Abstract
Objectives
The aim of the study was to assess the extent to which misoprostol alters mucosal or systemic immune responses following either buccal or vaginal administration.
Methods
This was a prospective, crossover pilot study of 15 healthy, reproductive age women. Women first received 800 μg misoprostol either via buccal or vaginal administration and were crossed over 1 month later to receive the drug via the other route. Cervicovaginal lavage samples, cervical Cytobrush samples, cervicovaginal swabs, urine and blood were obtained immediately prior to drug administration and the following day. Parameters assessed included urine and cervicovaginal misoprostol levels, whole blood cytokine responses (by ELISA) to immune stimulation with lipopolysaccharide, peripheral blood and cervical lymphocyte phenotyping by flow cytometry, cervicovaginal antimicrobial peptide measurement by ELISA, and vaginal microbial ecology assessment by 16S rRNA sequencing.
Results
Neither buccal nor vaginal misoprostol significantly altered local or systemic immune and microbiological parameters.
Conclusion
In this pilot study, we did not observe significant alteration of mucosal or systemic immunology or vaginal microbial ecology 1 day after drug administration following either the buccal or vaginal route.
Keywords: Abortion, Microbial ecology, Mucosal immunology, Prostaglandins
Introduction
Misoprostol is a synthetic prostaglandin (PG) E1 analogue used clinically to prevent gastric ulcerations in patients taking non-steroidal anti-inflammatory drugs [1]. It is also used to prevent postpartum haemorrhage [2], to induce labour in term pregnancies [3], and in the medical termination of pregnancy [4]. This agent binds to and activates each of the four transmembrane G protein-coupled E prostanoid (EP) receptors normally ligated by the endogenous lipid mediator PGE2.
Misoprostol has immunomodulatory effects [5–11] and, like PGE2, modulates innate and adaptive immune responses via its ability to increase intracellular levels of cAMP upon ligation of the EP2 and EP4 receptors [11–14]. It is generally administered orally (i.e. swallowed), buccally (i.e. allowed to absorb directly into the bloodstream through the mucosa of the cheek) or vaginally. The vaginal application of misoprostol to terminate pregnancy has garnered controversy because of concerns that the drug increases the susceptibility of women to infections of the reproductive tract following its use [15,16]. This has raised questions about whether single-dose misoprostol impairs innate and adaptive immunity and/or whether the route of administration of the drug is important in that regard. Because intravaginal misoprostol continues to be used for a number of indications, it is important to resolve controversies over putative effects on mucosal or systemic immunity.
We conducted a pilot study to define the extent to which a single buccal or vaginal dose of misoprostol administered to healthy, reproductive age women modulates cellular and mucosal immunity within the lower female reproductive tract, define the extent to which a single buccal or vaginal dose of misoprostol modulates systemic immune responses, and measure systemic and local misoprostol concentrations following a single buccal or vaginal dose of misoprostol.
Methods
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by Vanderbilt University Institutional Review Board (protocol number 140667); all participants provided written informed consent. The trial was registered at ClinicalTrials.gov (NCT02259309).
Recruitment
Inclusion and exclusion criteria are listed in Supplementary Table 1. Participants were recruited via Research Match, a national registry of individuals interested in volunteering for clinical research studies. Eighty-nine women responded to outreach efforts, and 72 of these were excluded. The primary reasons for exclusion were: not responding to contacts after study information was sent (n=28), being on hormonal contraception (n=22), and meeting medical exclusion criteria (n=8).
Enrolment
Seventeen women consented to participate in the study. Two individuals became ineligible during the screening period after consent was obtained. Fifteen women proceeded through screening and ultimately received initial study drug dosing. Of these, six identified as African American, seven as white, and two as Asian.
Study procedures
Participants were assigned to receive buccal or vaginal dosing of misoprostol on day 0 of the study and then scheduled to receive the other route of dosing at a second time point of the study (day 28). To reduce the likelihood of biological variability due to differences in menstrual cycle timing, each dosing was intended to be during the mid-luteal phase, determined by the results of ovulation testing kits provided to each participant. Therefore, the second dosing time point ranged from day 20 to day 36 after initial dosing depending on the date of ovulation of each participant. Buccal doses were retained for 30 min adjacent to the inner cheek within the oral cavity, with the participant swallowing remaining fragments after that time. Vaginal doses were inserted by a clinician into the vaginal fornices. Prior to and 1 day after each drug administration, peripheral blood, vaginal swab (Catch-All Sample Collection Swab; Epicentre, Madison, WI, USA), cervical Cytobrush and cervicovaginal lavage (CVL) samples were collected. Additionally, urine samples for misoprostol analysis were collected prior to the initial dose and 1 day after each dose.
Three participants did not receive the second dose of drug as scheduled. Two women started their menstrual cycle earlier than expected for them, so the mid-luteal phase was not able to be identified. One woman did not complete ovulation testing at home as scheduled. Each participant was contacted approximately 7 days after the final dose of misoprostol to assess any changes in medical conditions and to answer any questions.
Nugent scoring
Laboratory examination of vaginal smears and determination of the Nugent score [17] were assessed in a blinded fashion by one trained observer (LMR) using an Olympus BH-2 microscope (city, state, country). Results were verified by another observer (DMA).
Quantification of misoprostol in CVL and urine
CVL was analysed by liquid chromatography and tandem mass spectrometry using [2H4]misoprostol (Cayman Chemicals, Ann Arbor, MI, USA) as an internal standard. Urine (0.5 ml) was acidified to pH 3 with 1N HCl, and 10 ng of the internal standard [2H4]misoprostol (Cayman Chemicals) was added. Endogenous misoprostol and its deuterated standard were converted to the O-methyloxime derivative by treatment with 0.5 ml 16% (w/v) methyloxime HCl in 1.5 M sodium acetate buffer (pH 5). The urinary misoprostol level in each sample was normalised using the urinary creatinine level of the sample and was expressed in ng/mg creatinine.
CVL antimicrobial peptides
We measured levels of trappin-2 (also known as elafin) because it is an abundant antimicrobial peptide in cervicovaginal secretions and is implicated in the host defence against infection [18,19]. CVL fluid was collected and stored at −80°C with a protease inhibitor (cOmplete Mini; Roche, Indianapolis, IN, USA). CVL was assayed for the presence of trappin-2 using a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA) and following the manufacturer’s instructions.
Whole blood stimulation with endotoxin
Heparinised peripheral blood was collected from each patient and used in an ex vivo stimulation assay with lipopolysaccharide (LPS): 1 ml whole blood was stimulated for 4 h with or without 1000 ng/ml LPS. After stimulation, whole blood was centrifuged and plasma was collected for measurement of tumour necrosis factor (TNF)-α and interleukin (IL)-10 using commercially available ELISA kits (R&D Systems).
Preparation of cells for flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood after separation by Ficoll–Paque PLUS)(GE Healthcare, Piscataway, NJ, USA) density gradient. An aliquot of 1×106 cells was reserved for immediate flow cytometric staining and the rest was cryopreserved in fetal bovine serum with 10% dimethyl sulfoxide. Cervical Cytobrush samples were incubated with 2 ml 5 mM DL-dithiothreitol at 37°C for 15 min, washed with phosphate-buffered saline and enumerated. Fresh cervical cell samples and matched-subject PBMCs were stained with an amine-reactive viability dye (Aqua Live/Dead; Life Technologies, Carlsbad, CA, USA) for 10 min (22°C) followed by staining with a flow cytometry panel to assess immune activation: CD14-V500 (M5E2, BD), CD19-V500 (HIB19, BD), CD3-BV711 (UCHT1, BD), CD4-PcPCy5.5 (RPA-T4, BD), CD8-APCA750 (3B5; Life Technologies), CD57-FITC (NK-1, BD), PD-1-PE (EH12.2H7, BD), CD45RO-PETxR (UCHL1; Beckman Coulter, Brea, CA, USA), CD69-APC (FN50, BD), HLA-DR-V450 (G46-6, BD) and CD38-PECy7 (HIT2, BD). CD38-PECy7 and CD69-APC FMO controls were included to aid analysis and gating. Samples were analysed on a Fortessa (BD) flow cytometer (manufacturer, city, state, country) in the Vanderbilt Medical Center Flow Cytometry Shared Resource. The PD-1-PE and CD38-PECy7 channels were standardised with mid-range rainbow beads (BD) and compensation was set using single colour stained cells. Data analysis was performed using FACSDiva software (BD).
To assess the longitudinal effect of misoprostol administration, cryopreserved PBMCs from each subject at each of the four time points were thawed and stained with the identical panel described above, and analysed within the same flow cytometric run.
Microbial ecology
DNA was extracted from swabs by aseptically excising the swab material; bacterial DNA was directly extracted from swab material using the MoBio PowerSoil kit following manufacturer’s guidance (MoBio, Bio Laboratories, Carlsbad, CA, USA). Amplicon pyrosequencing (bTEFAP;) was originally described by Dowd et al. [20,21] and has been used to describe a wide range of environmental and health-related microbiomes [20–23]. The 16S universal eubacterial primers 341F CCTACGGGNGGCWGCAG and 785R GACTACHVGGGTATCTAATCC were used to evaluate the microbial ecology of swabs on the Illumina MiSeq (San Diego, CA, USA), with methods based on the bTEFAP. Data were processed using an analysis pipeline (MR DNA, Shallowater, TX, USA). Operational taxonomic units (OTUs) were defined by clustering at 3% divergence (97% similarity) and after removal of singleton sequences, and taxonomically classified using BLASTN against a curated RDPII/NCBI derived database [24]. Statistical analysis was performed using XLstat, NCSS 2007, ‘R’ and NCSS 2010. Alpha and beta diversity analysis was conducted as described previously [20,21,25–27] using Qiime (www.qiime.org). Significance reported for any analysis is defined as p<0.05.
Results
Adverse events
Adverse events were reported at clinic visits 1 day after each dosing (Supplementary Table 2). At the 7 day follow-up after the final dose of misoprostol, four participants reported changes in their menstrual cycle including heavier flow than normal, intermittent spotting, lighter flow than normal, and early menses.
Levels of misoprostol in blood and urine after administration
Drug levels in CVL fluid were detectable 1 day after vaginal administration (Figure 1A), but were undetectable after buccal administration. Systemic levels of misoprostol 1 day after exposure to the drug were negligible (Figure 1B).
Figure 1.
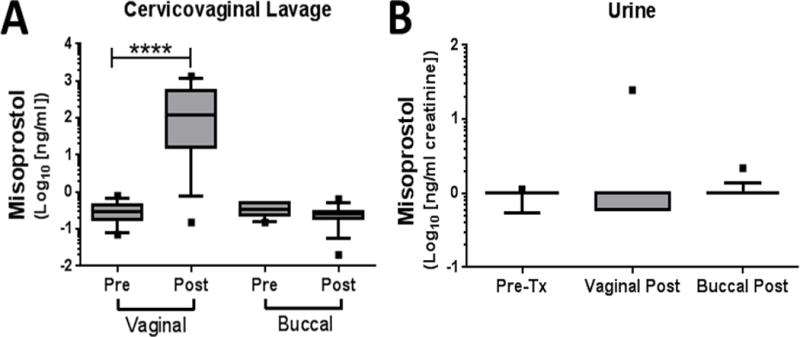
Local and systemic misoprostol concentrations before and after a single buccal or vaginal dose. Mass spectrometry was used to measure misoprostol in both (A) CVL and (B) urine before (pre) and 24 h after (post) administration. Data are shown as box-and-whisker plots (the line represents the median, the boxes represent the 25th–75th percentiles, the whiskers represent the 10th–90th percentiles, and the <10th and >90th percentile outliers are shown as dots). ****p<0.0001 by Mann–Whitney non-parametric testing.
Effects of misoprostol on whole blood response to endotoxin
To determine the extent to which buccal or vaginal single-dose misoprostol exposure alters systemic inflammatory responses to inflammatory stimuli, whole blood samples were stimulated with LPS. Pro- and anti-inflammatory responses were estimated by measuring the production of TNF-α and IL-10, respectively (Figure 2A, B). As illustrated, neither buccal nor vaginal misoprostol altered whole blood immune responses to LPS 24 h after administration.
Figure 2.
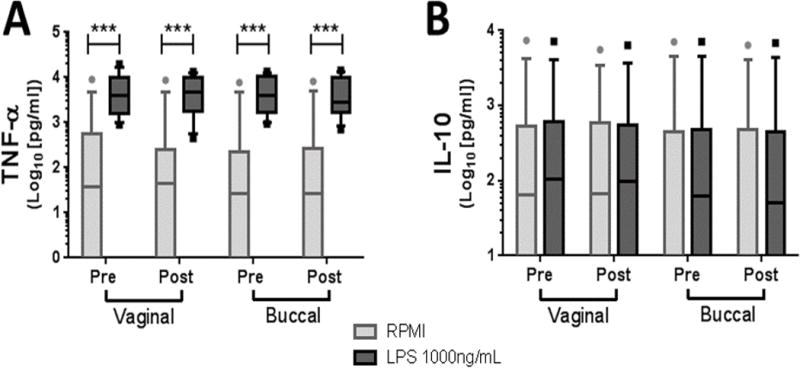
Misoprostol does not alter whole blood responses to LPS. Whole blood was stimulated for 4 h with LPS 1000 ng/ml, and extracellular TNF-α (A) and IL-10 (B) were measured by ELISA. ***p<0.001 by paired, non-parametric Wilcoxon matched pairs test between vehicle (RPMI medium) and LPS for each treatment route.
Effects of misoprostol on CVL antimicrobial peptide elafin/trappin-2
Levels of trappin-2 were assayed in the CVL as described in the Methods section. Misoprostol treatment had no effect on the CVL concentrations of this antimicrobial peptide (Figure 3).
Figure 3.
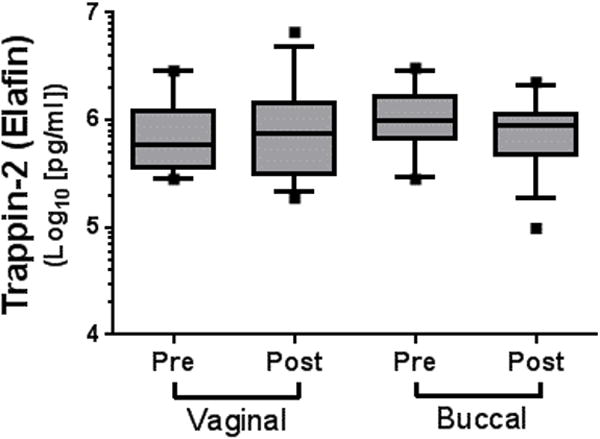
Misoprostol did not alter CVL levels of trappin-2 (elafin). Levels of trappin-2 were measured in CVL at baseline and 24 h after either buccal or vaginal administration of misoprostol 800 μg. Data are shown as box-and-whisker plots (the line represents the median, the boxes represent the 25th–75th percentiles, the whiskers represent the 10th–90th percentiles, and the <10th and >90th percentile outliers are shown as dots). The data were not statistically different.
Effect of misoprostol on circulating and cervical T cells
We evaluated markers of acute and chronic immune activation and senescence on peripheral blood T cells after administration of misoprostol. There were no significant changes in the fraction of CD4+ or CD8+ T cells expressing CD69, CD38, HLA-DR, CD57 or PD-1 on CD4+ or CD8+ after buccal or vaginal administration of misoprostol. Data showing the fraction of memory CD4+ T cells expressing CD38 and PD-1 are shown in Figure 4.
Figure 4.
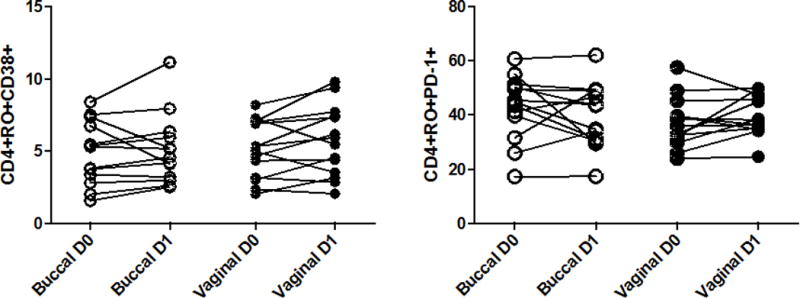
No effect of administration of misoprostol on T cell markers of activation. The expression of CD38 (A) and PD-1 (B) on peripheral blood CD4+ T cells was measured at baseline (D0) and 24 h after (D1) either buccal or vaginal administration of misoprostol 800 μg (p=NS for each comparison; Wilcoxon matched pairs test).
Over the course of the study, we collected 54 cervical Cytobrush specimens for flow cytometric analysis. These samples generally had low numbers of CD3+ T cells for analysis (range of isolated CD3+ T cells 0–1765). To reliably assess the percentage of activated T cells in the samples, we only performed statistical analysis on specimens without evidence of obvious blood contamination and with at least 100 viable CD3+ T cells in the lymphocyte gate (21 samples). Cervical CD4+ and CD8+ T cells had a predominant memory phenotype, with a higher frequency of CD4+CD45RO+ T cells (median CD4+CD45RO+ percentage 87.8%) compared with peripheral blood CD4+ T cells (median 63.1%; p<0.0001) (Figure 5A). Likewise, the percentage of CD8+CD45RO+ T cells was higher in cervical specimens compared with peripheral blood (79% vs 40%; p<0.0001) (Figure 5B). Cervical lymphocytes had a phenotype consistent with immune activation, with higher expression of CD69 (median CD4+CD45RO+CD69+ 48% vs 0.7% [p<0.0001] and median CD8+CD45RO+CD69+ 66% vs 9% [p<0.0001]) (Figure 5C, D) and HLA-DR (CD4+RO+DR+ 3.8% vs 2.2% [p=0.03] and CD8+RO+DR+ 7.8% vs 2.2% [p<0.01]) (Figure 5E, F). CD38 expression was no different between cervical and peripheral CD4+ memory T cells (p=0.17) but was higher on CD8+ cervical T cells (CD8+CD45RO+CD38+ 7.7% vs 3.8%; p=0.001). PD-1 is a cell surface marker associated with immune senescence when present on circulating T cells, but it is also expressed after acute activation. There was a high degree of PD-1 expression on cervical memory T cells (CD4+RO+PD-1+ 70% vs 41% [p<0.0001] and CD8+RO+PD-1+ 84% vs 43% [p<0.0001]) (Figure 5G, H).
Figure 5.
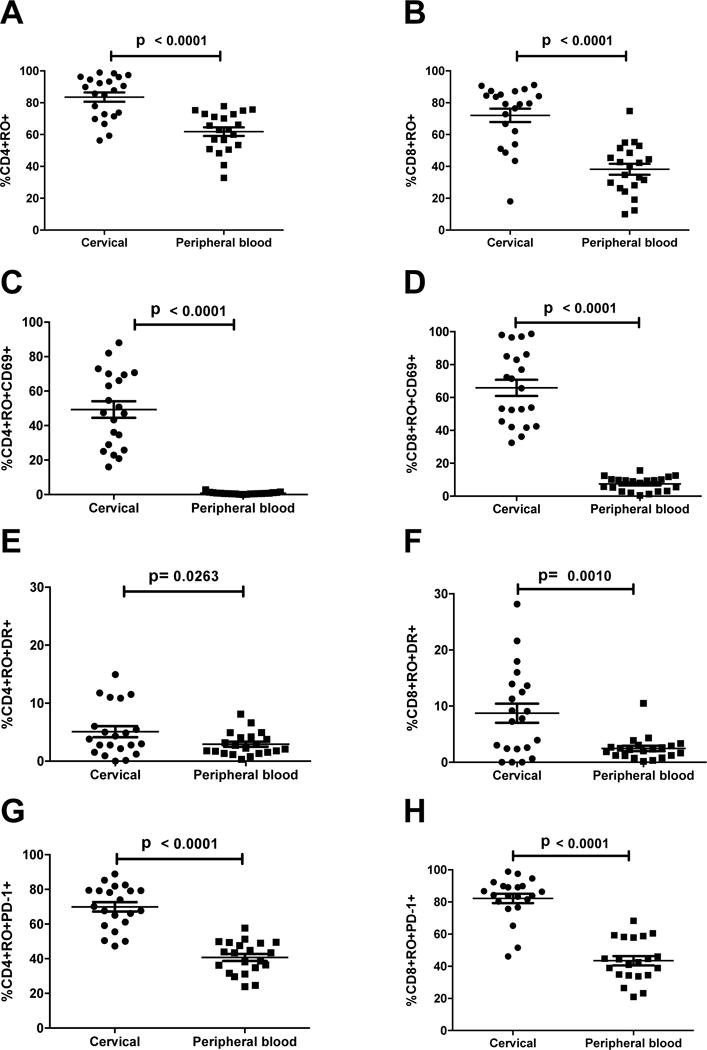
Cervical T cells predominantly display an activated memory phenotype compared with peripheral blood T cells. Comparison of memory population phenotype CD4+CD45RO+ (A), CD8+CD45RO+ (B), memory T cell activation CD4+CD45RO+CD69+ (C), CD8+CD45RO+CD69+ (D), CD4+CD45RO+DR+ (E), CD8+CD45RO+DR+ (F), and memory T cell activation/exhaustion CD4+CD45RO+PD-1+ (G), CD8+CD45RO+PD-1+ (H) between cervical and peripheral blood T cells. p-values were determined by Wilcoxon matched pairs test.
We assessed the effect of buccal or vaginal administration of misoprostol on lymphocyte activation in the cervix. Only six women had adequately cellular specimens on both day 0 and day 1 after either buccal or cervical misoprostol, to allow paired evaluation of cervical T cell activation. Based on this subset of individuals, we saw no changes in the activation state of lymphocytes as measured by CD69 or PD-1 expression (p=NS; Wilcoxon rank-sum test).
Impact of misoprostol on the prevalence of bacterial vaginosis and the cervicovaginal microbiome
Specimens were collected at baseline and 24 h after administration of misoprostol and subjected to analyses for microbial ecology and the presence of subclinical bacterial vaginosis by Nugent scoring. As illustrated in Supplementary Table 3, swabs were evaluated for evidence of bacterial vaginosis in 15 subjects, in a blinded fashion. No subjects had symptoms of bacterial vaginosis. However, microscopic evidence of bacterial vaginosis was demonstrated in two subjects at occasional time points (06LC, 10DD) and in two subjects at all times sampled (14KE, 12PE).
No bacterial genera were found that were significantly different between the buccal group and the vaginal group (controlled ANOVA, p=NS). There were no significant differences in OTU counts (Supplementary Table 4A) or Shannon indices (Supplementary Table 4B) between the groups. Based on UniFrac data, we used beta diversity principal coordinate analysis to generate a 3D scatterplot of the microbiome phylogenetic assemblage relationships among the groups (Supplementary Figure 1). Groups were not significantly different from one another. To provide a visual overview combined with analysis we used a dual hierarchal dendrogram (Supplementary Figure 2 and Supplementary Figure 3) to display the data for the predominant genera with clustering related to the different groups. Again, no bacterial genera were found that were significantly different between the buccal group and the vaginal group.
Notably, three subjects did appear to have an initial microbial environment significantly different from that of the remaining subjects (visualised at the far right of Supplementary Figure 3). However, these samples were all found to have asymptomatic bacterial vaginosis by independent (blinded) Nugent scoring (Supplementary Figure 3). Again, no bacterial genera were found that were significantly different between the buccal group and the vaginal group when these samples were excluded.
Discussion
Findings and interpretation
This pilot study suggests that single-dose misoprostol (800 μg) does not induce lasting (>24 h) effects on innate and/or adaptive immunity or the vaginal microbiome. Previously, studies have identified an impact of misoprostol on mucosal and systemic immunity. For example, the clinical use of misoprostol (800 μg daily for 12 weeks) has demonstrated beneficial effects in reducing immune rejection of transplanted kidneys, when the drug was combined with standard immunosuppressive agents [28]. However, specific effects on the immune system were not assessed and the drug was used for weeks.
In 2003, Waiser and colleagues conducted a small study in 20 healthy volunteers given up to 400 μg oral misoprostol on a single occasion; peripheral blood was sampled 4 h and 9 h after drug administration [10]. The effects of misoprostol on PBMCs, lymphocytes and phagocytes were determined ex vivo. The Waiser et al. study demonstrated that misoprostol induced a time- and dose-dependent reduction of anti-CD3-stimulated cell proliferation, with modest reduction in lymphocyte proliferation 4 h after administration, which returned to baseline 9 h after administration, and modest reductions of Th1 cytokine production after anti-CD3 stimulation (IL-2 and interferon-γ) 9 h after administration [10]. The drug also suppressed whole blood phagocytotic activity towards bacteria, in a dose-dependent manner [10]. These results are not directly comparable to those of our study, as they did not evaluate T cell phenotype, and we did not perform functional lymphocyte assays. Our studies also evaluated lymphocyte subsets at different times after administration of misoprostol and administered drug via mucosal routes, which might differ from oral ingestion with respect to immunomodulation. However, at 24 h after administration, we saw no difference in the activation or exhaustion phenotype of T cells in the peripheral blood or cervix.
A rationale for this pilot study was concern that the vaginal route of administration of misoprostol itself might increase the risk of infectious complications of abortion due to immunomodulation or changes to the vaginal microbiome [11,16]. Retrospective studies conducted by Planned Parenthood demonstrated that when practitioners shifted practice from vaginal misoprostol to buccal administration for medical abortion a significant decrease in infectious complications was observed [15,16]. However, because the change in route of misoprostol administration was accompanied by enhanced screening for sexually transmitted infections and prophylaxis of infectious complications with antibiotic administration [15,16], it remains uncertain whether changing routes of misoprostol administration per se had an impact on rates of infection.
Strengths and weaknesses of the study
Strengths of this pilot study include the number of assessments performed to define the impact of misoprostol on immunity and mucosal microbial ecology, as well as the crossover design, allowing each subject to participate in both buccal and vaginal misoprostol treatment arms. That said, we were limited in the number of assessments we could perform due to the pilot nature of the study.
Only non-pregnant women were enrolled in the study, which poses a potential limitation because the clinical use of misoprostol at the dose studied here (800 μg) is most common in the context of pregnancy termination. The crossover design of our study could not have been performed in pregnant women seeking abortion, as most subjects would not have been pregnant after the first route had been tested. The extent to which the normal immunomodulation associated with pregnancy might influence our results remains an open question.
We only measured a single antimicrobial peptide in the vagina, for example, and therefore cannot rule out effects on other antimicrobial peptides. We did not measure effects of misoprostol on the microbiome outside the vagina, which limits conclusions we can make about the impact of this agent on microbial ecology. A weakness related to the small number of subjects in our study limited the power to detect effects of misoprostol. The lack of evidence of an impact of misoprostol on the processes studied here does not mean that misoprostol lacks immunoregulatory actions, which have been suggested by previous works [5–11]. However, our data suggest that such effects may not be long-lived 24 h after a single dose.
Relevance of the findings: implications for clinicians and policy-makers
Misoprostol is widely used and generally safe. Prostaglandin analogues have a long track record of safe use when administered intravaginally. The extent to which the vaginal administration of 800 μg misoprostol impacts the risk of infectious diseases remains an unanswered question, though our data do not support strong, long-lasting effects on mucosal or systemic immunology and microbial ecology.
Unanswered questions and future research
Negative results from a pilot study should be interpreted with caution because of susceptibility of committing a type II error: failing to reject the null hypothesis (no difference between vaginal and buccal misoprostol) when it is actually false. Larger studies with more immune and microbial ecology parameters, observed across multiple time points will be needed to identify route-specific actions of misoprostol more conclusively.
Supplementary Material
Acknowledgments
Funding
Study team members from Gynuity Health Projects LLC assisted in the study design and in the writing of this report but did not participate in the collection, analysis or interpretation of data.
References
- 1.Lee OY, Kang DH, Lee DH, et al. A comparative study of DA-9601 and misoprostol for prevention of NSAID-associated gastroduodenal injury in patients undergoing chronic NSAID treatment. Arch Pharm Res. 2014;37:1308–16. doi: 10.1007/s12272-014-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheldon WR, Blum J, Durocher J, Winikoff B. Misoprostol for the prevention and treatment of postpartum hemorrhage. Expert Opin Investig Drugs. 2012;21:235–50. doi: 10.1517/13543784.2012.647405. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010;10:CD000941. doi: 10.1002/14651858.CD000941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong E, Tsereteli T, Nguyen NN, Winikoff B. A randomized controlled trial of different buccal misoprostol doses in mifepristone medical abortion. Contraception. 2012;86:251–6. doi: 10.1016/j.contraception.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Iyengar S, Contreras PC, Mick SJ, et al. Immune modifying effects of misoprostol and natural prostaglandins. Br J Rheumatol. 1991;30(Suppl. 2):71–4. [PubMed] [Google Scholar]
- 6.Widomski DL, Walsh RE, Baron DA, et al. Effects of the prostaglandin analogue misoprostol on inflammatory mediator release by human monocytes. Agents Actions. 1991;34:30–1. doi: 10.1007/BF01993229. [DOI] [PubMed] [Google Scholar]
- 7.Haynes DR, Whitehouse MW, Vernon-Roberts B. The prostaglandin E1 analogue, misoprostol, regulates inflammatory cytokines and immune functions in vitro like the natural prostaglandins E1, E2 and E3. Immunology. 1992;76:251–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis LD, Anwar N, Briggs JD, et al. Misoprostol in renal transplantation. Transplant Proc. 1993;25:602. [PubMed] [Google Scholar]
- 9.Rossetti RG, Seiler CM, Brathwaite K, Zurier RB. Effect of misoprostol on acute and chronic inflammation. Am J Ther. 1995;2:600–6. doi: 10.1097/00045391-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Waiser J, Bohler T, Stoll J, et al. The immunosuppressive potential of misoprostol – efficacy and variability. Clin Immunol. 2003;109:288–94. doi: 10.1016/j.clim.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Aronoff DM, Hao Y, Chung J, et al. Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii. J Immunol. 2008;180:8220–30. doi: 10.4049/jimmunol.180.12.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaslona Z, Okunishi K, Bourdonnay E, et al. Prostaglandin E2 suppresses allergic sensitization and lung inflammation by targeting the E prostanoid 2 receptor on T cells. J Allergy Clin Immunol. 2014;133:379–87. doi: 10.1016/j.jaci.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smallwood JI, Malawista SE. Misoprostol stimulates cAMP generation in human leukocytes: synergy with colchicine suggests a new potential for established drugs. Am J Ther. 1995;2:725–9. [PubMed] [Google Scholar]
- 14.Cheon H, Rho YH, Choi SJ, et al. Prostaglandin E2 augments IL-10 signaling and function. J Immunol. 2006;177:1092–100. doi: 10.4049/jimmunol.177.2.1092. [DOI] [PubMed] [Google Scholar]
- 15.Trussell J, Nucatola D, Fjerstad M, Lichtenberg ES. Reduction in infection-related mortality since modifications in the regimen of medical abortion. Contraception. 2014;89:193–6. doi: 10.1016/j.contraception.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fjerstad M, Trussell J, Sivin I, et al. Rates of serious infection after changes in regimens for medical abortion. N Engl J Med. 2009;361:145–51. doi: 10.1056/NEJMoa0809146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iqbal SM, Ball TB, Levinson P, et al. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS. 2009;23:1669–77. doi: 10.1097/QAD.0b013e32832ea643. [DOI] [PubMed] [Google Scholar]
- 19.Stock SJ, Duthie L, Tremaine T, et al. Elafin (SKALP/trappin-2/proteinase inhibitor-3) is produced by the cervix in pregnancy and cervicovaginal levels are diminished in bacterial vaginosis. Reprod Sci. 2009;16:1125–34. doi: 10.1177/1933719109341998. [DOI] [PubMed] [Google Scholar]
- 20.Dowd SE, Sun Y, Wolcott RD, et al. Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: bacterial diversity in the ileum of newly weaned Salmonella-infected pigs. Foodborne Pathog Dis. 2008;5:459–72. doi: 10.1089/fpd.2008.0107. [DOI] [PubMed] [Google Scholar]
- 21.Dowd SE, Callaway TR, Wolcott RD, et al. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) BMC Microbiol. 2008;8:125. doi: 10.1186/1471-2180-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callaway TR, Dowd SE, Edrington TS, et al. Evaluation of bacterial diversity in the rumen and feces of cattle fed different levels of dried distillers grains plus solubles using bacterial tag-encoded FLX amplicon pyrosequencing. J Anim Sci. 2010;88:3977–83. doi: 10.2527/jas.2010-2900. [DOI] [PubMed] [Google Scholar]
- 23.Williams WL, Tedeschi LO, Kononoff PJ, et al. Evaluation of in vitro gas production and rumen bacterial populations fermenting corn milling (co)products. J Dairy Sci. 2010;93:4735–43. doi: 10.3168/jds.2009-2920. [DOI] [PubMed] [Google Scholar]
- 24.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–72. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 26.Eren AM, Zozaya M, Taylor CM, et al. Exploring the diversity of Gardnerella vaginalis in the genitourinary tract microbiota of monogamous couples through subtle nucleotide variation. PLoS One. 2011;6:e26732. doi: 10.1371/journal.pone.0026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson KS, Dowd SE, Suchodolski JS, et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011;5:639–49. doi: 10.1038/ismej.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran M, Mozes MF, Maddux MS, et al. Prevention of acute graft rejection by the prostaglandin E1 analogue misoprostol in renal-transplant recipients treated with cyclosporine and prednisone. N Engl J Med. 1990;322:1183–8. doi: 10.1056/NEJM199004263221703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


