HBeAg not reported HBV DNA not detected (Inactive carrier) |
Week 12 | HBV DNA 82,600,000 IU/mL ALT 779 Bilirubin 90.8 μmol/L (treated with ETV) |
HBV flare with jaundice |
| 3–5 | Wang et al. | Cohort of 327 patients with HCV treated with DAAs in highly endemic HBV region. A total of 10 patients were coinfected with HBV (HBsAg(+)) and a further 124 patients had occult HBV (HBsAg(−) with detectable HBV DNA). 30% of the HBsAg(+) patients were found to have reactivation of HBV (not defined in the manuscript). No patient with occult HBV was noted to have HBV reactivation. | ||||||||
= This regimen includes interferon and ribavirin
= Significant outcome from HBV reactivation
IFN = Interferon
RBV = Ribavirin
TDF = Tenofovir
ETV = Entecavir
ALT = Alanine aminotransferase
IU = International units
Table 5.
Summary of the patient characteristics of the hepatitis B virus (HBV) reactivation cases notified to the United States Food and Drug Administration Adverse Event Reporting System (FAERS) and published in the literature.
| Isolated increased HBV DNA |
Mild hepatitis | Moderate-severe hepatitis | Severe hepatitis with jaundice |
Fulminant hepatic failure | ||
|---|---|---|---|---|---|---|
| n=15 | n=4 | n=2 | n=2 | n=3 | ||
| Age, years (Median, IQR) | 58.0 (54.0–69.0) | 59.0 (45.8–71.3) | 48.5 (46.3–50.8) | 56.0 (54.5–57.5) | 59.0 (58.0–66.0) | |
| Male (n, %) | 7 (46.7%) | 2 (50.0%) | 1 (50.0%) | 2 (100.0%) | 0 (0.0%) | |
| HCV genotype (n, %) | 1 | 4 (26.7%) | 1 (25.0%) | - | - | - |
| 1a | 3 (20.0%) | - | - | 1 (50.0%) | 1 (33.3%) | |
| 1b | 3 (20.0%) | 1 (25.0%) | - | 1 (50.0%) | - | |
| 3a | 1 (6.7%) | - | - | - | - | |
| 4d | - | - | 1 (50.0%) | - | - | |
| Unknown | 4 (26.7%) | 2 (50.0%) | 1 (50.0%) | - | 2 (66.7%) | |
| Fibrosis stage | Cirrhosis | 5 (33.3%) | 1 (25.0%) | 1 (50.0%) | - | 1 (33.3%) |
| Bridging fibrosis | 2 (13.3%) | - | - | - | - | |
| F0–2 METAVIR stage | 3 (20.0%) | 1 (25.0%) | 1 (50.0%) | 1 (50.0%) | 2 (66.7%) | |
| Unknown | 5 (33.3%) | 2 (50.0%) | - | 1 (50.0%) | - | |
| Past HCV treatment (n, %) | Naïve | 1 (6.7%) | - | - | - | 1 (33.3%) |
| IFN monotherapy | 0 (0.0%) | - | 1 (50.0%) | - | - | |
| IFN + RBV | 5 (33.3%) | - | 1 (50.0%) | 1 (50.0%) | 1 (33.3%) | |
| Unknown | 9 (60.0%) | 4 (100.0%) | - | 1 (50.0%) | 1 (33.3%) | |
| DAA regimen (n, %) | IFN + RBV + SMV | 1 (6.7%) | - | - | - | - |
| SOF + RBV | 2 (13.3%) | - | - | - | - | |
| SOF/LDV | 2 (13.3%) | 2 (50.0%) | 2 (100.0%) | 1 (50.0%) | - | |
| SOF + SMV | 2 (13.3%) | - | - | - | - | |
| SOF + SMV + RBV | - | - | - | - | 1 (33.3%) | |
| SOF + DCV + RBV | 1 (6.7%) | - | - | - | - | |
| DCV + ASV | 7 (46.7%) | 2 (50.0%) | - | - | 2 (66.7%) | |
| PrO + D + RBV | - | - | - | 1 (50.0%) | - | |
| HBsAg positive at baseline (n, %) | Yes | 14 (93.3%) | 2 (50.0%) | 1 (50.0%) | 2 (100.0%) | 2 (66.7%) |
| No | - | 1 (25.0%) | 1 (50.0%) | - | 1 (33.3%) | |
| Unknown | 1 (6.7%) | 1 (25.0%) | - | - | - | |
| HBeAg positive at baseline (n, %) | Yes | 0 (0.0%) | - | - | - | - |
| No | 10 (66.7%) | 3 (75.0%) | 1 (50.0%) | 1 (50.0%) | 2 (66.7%) | |
| Unknown | 5 (33.3%) | 1 (25.0%) | 1 (50.0%) | 1 (50.0%) | 1 (33.3%) | |
| Isolated anti-HBc (n, %) | Yes | 0 (0.0%) | 1 (25.0%) | 1 (50.0%) | - | 1 (33.3%) |
| No | 15 (100.0%) | 3 (75.0%) | 1 (50.0%) | 2 (100.0%) | 2 (66.7%) | |
| Unknown | - | - | - | - | - | |
| Baseline HBV DNA (IU/mL) | 22.5 (18.4–206.0) | 39.5 (21.9–64.7) | 355 (178–533) | 1,350 (675–2025) | 0 (0–709) | |
| Timing HBV reactivation, weeks (median, IQR) | 6.0 (4.0–8.5) | 7.0 (5.0–8.3) | 7.5 (6.8–8.3) | 9.0 (7.5–10.5) | 8.0 (7.5–9.5) | |
| Peak ALT (IU/mL) | 57.0 (44.5–60.5) | 309.0 (193.0–416.5) | 1221.5 (123.8–1319.3) | 1,521.5 (1,150.3–1892.8) | 2,263.0 (2,188.5–2,362.0) | |
| Peak HBV DNA level (IU/mL) | 3,563 (330–14,556) | 1406 (435–225,430) | 160,207,878 (151,026,103–169,389,653) | 42,150,000 (21,925,000–62,375,000) | 29,000,000 (27,059,432–140,094,322) |
= This regimen includes interferon and ribavirin
= Significant outcome from HBV reactivation
IFN = Interferon
RBV = Ribavirin
SMV = Simeprevir
SOF = Sofosbuvir
LDV = Ledipasvir
ASV = Asunaprevir
DCV = Daclatasvir
PrO + D = Paritaprevir/ritonavir/Ombitasvir + Dasabuvir
ALT = Alanine aminotransferase
IU = International units
IQR = Interquartile range
HBV reactivation did occur following the prior standard of care therapy for HCV, although the mechanism was more akin to a form of immune reconstitution. A meta-analysis of treatment outcomes following peg-IFNα plus RBV demonstrated that HBV reactivation occurred in 25% overall, and the risk was highest in individuals who did not clear HCV during therapy (odds ratio 3.36) (143). However, the majority of these cases of HBV reactivation were mild with regards to increases in HBV replication and also clinical presentation. A very recent systemic review and meta-analysis of HBV reactivation in HBV/HCV coinfected individuals found that the incidence was similar in DAA-based regimens (12.2%) and IFN-based regimens (14.5%) (144). However, clinical manifestations and timing of the HBV reactivation were starkly different. HBV reactivation occurred much earlier during DAA therapy (ranging from week 4–12 of DAA therapy compared to near the end of treatment or during follow-up with IFN-based therapy) and presentation was more severe with more cases of icteric hepatitis and fulminant liver failure. It should be noted that these cases represent only those reported to the FDA or published in the literature. Furthermore, HBV reactivation was not predicted to be a sequelae of DAA therapy; therefore testing and monitoring may not have routinely been performed, and the true incidence is not known.
10.2 HBV reactivation in other situations
10.2.1 HBV reactivation in the setting of immunosuppression and immune reconstitution
HBV reactivation in individuals who have either previously cleared HBV or have low or undetectable HBV DNA levels has been frequently described, particularly in patients undergoing immunosuppressive therapy for cancer, organ transplantation and autoimmune disease (145–148). It is of major clinical importance leading to significant and can lead to life-threatening sequelae.
HBV reactivation may present in several clinical ways, related to the phases of HBV reactivation (Table 6) (149). Firstly, there is an abrupt increase in HBV replication, usually defined as >1 log10IU/mL increase in HBV DNA levels, which occurs after initiating immunosuppressive therapy. This is often accompanied by HBeAg seroreversion (reappearance) and in some cases HBsAg seroreversion in individuals who are HBeAg and HBsAg negative, respectively. The second phase is characterized by disease activity and hepatic injury, and is usually initiated upon withdrawal or reduction of immunosuppression, mediated by host immune reconstitution. Elevations in aminotransferases are common owing to the hepatocellular injury that ensues, and may be associated with jaundice, symptoms of hepatitis or in severe cases fulminant liver failure and death. Concurrently, often a fall in HBV DNA levels is observed as the host attempts to control HBV replication. The final phase is the recovery phase, which occurs as liver injury resolves and HBV activity returns to baseline levels. However, not all individuals progress through all three phases of reactivation, and not all patients will undergo HBV reactivation even with immunosuppressive therapy. Some individuals develop reactivation without liver injury, and this scenario often leads to persistent HBV replication requiring therapy. Meanwhile others develop severe immune reconstitution liver injury without recovery leading to fulminant hepatic failure that may require liver transplantation or may even be fatal.
Table 6.
Phases and characteristics of hepatitis B virus (HBV) reactivation.
| HBV reactivation phase | Characteristics |
|---|---|
| Increased HBV replication | Rapid rise (>1 log10IU/mL) in HBV DNA HBeAg re-appearance (seroreversion) in HBeAg negative individuals HBsAg re-appearance (seroreversion) in individuals with past HBV infection |
| Necroinflammatory host response | Host response leads to liver injury
|
| Recovery | Host re-gains controls of HBV replication
|
IU = International units
ALT = Alanine aminotransferase
Pooled results of a systematic review have demonstrated that HBV reactivation following immunosuppressive therapy for cancer causes hepatitis in 33.4%, liver failure in 13.0% and leads to death in 6.7% (150). A more recent meta-analysis and systematic review of HBV reactivation following chemotherapy for solid organ cancers found that HBV reactivation ranged from 4–68%. Owing to this significant risk and potentially severe consequences, HBV prophylaxis is now recommended according to the HBV status and the immunosuppressive regimen (151). This strategy reduces the risk of HBV reactivation (odds ratio 0.12), HBV hepatitis (4%, odds ratio 0.18), fulminant hepatic failure (0%) and death (2%) (150, 152).
The type of immunosuppressive regimen also appears to play a role in HBV reactivation, with anti-CD20-based regimens (rituximab) associated with the highest risk of HBV reactivation, including in individuals with a history of past HBV infection only (153). Immunosuppressive regimens containing high dose prednisolone also increases the risk and severity of HBV reactivation (154). HBV reactivation has also been reported in 30% of patients following transarterial chemoembolization, a locally ablative palliative therapy for HCC (155). Anti-TNF monoclonal antibody therapy and other biologics, such as interleukin (IL) inhibitors, are becoming increasingly more common to treat a wide range of autoimmune diseases. In a systemic analysis of 257 cases of individuals receiving anti-TNF therapy with concurrent positive HBV markers, HBV reactivation was noted in 39.3% of the 89 HBsAg positive individuals (156). Of these individuals, 34.3% developed hepatitis with an alanine aminotransferase of more than 5 times the upper limit of normal, and 14.3% have evidence of liver failure. In the remaining HBsAg negative individuals, the risk of HBV reactivation was 7-fold lower. There are less data available for IL-6 and IL-12/IL-23 inhibitors and T cell activation inhibitors (157). However based on these limited data these agents are generally considered low-risk agents, although HBV reactivation has been reported with these agents. Therefore, HBV testing is recommended in patients planned for any class of biologic therapy, and they should be offered HBV prophylaxis according to guidelines (151, 158).
10.2 HBV reactivation in the setting of pregnancy
Pregnancy is considered a tolerogenic state as pregnant women require mechanisms to prevent rejection of the developing fetus (159). These maternal immunological changes have been associated with the development of HBV reactivation both during pregnancy and more commonly in the post-partum period. As observed in HBV reactivation during immunosuppressive therapy, the HBV reactivation with HBV flare is though to be due to immune reconstitution following delivery of the fetus. In a prospective study of 126 pregnant women with CHB, 1.5% developed HBV reactivation with an ALT flare during pregnancy, whilst 25.0% developed HBV reactivation with hepatitis in the post-partum period (160). A multivariate analysis demonstrated that younger age, gravida and parity were associated with HBV reactivation with an ALT flare. Univariate analysis also demonstrated that HBeAg status was associated with HBV flare during pregnancy (odds ratio 2.56), however this just failed to meet statistical significance in the multivariate analysis (p=0.051). For the majority of women, HBV flares were mild and resolved spontaneously.
11. Conclusion
HCV and HBV coinfection is not uncommon owing to shared transmission routes; however the prevalence varies considerably worldwide. Both HCV and HBV are hepatotropic viruses that require host machinery to carry out viral replication, and have developed strategies to evade and thwart normal host antiviral immune responses that allow the development of persistent infection. In HCV, this is characterized by failure of innate immune signaling via the PRRs, NK cell dysfunction, and upregulation and subsequently exhaustion of ISGs. This innate immune dysfunction also contributes to the failure of adaptive immunity, with weak CD4 T cell responses early during the course of infection that leads to a lack of invigoration of CD8 T cell responses. Furthermore, there is failure of virus-specific CD8 T cell responses, poor proliferation and effector function, and exhaustion of CD8 T cells. Humoral responses may play a small role in preventing host viral clearance. In HBV, initial innate immune responses appear to be less important, but similar T cell dysfunction is reported. Inadequate B cell responses are more prominent in HBV than HCV. In addition to these host-viral interactions, there is also viral-viral interference that leads to changes in each replicating virus, although whether this is a direct or indirect effect is open for debate. The development of IFN-free all oral DAA therapy for HCV has dramatically changed the treatment landscape for HCV, with rapid clearance of HCV from the circulation and cure rates approaching 100%. DAA therapy has been associated with reversal of many of the domains of innate and adaptive immune failure in CHC. Since DAAs specifically target HCV proteins that are essential for HCV replication, they have no activity in HBV. This has become relevant in HCV/HBV coinfected individuals as there have now been reports of HBV reactivation in individuals treated with IFN-free DAA therapies, a phenomenon well described during immunosuppressive therapy or during immunosuppressive states but not thought to be relevant during DAA therapy. A recent meta-analysis of HBV reactivation during peg-IFNα plus RBV and DAA therapy, found that the incidence of HBV reactivation was similar with both treatment regimens, but patterns of reactivation were markedly different with the two treatment regimens. HBV reactivation with peg-IFNα plus ribavirin occurred very late in the course of therapy or more frequently during the follow-up period and was generally milder in clinical presentation. In contrast, HBV reactivation with DAA therapy exclusively occurred during HCV therapy in all cases, and the clinical presentation was more severe. This HBV reactivation has occurred in individuals with inactive CHB, occult HBV and in those with past infection only, and has resulted in clinically significant liver failure and death, culminating in an alert from the FDA, and testing guidelines and recommendations in HCV/HBV coinfected individuals undergoing DAA therapy.
12. Expert Opinion
HBV reactivation during DAA therapy in individuals with CHC and either HBV coinfection or past HBV infection has been a somewhat unexpected sequelae given the specific mechanism of action of the DAAs against HCV proteins and the lack of known direct immune modulation by these agents. HBV reactivation is a well-known phenomenon that occurs as a result of immune reconstitution during immunosuppressive therapy or during conditions that cause immunosuppression, such as pregnancy.
HBV reactivation can be divided into three main stages, although individuals may not progress through all stages. The first stage is characterized by a rapid increase in HBV replication above baseline, suggesting that prior immune control of HBV has been lost. This phase usually coincides with commencement of immunosuppression. Once the immune suppression is reversed, immune reconstitution follows with restoration of immune function. The last phase is the recovery phase, where a HBV replication and disease activity returns to baseline levels. However, why DAAs would also lead to HBV reactivation is unknown. It is plausible that the mechanism of HBV reactivation during DAA therapy also involves immune reconstitution, but may involve other host and viral factors such as increased replication space for HBV following HCV eradication and direct viral-viral interactions.
It is less likely that HBV reactivation is occurring as a consequence of increased replication space for HBV.
HCV and HBV are both dependent on host machinery in order to carry out replication, although the precise host machinery required for each virus and any overlap are unknown. Although the percentage of hepatocytes infected with either or both viruses varies considerably, most studies report up to 45% and 27% of hepatocytes infected with HCV or HBV, respectively (100, 102, 103). This suggests that there is still a large mass of uninfected hepatocytes that could easily support HCV and HBV infection, and therefore liberation of host machinery following eradication of the percentage of hepatocytes infected with HCV is less likely to cause a meaningful increase in HBV replication.
Another explanation may be reversal of the inhibitory effects of HCV on HBV replication. Early in vitro studies demonstrated that the overexpression of HCV core, NS5a and E2 proteins inhibited HBV replication and gene expression. Therefore, eradication of HCV with DAAs may remove this inhibition and allow HBV to replicate. However, for this scenario to occur, this would likely require the majority of hepatocytes to be infected with both viruses in order to produce sufficient direct inhibition of HBV replication. Furthermore, more recent studies using in vitro replicating virus cell culture models demonstrated that HCV and HBV were able to infect the same hepatocyte without appreciable viral interference as assessed by specific inhibition of each virus. However, in vitro cell culture models, in vivo chimpanzee models and human studies have all demonstrated that the HCV and HBV levels in the setting of HCV/HBV coinfection are lower compared to monoinfection with either virus alone, suggesting there is some viral-viral interaction. Whilst there may not be a direct effect of HCV on HBV replication, there may be other indirect host and/or immune factors that modulate indirect viral-viral interactions.
Expanding on this further, the more likely scenario is that HBV reactivation occurs as a consequence of modulation of host antiviral responses. In contrast to HCV, HBV infection does not induce a strong type I interferon response and chronically elevated ISG expression is not a feature. This hepatic induction of ISGs and other immune effectors such as IL-10 in CHC may create an antiviral milieu that may suppress or control HBV replication. Owing to the superior restoration of HCV-induced innate and adaptive immune dysfunction with DAAs, this immune reconstitution may result in increased host immune surveillance and reduction of suppressive factors towards HBV, leading to an increase in HBV replication. The newly invigorated immune system may allow for improved detection of replicating HBV, which may in turn lead to more potent, proliferative and sustained host innate and importantly adaptive immune responses directed towards HBV, causing significant hepatic damage, resulting in HBV flares with varying severity of liver injury (Figure 1). In other individuals, the host response may lead to spontaneous control of HBV replication. Identifying what factors may drive this is critical to further our understanding of HBV reactivation and recognition of counterproductive host responses that lead to detrimental outcomes is paramount. Evaluation of circulating cytokines/chemokines and T cell responses will no doubt help shed some light on mechanisms and/or biomarkers that are associated with reactivation and severe liver injury that could be followed longitudinally during DAA therapy to predict HBV reactivation. As cell culture models have limitations, including limited genetic phenotype and transformation, and small animal models of HCV and HBV are largely lacking, ex vivo human peripheral samples and/or liver tissue obtained from patients with a spectrum of HBV outcomes during DAA therapy are likely to yield the most interesting results. Furthermore, cross-talk between other cell types may play key roles that would otherwise not be studies in monoculture cell culture models.
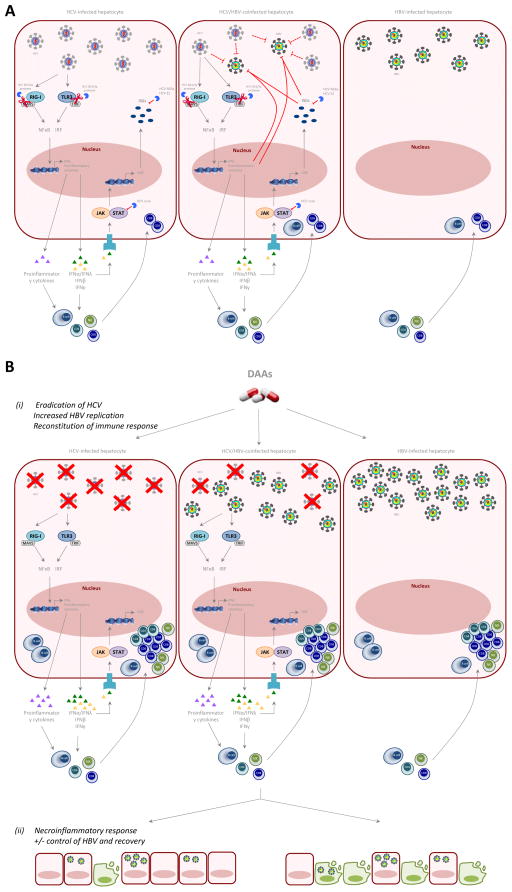
Figure 1.
Proposed mechanism of HBV reactivation during DAA therapy for HCV.
(A) In HCV/HBV hepatocytes there is relative dual inhibition of both HCV and HBV replication possibly through host-viral interactions compared to monoinfected cells. HCV evades host innate immune responses leading to NK cell dysfunction and exhausted ISG phenotype, failure of early CD4 T cell responses and subsequent poor CD8 T cell responses. In HBV monoinfected cells, HBV induces very little innate immune responses, and there is failure of CD4 and CD8 T cell responses.
(B) Following DAA therapy, HCV is rapidly eradicated within 2–4 weeks leading to restoration of innate and adaptive immune responses. ISGs, anti-viral cytokine and anti-inflammatory IL10 upregulation is reversed +/- reversal of direct viral-viral interactions +/− liberation of host machinery, allowing HBV reactivation. The immune restoration causes immune reconstitution and increased host immune surveillance in some individuals leading to HBV flares +/− hepatic failure.
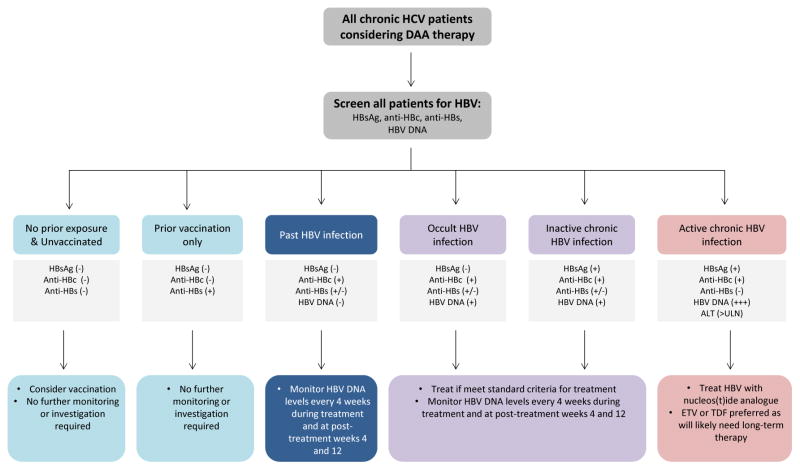
These cases clearly demonstrate that HBV reactivation does occur with potentially devastating consequences during DAA therapy. Figure 2 depicts a suggested algorithm for HBV screening and monitoring in HCV/HBV coinfected individuals considering DAA therapy. Although the true incidence of HBV reactivation during DAA therapy for CHC is not yet known, it appears that this is a relatively uncommon phenomenon and probably does not justify widespread HBV prophylaxis with nucleos(t)ide analogues for all CHC patients with HBV exposure. However, it is critical to perform testing for HBV when considering antiviral DAA therapy in any HCV positive individual. All individuals previously exposed to HBV are at risk, irrespective of the status of their HBV; inactive carriers, occult HBV and past HBV infection are all at risk. Therefore, once HBsAg and/or anti-HBc are detected, these individuals should be monitored carefully at least every 4 weeks for evidence of HBV reactivation. Early recognition of HBV reactivation and timely administration of anti-HBV may mitigate hepatic injury, and reduce morbidity and mortality.
Figure 2.
Proposed algorithm for hepatitis B virus (HBV) assessment and monitoring during direct-acting antiviral therapy for chronic hepatitis C virus (HCV) infection.
ULN = upper limit normal
ETV = entecavir
TDF = tenofovir
Article highlights.
HCV/HBV coinfection is common, particularly in areas where HBV is endemic
Newer direct-acting antiviral agents (DAAs) are extremely effective in eradicating HCV (>95%) but have no activity against HBV, in contrast to IFN-based therapy
In vitro studies and in vivo studies have suggested viral interference between HCV and HBV replication, most likely mediated by host factors, although some studies have demonstrated inhibitory effects of viral proteins on the other virus
DAA therapy has been associated with HBV reactivation in HCV patients infected with HBV and HCV patients with a history of past HBV infection
HBV reactivation during DAA therapy occurred during therapy and was associated with a more severe clinical phenotype, including fulminant liver failure requiring liver transplantation and death
All HCV patients should be assessed for HBV coinfection or HBV past infection, and should undergo regular monitoring of HBV DNA and ALT during DAA therapy
This box summarizes key points contained in the article
Acknowledgments
Funding
This paper has not been funded.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as: * of interest ** of considerable interest
- 1**.(FAERS) TFaDAAERS. [Accessed 18 February 2017];FDA Drug Safety Communication: FDA warns about risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. 2016 Oct; https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.html. Communication from the FDA first reporting risk of HBV reactivation during DAA therapy and recommendation for box warning.
- 2*.Balagopal A, Thio CL. Editorial Commentary: Another Call to Cure Hepatitis B. Clin Infect Dis. 2015;61:1307–1309. doi: 10.1093/cid/civ475. Publication describing a case of HBV reactivation during DAA therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Collins JM, Raphael KL, Terry C, Cartwright EJ, Pillai A, Anania FA, Farley MM. Hepatitis B Virus Reactivation During Successful Treatment of Hepatitis C Virus With Sofosbuvir and Simeprevir. Clin Infect Dis. 2015;61:1304–1306. doi: 10.1093/cid/civ474. Publication describing a case of HBV reactivation during DAA therapy. [DOI] [PubMed] [Google Scholar]
- 4*.De Monte A, Courjon J, Anty R, Cua E, Naqvi A, Mondain V, Cottalorda J, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: Reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27–30. doi: 10.1016/j.jcv.2016.02.026. Publication describing a case of HBV reactivation during DAA therapy. [DOI] [PubMed] [Google Scholar]
- 5*.Ende AR, Kim NH, Yeh MM, Harper J, Landis CS. Fulminant hepatitis B reactivation leading to liver transplantation in a patient with chronic hepatitis C treated with simeprevir and sofosbuvir: a case report. J Med Case Rep. 2015;9:164. doi: 10.1186/s13256-015-0630-8. Publication describing a case of HBV reactivation during DAA therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Wang C, Ji D, Chen J, Shao Q, Li B, Liu J, Wu V, et al. Hepatitis due to Reactivation of Hepatitis B Virus in Endemic Areas Among Patients With Hepatitis C Treated With Direct-acting Antiviral Agents. Clin Gastroenterol Hepatol. 2017;15:132–136. doi: 10.1016/j.cgh.2016.06.023. Publication describing a case of HBV reactivation during DAA therapy. [DOI] [PubMed] [Google Scholar]
- 7*.Takayama H, Sato T, Ikeda F, Fujiki S. Reactivation of hepatitis B virus during interferon-free therapy with daclatasvir and asunaprevir in patient with hepatitis B virus/hepatitis C virus co-infection. Hepatol Res. 2016;46:489–491. doi: 10.1111/hepr.12578. Publication describing a case of HBV reactivation during DAA therapy. [DOI] [PubMed] [Google Scholar]
- 8*.Kimura H, Ohkawa K, Sakakibara M, Imanaka K, Matsunaga T, Miyazaki M, Miyagi T, et al. Sustained hepatitis C virus RNA clearance accompanied by elevation of hepatitis B virus DNA after short-term peginterferon-α, ribavirin and simeprevir therapy in a chronic hepatitis patient having dual infection with hepatitis B and C viruses. Kanzo. 2015;56:422–427. Publication describing a case of HBV reactivation during DAA therapy. [Google Scholar]
- 9.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 10.Ogata N, Alter HJ, Miller RH, Purcell RH. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991;88:3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinhauer DA, Domingo E, Holland JJ. Lack of evidence for proofreading mechanisms associated with an RNA virus polymerase. Gene. 1992;122:281–288. doi: 10.1016/0378-1119(92)90216-c. [DOI] [PubMed] [Google Scholar]
- 12*.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. Recent large study depicting global HCV prevalence and HCV genotype distribution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gidding HF, Topp L, Middleton M, Robinson K, Hellard M, McCaughan G, Maher L, et al. The epidemiology of hepatitis C in Australia: notifications, treatment uptake and liver transplantations, 1997–2006. J Gastroenterol Hepatol. 2009;24:1648–1654. doi: 10.1111/j.1440-1746.2009.05910.x. [DOI] [PubMed] [Google Scholar]
- 14.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen TS. Hepadnaviral X Protein:Review of Recent Progress. J Biomed Sci. 1996;3:20–30. doi: 10.1007/BF02253575. [DOI] [PubMed] [Google Scholar]
- 16*.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. First publication identifying and characterizing the receptor required for HBV entry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newbold JE, Xin H, Tencza M, Sherman G, Dean J, Bowden S, Locarnini S. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J Virol. 1995;69:3350–3357. doi: 10.1128/jvi.69.6.3350-3357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 19.Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55(Suppl 1):S10–15. doi: 10.1093/cid/cis361. [DOI] [PubMed] [Google Scholar]
- 20*.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. Recent systemic review from the Global Burden of Disease Study Group describing the regional prevalences of HBV. [DOI] [PubMed] [Google Scholar]
- 21.Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–774. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- 22.Wasley A, Grytdal S, Gallagher K Centers for Disease C, Prevention. Surveillance for acute viral hepatitis--United States, 2006. MMWR Surveill Summ. 2008;57:1–24. [PubMed] [Google Scholar]
- 23.Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology. 1987;92:1844–1850. doi: 10.1016/0016-5085(87)90614-7. [DOI] [PubMed] [Google Scholar]
- 24*.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. Recent systemic review from the Global Burden of Disease Study Group describing the regional prevalences of HCV. [DOI] [PubMed] [Google Scholar]
- 25.Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 26.Tyson GL, Kramer JR, Duan Z, Davila JA, Richardson PA, El-Serag HB. Prevalence and predictors of hepatitis B virus coinfection in a United States cohort of hepatitis C virus-infected patients. Hepatology. 2013;58:538–545. doi: 10.1002/hep.26400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caccamo G, Saffioti F, Raimondo G. Hepatitis B virus and hepatitis C virus dual infection. World J Gastroenterol. 2014;20:14559–14567. doi: 10.3748/wjg.v20.i40.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mekky MA, Nasr AM, Saleh MA, Wasif NK, Khalaf M, Aboalam H, Haredy M. Virologic and histologic characterisation of dual hepatitis B and C co-infection in Egyptian patients. Arab J Gastroenterol. 2013;14:143–147. doi: 10.1016/j.ajg.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Senturk H, Tahan V, Canbakan B, Uraz S, Ulger Y, Ozaras R, Tabak F, et al. Chronic hepatitis C responds poorly to combination therapy in chronic hepatis B carriers. Neth J Med. 2008;66:191–195. [PubMed] [Google Scholar]
- 30.Yan LB, Rao HY, Ma YJ, Bai L, Chen EQ, Du LY, Yang RF, et al. Hepatitis B virus infection in Chinese patients with hepatitis C virus infection: prevalence, clinical characteristics, viral interactions and host genotypes: a nationwide cross-sectional study. BMJ Open. 2016;6:e012016. doi: 10.1136/bmjopen-2016-012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pramoolsinsap C, Sirikulchayanonta V, Busakorn W, Poovorawan Y, Hirsch P, Theamboonlers A, Lerdverasirikul P. Coinfections with hepatitis g and/or c virus in hepatitis B-related chronic liver disease. Southeast Asian J Trop Med Public Health. 1999;30:741–749. [PubMed] [Google Scholar]
- 32.Xess A, Kumar M, Minz S, Sharma HP, Shahi SK. Prevalence of hepatitis B and hepatitis C virus coinfection in chronic liver disease. Indian J Pathol Microbiol. 2001;44:253–255. [PubMed] [Google Scholar]
- 33.Fong TL, Di Bisceglie AM, Waggoner JG, Banks SM, Hoofnagle JH. The significance of antibody to hepatitis C virus in patients with chronic hepatitis B. Hepatology. 1991;14:64–67. doi: 10.1002/hep.1840140111. [DOI] [PubMed] [Google Scholar]
- 34.Liaw YF, Chu CM, Chang-Chien CS, Wui CS. Simultaneous acute infections with hepatitis non-A, non-B, and B viruses. Dig Dis Sci. 1982;27:762–764. doi: 10.1007/BF01393772. [DOI] [PubMed] [Google Scholar]
- 35.Baginski I, Chemin I, Hantz O, Pichoud C, Jullien AM, Chevre JC, Li JS, et al. Transmission of serologically silent hepatitis B virus along with hepatitis C virus in two cases of posttransfusion hepatitis. Transfusion. 1992;32:215–220. doi: 10.1046/j.1537-2995.1992.32392213803.x. [DOI] [PubMed] [Google Scholar]
- 36.Alberti A, Pontisso P, Chemello L, Fattovich G, Benvegnu L, Belussi F, De Mitri MS. The interaction between hepatitis B virus and hepatitis C virus in acute and chronic liver disease. J Hepatol. 1995;22:38–41. [PubMed] [Google Scholar]
- 37.Mimms LT, Mosley JW, Hollinger FB, Aach RD, Stevens CE, Cunningham M, Vallari DV, et al. Effect of concurrent acute infection with hepatitis C virus on acute hepatitis B virus infection. BMJ. 1993;307:1095–1097. doi: 10.1136/bmj.307.6912.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liaw YF, Chen YC, Sheen IS, Chien RN, Yeh CT, Chu CM. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology. 2004;126:1024–1029. doi: 10.1053/j.gastro.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Liaw YF. Hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. J Gastroenterol. 2002;37(Suppl 13):65–68. doi: 10.1007/BF02990102. [DOI] [PubMed] [Google Scholar]
- 40.Wu JC, Chen CL, Hou MC, Chen TZ, Lee SD, Lo KJ. Multiple viral infection as the most common cause of fulminant and subfulminant viral hepatitis in an area endemic for hepatitis B: application and limitations of the polymerase chain reaction. Hepatology. 1994;19:836–840. [PubMed] [Google Scholar]
- 41.Chu CM, Sheen IS, Liaw YF. The role of hepatitis C virus in fulminant viral hepatitis in an area with endemic hepatitis A and B. Gastroenterology. 1994;107:189–195. doi: 10.1016/0016-5085(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 42.Chu CM, Yeh CT, Liaw YF. Fulminant hepatic failure in acute hepatitis C: increased risk in chronic carriers of hepatitis B virus. Gut. 1999;45:613–617. doi: 10.1136/gut.45.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liaw YF, Yeh CT, Tsai SL. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am J Gastroenterol. 2000;95:2978–2980. doi: 10.1111/j.1572-0241.2000.02337.x. [DOI] [PubMed] [Google Scholar]
- 44.Sagnelli E, Coppola N, Messina V, Di Caprio D, Marrocco C, Marotta A, Onofrio M, et al. HBV superinfection in hepatitis C virus chronic carriers, viral interaction, and clinical course. Hepatology. 2002;36:1285–1291. doi: 10.1053/jhep.2002.36509. [DOI] [PubMed] [Google Scholar]
- 45.Sagnelli E, Pasquale G, Coppola N, Scarano F, Marrocco C, Scolastico C, Santantonio T, et al. Influence of chronic coinfection with hepatitis B and C virus on liver histology. Infection. 2004;32:144–148. doi: 10.1007/s15010-004-3080-6. [DOI] [PubMed] [Google Scholar]
- 46.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22–26. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda R, Ishimura N, Hamamoto S, Moritani M, Uchida Y, Ishihara S, Akagi S, et al. Co-infection by serologically-silent hepatitis B virus may contribute to poor interferon response in patients with chronic hepatitis C by down-regulation of type-I interferon receptor gene expression in the liver. J Med Virol. 2001;63:220–227. doi: 10.1002/1096-9071(200103)63:3<220::aid-jmv1004>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Paterlini P, Driss F, Nalpas B, Pisi E, Franco D, Berthelot P, Brechot C. Persistence of hepatitis B and hepatitis C viral genomes in primary liver cancers from HBsAg-negative patients: a study of a low-endemic area. Hepatology. 1993;17:20–29. [PubMed] [Google Scholar]
- 49*.De Maria N, Colantoni A, Friedlander L, Leandro G, Idilman R, Harig J, Van Thiel DH. The impact of previous HBV infection on the course of chronic hepatitis C. Am J Gastroenterol. 2000;95:3529–3536. doi: 10.1111/j.1572-0241.2000.03371.x. Publication describing the impact of HCV/HBV coinfection of the natural history of HCV. [DOI] [PubMed] [Google Scholar]
- 50.Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102–110. doi: 10.1053/j.gastro.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 51**.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. Review article summarizing innate immune response to viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foy E, Li K, Sumpter R, Jr, Loo YM, Johnson CL, Wang C, Fish PM, et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci U S A. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bode JG, Ludwig S, Ehrhardt C, Albrecht U, Erhardt A, Schaper F, Heinrich PC, et al. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003;17:488–490. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- 55.Heim MH, Moradpour D, Blum HE. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J Virol. 1999;73:8469–8475. doi: 10.1128/jvi.73.10.8469-8475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor DR, Shi ST, Romano PR, Barber GN, Lai MM. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez-Anon M, Cervera-Deval J, Garcia-Vila JH, Bordon-Ferre F, Ambit-Capdevila S, Piqueras-Olmeda R, Jornet-Fayos J, et al. Characterization of solid liver lesions with color and pulsed Doppler imaging. Abdom Imaging. 1999;24:137–143. doi: 10.1007/s002619900462. [DOI] [PubMed] [Google Scholar]
- 58**.Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi: 10.1038/nm.3251. Review article summarizing innate and adaptive immune response to viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, McHutchison JG, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 60*.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. Review article summarizing innate and adaptive immune response to HCV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Penna A, Del Prete G, Cavalli A, Bertoletti A, D’Elios MM, Sorrentino R, D’Amato M, et al. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B. Hepatology. 1997;25:1022–1027. doi: 10.1002/hep.510250438. [DOI] [PubMed] [Google Scholar]
- 62.Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, Chisari FV. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol. 2009;83:9652–9662. doi: 10.1128/JVI.00867-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Folgori A, Spada E, Pezzanera M, Ruggeri L, Mele A, Garbuglia AR, Perrone MP, et al. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–1019. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med. 2005;201:1709–1714. doi: 10.1084/jem.20050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarobe P, Lasarte JJ, Casares N, Lopez-Diaz de Cerio A, Baixeras E, Labarga P, Garcia N, et al. Abnormal priming of CD4(+) T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J Virol. 2002;76:5062–5070. doi: 10.1128/JVI.76.10.5062-5070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509–523. doi: 10.1038/nri.2016.69. Review article summarizing immune responses to viral infection in the acute and chronic phases. [DOI] [PubMed] [Google Scholar]
- 70.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang G, Liu A, Xie Q, Guo TB, Wan B, Zhou B, Zhang JZ. Association of CD4+CD25+Foxp3+ regulatory T cells with chronic activity and viral clearance in patients with hepatitis B. Int Immunol. 2007;19:133–140. doi: 10.1093/intimm/dxl130. [DOI] [PubMed] [Google Scholar]
- 72.Bolacchi F, Sinistro A, Ciaprini C, Demin F, Capozzi M, Carducci FC, Drapeau CM, et al. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188–196. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farci P, Alter HJ, Govindarajan S, Wong DC, Engle R, Lesniewski RR, Mushahwar IK, et al. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;258:135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- 74.Neumann-Haefelin C, Timm J, Spangenberg HC, Wischniowski N, Nazarova N, Kersting N, Roggendorf M, et al. Virological and immunological determinants of intrahepatic virus-specific CD8+ T-cell failure in chronic hepatitis C virus infection. Hepatology. 2008;47:1824–1836. doi: 10.1002/hep.22242. [DOI] [PubMed] [Google Scholar]
- 75.Wahid A, Dubuisson J. Virus-neutralizing antibodies to hepatitis C virus. J Viral Hepat. 2013;20:369–376. doi: 10.1111/jvh.12094. [DOI] [PubMed] [Google Scholar]
- 76.Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, Lascar RM, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 77.Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 2010;58:258–266. doi: 10.1016/j.patbio.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78*.Hosel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, Tedjokusumo R, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. Publication demonstrating that innate immune responses are weak in HBV. [DOI] [PubMed] [Google Scholar]
- 79.Kumar M, Jung SY, Hodgson AJ, Madden CR, Qin J, Slagle BL. Hepatitis B virus regulatory HBx protein binds to adaptor protein IPS-1 and inhibits the activation of beta interferon. J Virol. 2011;85:987–995. doi: 10.1128/JVI.01825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 81.Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R, Rodgers S, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45:102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 82.Thompson AJ, Colledge D, Rodgers S, Wilson R, Revill P, Desmond P, Mansell A, et al. Stimulation of the interleukin-1 receptor and Toll-like receptor 2 inhibits hepatitis B virus replication in hepatoma cell lines in vitro. Antivir Ther. 2009;14:797–808. doi: 10.3851/IMP1294. [DOI] [PubMed] [Google Scholar]
- 83.Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gane EJ, Lim YS, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, Roberts S, et al. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol. 2015;63:320–328. doi: 10.1016/j.jhep.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 85.Peppa D, Micco L, Javaid A, Kennedy PT, Schurich A, Dunn C, Pallant C, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6:e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 87*.Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol. 2010;184:287–295. doi: 10.4049/jimmunol.0902761. Publication demonstrating the importance of T cell function in HBV replication. [DOI] [PubMed] [Google Scholar]
- 88.Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51:581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 89*.Schuttler CG, Fiedler N, Schmidt K, Repp R, Gerlich WH, Schaefer S. Suppression of hepatitis B virus enhancer 1 and 2 by hepatitis C virus core protein. J Hepatol. 2002;37:855–862. doi: 10.1016/s0168-8278(02)00296-9. Publication reporting direct interference by HCV proteins on HBV replication. [DOI] [PubMed] [Google Scholar]
- 90.Alberti A, Diana S, Sculard GH, Eddleston AL, Williams R. Detection of a new antibody system reacting with Dane particles in hepatitis B virus infection. Br Med J. 1978;2:1056–1058. doi: 10.1136/bmj.2.6144.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grady GF, Lee VA, Prince AM, Gitnick GL, Fawaz KA, Vyas GN, Levitt MD, et al. Hepatitis B immune globulin for accidental exposures among medical personnel: final report of a multicenter controlled trial. J Infect Dis. 1978;138:625–638. doi: 10.1093/infdis/138.5.625. [DOI] [PubMed] [Google Scholar]
- 92.Glebe D, Lorenz H, Gerlich WH, Butler SD, Tochkov IA, Tennant BC, Cote P, et al. Correlation of virus and host response markers with circulating immune complexes during acute and chronic woodchuck hepatitis virus infection. J Virol. 2009;83:1579–1591. doi: 10.1128/JVI.01934-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mason W, Litwin S. Pathogenesis of Hepadnavirus Infections. In: Lai C, Locarnini S, editors. Hepatitis B Virus. London, UK: International Medical Press; 2002. pp. 99–114. [Google Scholar]
- 94.Xu X, Shang Q, Chen X, Nie W, Zou Z, Huang A, Meng M, et al. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol Immunol. 2015;12:309–316. doi: 10.1038/cmi.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shih CM, Lo SJ, Miyamura T, Chen SY, Lee YH. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J Virol. 1993;67:5823–5832. doi: 10.1128/jvi.67.10.5823-5832.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shih CM, Chen CM, Chen SY, Lee YH. Modulation of the trans-suppression activity of hepatitis C virus core protein by phosphorylation. J Virol. 1995;69:1160–1171. doi: 10.1128/jvi.69.2.1160-1171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97*.Chen SY, Kao CF, Chen CM, Shih CM, Hsu MJ, Chao CH, Wang SH, et al. Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein. J Biol Chem. 2003;278:591–607. doi: 10.1074/jbc.M204241200. Publication reporting direct interference by HCV proteins on HBV replication. [DOI] [PubMed] [Google Scholar]
- 98.Pan Y, Wei W, Kang L, Wang Z, Fang J, Zhu Y, Wu J. NS5A protein of HCV enhances HBV replication and resistance to interferon response. Biochem Biophys Res Commun. 2007;359:70–75. doi: 10.1016/j.bbrc.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 99.Guo H, Zhou T, Jiang D, Cuconati A, Xiao GH, Block TM, Guo JT. Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-akt signal transduction pathway. J Virol. 2007;81:10072–10080. doi: 10.1128/JVI.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kandathil AJ, Graw F, Quinn J, Hwang HS, Torbenson M, Perelson AS, Ray SC, et al. Use of laser capture microdissection to map hepatitis C virus-positive hepatocytes in human liver. Gastroenterology. 2013;145:1404–1413. e1401–1410. doi: 10.1053/j.gastro.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agnello V, Abel G, Knight GB, Muchmore E. Detection of widespread hepatocyte infection in chronic hepatitis C. Hepatology. 1998;28:573–584. doi: 10.1002/hep.510280240. [DOI] [PubMed] [Google Scholar]
- 102.Rodriguez-Inigo E, Mariscal L, Bartolome J, Castillo I, Navacerrada C, Ortiz-Movilla N, Pardo M, et al. Distribution of hepatitis B virus in the liver of chronic hepatitis C patients with occult hepatitis B virus infection. J Med Virol. 2003;70:571–580. doi: 10.1002/jmv.10432. [DOI] [PubMed] [Google Scholar]
- 103.Cheng PN, Liu WC, Tsai HW, Wu IC, Chang TT, Young KC. Association of intrahepatic cccDNA reduction with the improvement of liver histology in chronic hepatitis B patients receiving oral antiviral agents. J Med Virol. 2011;83:602–607. doi: 10.1002/jmv.22014. [DOI] [PubMed] [Google Scholar]
- 104.Eyre NS, Phillips RJ, Bowden S, Yip E, Dewar B, Locarnini SA, Beard MR. Hepatitis B virus and hepatitis C virus interaction in Huh-7 cells. J Hepatol. 2009;51:446–457. doi: 10.1016/j.jhep.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 105**.Bellecave P, Gouttenoire J, Gajer M, Brass V, Koutsoudakis G, Blum HE, Bartenschlager R, et al. Hepatitis B and C virus coinfection: a novel model system reveals the absence of direct viral interference. Hepatology. 2009;50:46–55. doi: 10.1002/hep.22951. Publication reporting the HCV and HBV can simultaneously coinfect the same hepatocyte without direct viral interference. [DOI] [PubMed] [Google Scholar]
- 106.Rodriguez-Inigo E, Bartolome J, Ortiz-Movilla N, Platero C, Lopez-Alcorocho JM, Pardo M, Castillo I, et al. Hepatitis C virus (HCV) and hepatitis B virus (HBV) can coinfect the same hepatocyte in the liver of patients with chronic HCV and occult HBV infection. J Virol. 2005;79:15578–15581. doi: 10.1128/JVI.79.24.15578-15581.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107**.Wieland SF, Asabe S, Engle RE, Purcell RH, Chisari FV. Limited hepatitis B virus replication space in the chronically hepatitis C virus-infected liver. J Virol. 2014;88:5184–5188. doi: 10.1128/JVI.03553-13. Publication suggesting limited replication space in a chimpanzee model of HCV/HBV coinfection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brotman B, Prince AM, Huima T, Richardson L, van den Ende MC, Pfeifer U. Interference between non-A, non-B and hepatitis B virus infection in chimpanzees. J Med Virol. 1983;11:191–205. doi: 10.1002/jmv.1890110303. [DOI] [PubMed] [Google Scholar]
- 109.Bradley DW, Maynard JE, McCaustland KA, Murphy BL, Cook EH, Ebert JW. Non-A, non-B hepatitis in chimpanzees: interference with acute hepatitis A virus and chronic hepatitis B virus infections. J Med Virol. 1983;11:207–213. doi: 10.1002/jmv.1890110304. [DOI] [PubMed] [Google Scholar]
- 110.Liaw YF, Lin SM, Sheen IS, Chu CM. Acute hepatitis C virus superinfection followed by spontaneous HBeAg seroconversion and HBsAg elimination. Infection. 1991;19:250–251. doi: 10.1007/BF01644957. [DOI] [PubMed] [Google Scholar]
- 111.Fattovich G, Tagger A, Brollo L, Giustina G, Pontisso P, Realdi G, Alberti A, et al. Hepatitis C virus infection in chronic hepatitis B virus carriers. J Infect Dis. 1991;163:400–402. doi: 10.1093/infdis/163.2.400. [DOI] [PubMed] [Google Scholar]
- 112.Sato S, Fujiyama S, Tanaka M, Yamasaki K, Kuramoto I, Kawano S, Sato T, et al. Coinfection of hepatitis C virus in patients with chronic hepatitis B infection. J Hepatol. 1994;21:159–166. doi: 10.1016/s0168-8278(05)80389-7. [DOI] [PubMed] [Google Scholar]
- 113.Sheen IS, Liaw YF, Chu CM, Pao CC. Role of hepatitis C virus infection in spontaneous hepatitis B surface antigen clearance during chronic hepatitis B virus infection. J Infect Dis. 1992;165:831–834. doi: 10.1093/infdis/165.5.831. [DOI] [PubMed] [Google Scholar]
- 114.Chu CM, Yeh CT, Liaw YF. Low-level viremia and intracellular expression of hepatitis B surface antigen (HBsAg) in HBsAg carriers with concurrent hepatitis C virus infection. J Clin Microbiol. 1998;36:2084–2086. doi: 10.1128/jcm.36.7.2084-2086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jardi R, Rodriguez F, Buti M, Costa X, Cotrina M, Galimany R, Esteban R, et al. Role of hepatitis B, C, and D viruses in dual and triple infection: influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology. 2001;34:404–410. doi: 10.1053/jhep.2001.26511. [DOI] [PubMed] [Google Scholar]
- 116**.Chen F, Zhang J, Wen B, Luo S, Lin Y, Ou W, Guo F, et al. HBV/HCV dual infection impacts viral load, antibody response, and cytokine expression differently from HBV or HCV single infection. Sci Rep. 2016;6:39409. doi: 10.1038/srep39409. Publication describing viral, antibody and cytokine responses during dual HCV/HBV coinfection compared to monoinfection with either virus alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ohkawa K, Hayashi N, Yuki N, Hagiwara H, Kato M, Yamamoto K, Eguchi H, et al. Hepatitis C virus antibody and hepatitis C virus replication in chronic hepatitis B patients. J Hepatol. 1994;21:509–514. doi: 10.1016/s0168-8278(94)80094-4. [DOI] [PubMed] [Google Scholar]
- 118.Ohkawa K, Hayashi N, Yuki N, Masuzawa M, Kato M, Yamamoto K, Hosotsubo H, et al. Long-term follow-up of hepatitis B virus and hepatitis C virus replicative levels in chronic hepatitis patients coinfected with both viruses. J Med Virol. 1995;46:258–264. doi: 10.1002/jmv.1890460316. [DOI] [PubMed] [Google Scholar]
- 119.Crespo J, Lozano JL, de la Cruz F, Rodrigo L, Rodriguez M, San Miguel G, Artinano E, et al. Prevalence and significance of hepatitis C viremia in chronic active hepatitis B. Am J Gastroenterol. 1994;89:1147–1151. [PubMed] [Google Scholar]
- 120.Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, Tran van Nhieu J, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 121.Yu G, Chi X, Wu R, Wang X, Gao X, Kong F, Feng X, et al. Replication Inhibition of Hepatitis B Virus and Hepatitis C Virus in Co-Infected Patients in Chinese Population. PLoS One. 2015;10:e0139015. doi: 10.1371/journal.pone.0139015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pontisso P, Gerotto M, Ruvoletto MG, Fattovich G, Chemello L, Tisminetzky S, Baralle F, et al. Hepatitis C genotypes in patients with dual hepatitis B and C virus infection. J Med Virol. 1996;48:157–160. doi: 10.1002/(SICI)1096-9071(199602)48:2<157::AID-JMV7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 123**.Raimondo G, Brunetto MR, Pontisso P, Smedile A, Maina AM, Saitta C, Squadrito G, et al. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology. 2006;43:100–107. doi: 10.1002/hep.20944. Important publication that demonstrates that there are wide fluctuations in viral dominance and viral loads during longitudinal monitoring of HCV/HBV coinfected individuals. [DOI] [PubMed] [Google Scholar]
- 124.Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347–354. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 125.Agrawal A, Samrat SK, Agrawal B, Tyrrell DL, Kumar R. Co-incubation with core proteins of HBV and HCV leads to modulation of human dendritic cells. Viral Immunol. 2014;27:412–417. doi: 10.1089/vim.2014.0056. [DOI] [PubMed] [Google Scholar]
- 126.Chu CM, Sheen IS, Liaw YF. Low-level hepatitis C viremia and humoral immune response to NS4 in chronic hepatitis B virus-hepatitis C virus coinfection. Scand J Gastroenterol. 2004;39:778–782. doi: 10.1080/00365520410006332. [DOI] [PubMed] [Google Scholar]
- 127.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25(Suppl 1):3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 128.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Delmas J, Schorr O, Jamard C, Gibbs C, Trepo C, Hantz O, Zoulim F. Inhibitory effect of adefovir on viral DNA synthesis and covalently closed circular DNA formation in duck hepatitis B virus-infected hepatocytes in vivo and in vitro. Antimicrob Agents Chemother. 2002;46:425–433. doi: 10.1128/AAC.46.2.425-433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 131.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 132.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 133.Holmes JA, Desmond PV, Thompson AJ. Interferon-free combination therapies for the treatment of hepatitis C: current insights. Hepatic Medicine: Evidence and Research. 2015 doi: 10.2147/HMER.S55864. Accepted for publication 23rd March 2015, DOI pending. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rijckborst V, Janssen HL. The Role of Interferon in Hepatitis B Therapy. Curr Hepat Rep. 2010;9:231–238. doi: 10.1007/s11901-010-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology. 2015;61:712–721. doi: 10.1002/hep.27323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, Porcella S, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest. 2014;124:3352–3363. doi: 10.1172/JCI75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137**.Meissner EG, Kohli A, Virtaneva K, Sturdevant D, Martens C, Porcella SF, McHutchison JG, et al. Achieving sustained virologic response after interferon-free hepatitis C virus treatment correlates with hepatic interferon gene expression changes independent of cirrhosis. J Viral Hepat. 2016;23:496–505. doi: 10.1111/jvh.12510. Important publication demonstrating restoration of type I interferon responses following DAA therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138**.Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, Ghany M, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015;149:190–200. e192. doi: 10.1053/j.gastro.2015.03.004. Important publication demonstrating restoration of NK function following DAA therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HL, de Knegt RJ, et al. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J Infect Dis. 2016;213:216–223. doi: 10.1093/infdis/jiv391. [DOI] [PubMed] [Google Scholar]
- 140.Burchill MA, Golden-Mason L, Wind-Rotolo M, Rosen HR. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat. 2015;22:983–991. doi: 10.1111/jvh.12465. [DOI] [PubMed] [Google Scholar]
- 141**.Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, Bocher WO, et al. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol. 2014;61:538–543. doi: 10.1016/j.jhep.2014.05.043. Important publication demonstrating restoration of NK function following DAA therapy. [DOI] [PubMed] [Google Scholar]
- 142*.Ou P, Fang Z, Chen J. Hepatitis B reactivation in a chronic hepatitis C patient treated with ledipasvir and sofosbuvir: A case report. Clin Res Hepatol Gastroenterol. 2016 doi: 10.1016/j.clinre.2016.08.001. Publication describing a case of HBV reactivation during DAA therapy. [DOI] [PubMed] [Google Scholar]
- 143.Liu JY, Sheng YJ, Hu HD, Zhong Q, Wang J, Tong SW, Zhou Z, et al. The influence of hepatitis B virus on antiviral treatment with interferon and ribavirin in Asian patients with hepatitis C virus/hepatitis B virus coinfection: a meta-analysis. Virol J. 2012;9:186. doi: 10.1186/1743-422X-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144**.Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, et al. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: A systematic review and meta-analysis. Hepatology. 2017 doi: 10.1002/hep.29109. Important meta-analysis comparing HBV reactivation during interferon+ribavirin therapy and DAA therapy showing differences in patterns of HBV reactivation but not prevalence. [DOI] [PubMed] [Google Scholar]
- 145*.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–188. doi: 10.1016/0016-5085(91)90599-g. Prospective study characterizing the risk of HBV reactivation during immune suppressive therapy for cancer. [DOI] [PubMed] [Google Scholar]
- 146*.Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, Hui P, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::aid-jmv1>3.0.co;2-0. Prospective study characterizing the risk of HBV reactivation during immune suppressive therapy for cancer. [DOI] [PubMed] [Google Scholar]
- 147.Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009–1022. doi: 10.1053/gast.2001.22461. [DOI] [PubMed] [Google Scholar]
- 148.Vento S, Cainelli F, Longhi MS. Reactivation of replication of hepatitis B and C viruses after immunosuppressive therapy: an unresolved issue. Lancet Oncol. 2002;3:333–340. doi: 10.1016/s1470-2045(02)00773-8. [DOI] [PubMed] [Google Scholar]
- 149.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156–165. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 150.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151*.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT American Gastroenterological Association I. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–219. doi: 10.1053/j.gastro.2014.10.039. quiz e216–217. Publication reporting guidelines for the management of HBV reactivation during immunosuppressive therapy. [DOI] [PubMed] [Google Scholar]
- 152.Paul S, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis. Ann Intern Med. 2016;164:30–40. doi: 10.7326/M15-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, Liu CY, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765–2772. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 154.Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, Kao WY, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320–1328. doi: 10.1053/jhep.2003.50220. [DOI] [PubMed] [Google Scholar]
- 155.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, Cho SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43:233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 156*.Perez-Alvarez R, Diaz-Lagares C, Garcia-Hernandez F, Lopez-Roses L, Brito-Zeron P, Perez-de-Lis M, Retamozo S, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011;90:359–371. doi: 10.1097/MD.0b013e3182380a76. Study characterizing the risk of HBV reactivation during anti-TNF therapy in 257 cases reported in the literature. [DOI] [PubMed] [Google Scholar]
- 157*.Koutsianas C, Thomas K, Vassilopoulos D. Hepatitis B Reactivation in Rheumatic Diseases: Screening and Prevention. Rheum Dis Clin North Am. 2017;43:133–149. doi: 10.1016/j.rdc.2016.09.012. Study summarizing the risk of HBV reactivation during immunsuppressive regimens in Rheumatic DIsease. [DOI] [PubMed] [Google Scholar]
- 158.Bessone F, Dirchwolf M. Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations. World J Hepatol. 2016;8:385–394. doi: 10.4254/wjh.v8.i8.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 160*.Giles M, Visvanathan K, Lewin S, Bowden S, Locarnini S, Spelman T, Sasadeusz J. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut. 2015;64:1810–1815. doi: 10.1136/gutjnl-2014-308211. Study characterizing clinical and virological predictors of HBV flares in pregnancy. [DOI] [PubMed] [Google Scholar]