Abstract
Pyrido[3,4]homotropane (PHT) is a conformationally rigid, high affinity analogue of nicotine. (+)-PHT was previously shown to be 266 times more potent than (−)-PHT for inhibition of [3H]epibatidine binding to nAChRs but had no antinociceptive activity in mouse tail-flick or hot-plate tests and was not a nicotinic antagonist even when administered intrathecally. While (−)-PHT had no agonist activity, it was a potent, nicotinic antagonist in the test. Here, electrophysiological studies with rat nAChRs show (+)-PHT to be a low eficacy partial agonist selective for α4β2-nAChRs, relative to α3β4-nAChRs (15-fold) and α7-nAChRs (45-fold). (−)-PHT was an antagonist with selectivity for α3β4, relative to α4β2-(3-fold) and α7- (11-fold) nAChRs. In [3H]DA release studies in mice, (+)-PHT was 10-fold more potent than (−)-PHT at α4β2*-nAChRs and 30-fold more potent at α6β2*-nAChRs. Studies using α5KO mice suggested that much of the activity at α4β2*-nAChRs is mediated by the α4β2α5-nAChR subtype. In conditioned place preference studies, (−)-PHT was more potent than (+)-PHT in blocking nicotine reward. Off-target screens showed (+)- and (−)-PHT to be highly selective for nAChRs. The high potency, full agonism of (+)- and (−)-PHT at α6*-nAChR contrasts with the partial agonism observed for α4*-nAChR, making these ligands intriguing probes for learning more about the pharmacophores for various nAChRs.
Keywords: (−)- and (+)-Pyrido[3,4]homotropane; (−)- and (+)-PHT; α6β2-nicotine agonist; nicotinic receptors; [3H]dopamine release studies; electrophysiological studies; conditioned place preference studies
Graphical Abstract

Nicotinic acetylcholine receptor (nAChR) ligands are of interest as potential pharmacotherapies for treating addiction (nicotine, alcohol, methamphetamine, and cocaine), Parkinson’s disease, schizophrenia, depression, Alzheimer’s disease, attention deficit/hyperactivity disorder, pain, and inflammation (see ref 1 for a recent review). Since the α4β2-and α7-nAChRs are the major nAChR subtypes found in the brain, most of the previous efforts in drug discovery and development have been directed toward these subtypes. However, since the α6 subunit is predominantly expressed in the ventral tegmental area and the mesolimbic system, nAChRs containing this subunit are also of interest as targets for drug development.2–5 Drug discovery studies using the α6*-nAChR as target have been limited due to difficulties in expressing the α6 subunit in heterologous systems and the lack of suitable small molecule standard compounds with which to characterize the α6*-nAChRs.
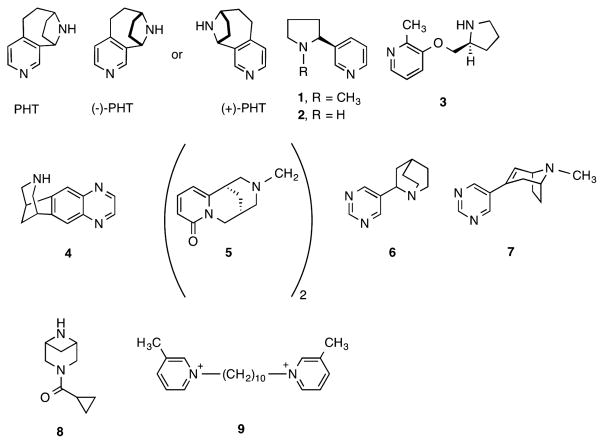
In the late 1980s and in a follow-up study in 2005, Kanne et al. reported that racemic pyrido[3,4]homotropane (PHT), a conformationally rigid analogue of nicotine (1) or nor-nicotine (2) (Figure 1) had good affinity for nAChR labeled by [3H]nicotine and other radioligands and possessed activity similar to nicotine in a prostration assay.6–8 In 2006 we synthesized (+)- and (−)-PHT (Figure 1) and reported that there was a large difference in the ability of the two enantiomers to compete with [3H]epibatidine binding in rat brain.9 In addition, their in vivo pharmacological properties in mice were not consistent with their nAChR binding affinities. We speculated that this inconsistency could be due to binding to other nAChR subtypes, differential binding to various conformations of nAChRs (desensitized or inactive state, for example) or simply binding to non-nicotinic receptors.9 In the present study we investigated the activity and selectivity of (+)and (−)-PHT using in vitro and in vivo experiments.
Figure 1.
Structures of PHT, (−)-PHT, (+)-PHT, 1 (nicotine), 2 (nor-nicotine), 3 (ABT-089), 4 (varenicline), 5 (CC-4), compounds 6, 7, 8 (TC-8831), and 9 (bPiDi).
RESULTS AND DISCUSSION
The nAChR subtype selectivity of (+)- and (−)-PHT was initially assessed in an electrophysiological assay using rat α4β2-, α3β4-, and α7-nAChR’s expressed in Xenopus oocytes and assayed by a two-electrode voltage clamp. To test for agonist activity, currents in response to 100 μM applications of each compound were normalized to the response to ACh. Results from this screen, shown in Table 1, suggested that (+)-PHT is a low efficacy partial agonist of the α4β2 and α3β4-nAChRs. No agonist activity was observed for (+)-PHT at α7-nAChRs. (−)-PHT displayed little or no agonist activity at the α4β2, α3β4, and α7-nAChRs.
Table 1.
In Vitro Single Concentration Functional Assays for (+)- and (−)-PHTa
| cmpd | agonist activity (% of maximal ACh response)
|
antagonism (% of EC50 ACh remaining)
|
||||
|---|---|---|---|---|---|---|
| α4β2 | α3β4 | α7 | α4β2 | α3β4 | α7 | |
| (+)-PHT | 3.2 ± 0.4 | 7.7 ± 0.9 | 0 | 17 ± 3 | 93 ± 3 | 87± 1 |
| (−)-PHT | 0 | 0 | 1.1± 0.2 | 41 ± 4 | 32 ± 3 | 84± 10 |
Current responses of rat nAChRs expressed in Xenopus oocytes were recorded under a two-electrode voltage clamp. Agonist activity was assessed by comparing the response to 100 μM of each compound to the response to acetylcholine applied at an EC20 concentration (20 μM for α4β2, 110 μM for α3β4) or an EC50 concentration (300 μM for α7). Antagonist activities assessed by comparing the current response to an EC50 concentration of acetylcholine (70 μM for α4β2, 200 μM for α3β4, 300 μM for α7) in the presence of 100 μM of each compound are compared to the response to acetylcholine alone.
To test for antagonist properties, the current response to an EC50 concentration of ACh in the presence of 100 μM (+)- or (−)-PHT was determined. Results from this screen (Table 1) suggested that (+)-PHT may be selective for α4β2- relative to the α3β4 and α7-nAChRs and that (−)-PHT appeared to be selective for α3β4- and α4β2-, relative to α7-nAChRs.
In order to gain more detailed information on the subtype selectivity of the agonist and antagonist activity of (+)- and (−)-PHT, concentration–response and concentration–inhibition curves were generated (Table 2). We found (+)-PHT to be a low efficacy partial agonist at the α4β2- and α3β4-nAChRs, yielding maximal responses that were 4.6% and 8.4% of the maximal response to ACh, respectively. A comparison of EC50 values showed (+)-PHT to be 12-fold more potent at the α4β2-nAChR, as compared to the α3β4-nAChR. A comparison of IC50 values showed that as an antagonist (+)-PHT is 15-fold selective for α4β2- relative to the α3β4-nAChR and 45-fold selective for α4β2- relative to the α7-nAChR. In contrast, (−)-PHT was a modestly selective antagonist of α3β4- relative to the α4β2-nAChR (3-fold) and 11-fold selective for α3β4-relative to the α7-nAChR. We were not able to assess agonist or antagonist activity of (+)- or (−)-PHT at α6-containing nAChRs using the Xenopus oocyte assay due to the difficulty of expressing wild-type α6-containing nAChRs in heterologous systems. Use of chimeric or concatameric constructs is usually required to achieve functional expression of α6-containing nAChRs,10,11 which can be a concern when trying to assess agonist efficacy. Instead, we turned to a striatal synaptosome [3H]dopamine release assay to assess the activity of (+)- and (−)-PHT at α6-containing nAChRs.
Table 2.
Comparison of Agonist Potency and Efficacy Values, And Antagonist Potency Values, for (+)- and (−)-PHT at α4β2-, α3β4-, and α7-nAChRsa
| cmpd | agonist activity
|
antagonism, IC50 (μM)
|
|||||
|---|---|---|---|---|---|---|---|
|
α4β2
|
α3β4
|
||||||
| EC50 (μM) | Emax (% ACh) | EC50 (μM) | Emax (% ACh) | α4β2 | α3β4 | α7 | |
| (+)-PHT | 4 ± 1 | 4.6 ± 0.4 | 50 ± 6 | 8.4 ± 0.5 | 16 ± 3 | 242 ± 13 | 720 ± 290 |
| (−)-PHT | 99 ± 13 | 33 ± 6 | 353 ± 34 | ||||
Current responses of rat nAChRs expressed in Xenopus oocytes were recorded under a two-electrode voltage clamp. EC50 and Emax values for (+)-PHT activation of α4β2- and α3β4-nAChRs are derived from concentration–response curves. Emax values are expressed as a percentage of the Emax for acetylcholine. IC50 values for (+)-PHT and (−)-PHT inhibition of α4β2-, α3β4-, and α7-nAChRs are derived from concentration–inhibition curves, in which the current response to an EC50 concentration of acetylcholine (70 μM for α4β2, 200 μM for α3β4, 300 μM for α7) in the presence of various concentrations of each compound are compared to the response to acetylcholine alone.
We performed [3H]dopamine release studies for (+)- and (−)-PHT using wild type and α5KO* mice, and the results are provided in Table 3. Maximal responses were normalized to that measured with 10 μM nicotine. In wild type mice (+)-PHT has EC50 values of 0.4 and 0.13 μM for the α-CtxMII resistant (α4β2*-nAChR) and sensitive (α6β2*-nAChR) response compared to 4 and 4.5 μM for (−)-PHT. Thus, (+)-PHT is 10-fold more potent than (−)-PHT at the α4β2*-nAChR and 30-fold more potent for the α6β2*-nAChR.
Table 3.
Dopamine Release from Striatal Synaptosomes Stimulated by (+)- and (−)-PHTa
| (+) PHT res α4β2 | (+) PHT sens α6β2 | (+) PHT α4β2 in α5KO* | (+) PHT α6β2 in α5KO | (−) PHT res α4β2 | (−) PHT sens α6β2 | (−) PHT α4β2 in α5KO* | (−) PHT α6β2 in α5KO | |
|---|---|---|---|---|---|---|---|---|
| EC50 | 0.4 μM | 0.13 μM | 2 μM | 0.20 μM | 4 μM | 4.5 μM | 0.7 μM | 2 μM |
| sem | ±0.2 μM | ±0.04 μM | ±3 μM | ±0.06 μM | ±3 μM | ±0.6 μM | ±0.8 μM | ±2 μM |
| maxb (% nic) | 33% | 94% | 13% | 125% | 17% | 109% | 10% | 96% |
| sem | ±5% | ±18% | ±4% | ±8% | ±3% | ±25% | ±2% | ±12% |
| binding Kic | 1.7 nM | 28 nM | 35 nM | 629 nM | ||||
| sem | ±0.2 nM | ±3.1 nM | ±1.8 nM | ±19 nM |
EC50 from n = 3–4 experiments; DA release from mouse striatal synaptosomes; res = αCtxMII resistant (response); sens = αCtxMII sensitive (response).
Max (% nic) from n = 7–8 experiments; comparison of 10 μM nic response to 10 μM (+) PHT or 100 μM (−) PHT.
Binding Ki values from inhibition of [125I]epibatidine binding to membranes prepared from mouse cortex (α4β2 sites only) or membranes prepared from striatum and olfactory tubercle from α4KO mice (α6β2 sites only).
Both isomers are more efficacious at the α6β2*-nAChR and are about as efficacious as nicotine. Both isomers are partial agonists for α4β2*-nAChR with the (+)- and (−)-isomers being 1/3 and 1/6, respectively, as efficacious as nicotine.
In the [125I]epibatidine binding studies, both isomers have higher affinity for the α4β2*- than the α6β2*-nAChR. (+)-PHT has Ki values of 1.7 and 28 nM for the αCtxMII resistant (α4β2*-nAChR) and αCtxMII sensitive (α6β2*-nAChR), respectively, compared to 35 and 629 nM for the (−)-PHT at the α4β2* and α6β2*-nAChRs, respectively.
In the α5KO* mice (+)-PHT has EC50 values of 2 and 0.2 μM, respectively, at the α4β2*- and α6β2*-nAChR, respectively, compared to EC50 values of 0.7 and 2 μM at the α4β2*-and α6β2*-nAChR, respectively, for (−)-PHT. These results show that deletion of the α5 subunit greatly reduces α-CtxMII-resistant activity of both isomers suggesting that much of the activity at the α4β2*-nAChR is mediated by the α4β2α5-nAChR subtype. These results are similar to those found for nicotine in the striatum, but the effect of the α5 gene deletion on (+)- and (−)-PHT is even greater than it is for nicotine.12
Since the in vitro data indicate that both (+)- and (−)-PHT modulate nicotinic receptors function and DA release in the striatum, we evaluated the effects of (+)- and (−)-PHT in vivo in the conditioned place preference (CPP) test. As shown in Figure 2A, (+)-PHT induced a dose-related preference when given s.c. to mice. Post hoc analysis showed that (+)-PHT significantly increased CPP scores at a dose of 3 mg/kg, whereas it did not at doses of 0.1 and 1 mg/kg. In contrast, all doses of (−)-PHT tested were ineffective in inducing significant preference in the CPP test (Figure 2C). In the antagonism studies, in mice given (+)-PHT before each drug pairing with nicotine (0.5 mg/kg, s.c.), preference scores in comparison to nicotine alone (Figure 2B) were significantly reduced after pretreatment with 1 mg/kg of the drug. In addition, nicotine-induced CPP was blocked by pretreatment with (−)-PHT in a dose-dependent manner with the dose of 0.05 mg/kg completely preventing nicotine preference (Figure 2D).
Figure 2.
Effects of (+)-PHT and (−)-PHT on development of nicotine-induced CPP in the mouse. (A) Dose–response curve for (+)-PHT in the CPP test given s.c. 15 min before conditioning. (B) Blockade of nicotine-induced CPP by (+)-PHT. The drug was given 15 min before nicotine (0.5 mg/kg, s.c.) during conditioning. (C) Dose–response curve for (−)-PHT in the CPP test given s.c. 15 min before conditioning. (D) Blockade of nicotine-induced CPP by (−)-PHT. The drug was given 15 min before nicotine (0.5 mg/kg, s.c.) during conditioning. Each point represents the mean ± SEM of 8 mice per group. * denotes p < 0.05 vs the vehicle control groups. Veh = Vehicle.
(+)-PHT (10 μM) was inactive in radioligand binding assays for the 5HT1A, 5HT2A, 5HT2C, DAD1, DAD2, DAD3, DAD4, and μ, δ, and κ opioid receptors, and the DAT and SERT. (+)-PHT (10 μM) inhibited 61% of specific [125I]RTI-55 binding to the NET. (+)-PHT (10 μM) was inactive at off site targets in a 62 receptor/enzyme screen (NovaScreen).
(−)-PHT (10 μM) was inactive in radioligand binding assays for the 5HT1A, 5HT2A, 5HT2C, DAD1, DAD2, DAD3, DAD4, and μ, δ, and κ opioid receptors, and the DAT, NET, and SERT. (−)-PHT (10 μM) was inactive at off site targets in a 62 receptor/enzyme screen (NovaScreen).
CHRNA6, the gene encoding for the α6 subunit, is located on chromosome 8b11.21 and is predominantly expressed in visual pathways, the ventral tegmental area, and the mesolimbic system.2–5 These anatomical distributions identified by in situ hybridization studies and physiological roles of α6*-nAChRs, using subunit-null transgenic animals and peptide ligands, suggest the relevance of these nicotinic subtypes in drug addiction (nicotine, alcohol, and others) and other CNS disorders, making them potential targets for pharmacotherapy development.
The paucity of suitable pharmacological tools has made it difficult to conduct studies with small molecule α6*-nAChR ligands. Most of the tools available are peptides. The discovery of α-Ctx MII allowed positive identification of the α3β2*- and α6β2*-nAChRs13 and the development of the only radioligand currently available: [125I]α-Ctx MII.14,15 A subtraction approach was used to identify an α-CtxMII-selective nAChR population that mediates dopamine release. By measuring the functional response in the presence and absence of α-Ctx MII, the activity of α-Ctx MII-sensitivity can be distinguished from that of α4β2*-nAChR.10,12,15,16 Even though α-Ctx MII has been highly useful in the characterization of α6*-nAChR, its pharmacological properties have some limitations. In addition to lack of CNS availability via systemic administration and stability, α-Ctx MII interacts with α3β2*-nAChR with similar potency with α6β2*-nAChRs. The lack of selectivity was overcome by the development of the more α6β2*-nAChR selective α-conotoxin PIA (α-CtxPIA).16 However, α-CtxPIA is also a peptide and lacks CNS availability after systemic administration. In addition, expression of α6 with α4 and β2 and/or β3 subunits in oocytes yielded functional receptors with different pharmacological properties than α4β2-nAChRs, such as enhanced acetylcholine (ACh) sensitivity, slower desensitization, and different desensitization rate and potency for nicotine.17 Clearly small molecule-based α6*-nAChR ligands and their radioligand analogues are needed to reliably distinguish between α6*-nAChR and other nAChR subtypes as well as the various α6*-nAChR subtypes.
Using the tools available, the α6*-nAChR properties of nicotine and several other nicotine ligands have been studied. This information is summarized below in order to show the lack of suitable small molecules selective α6β2*-nAChR agonists. Nicotine is reported to have 70% partial agonist activity at α4α6β2-nAChRs and could desensitize the α4β2-nAChR and activate the α4α6β2-nAChR.17 2-Methyl-3-[(2S)-pyrrolidin-2-yl]methoxypyridine (pozanicline, ABT-089, 3) (Figure 1), which is a potent partial agonist at α4β2*-nAChR, increases in vitro striatal DA release via activation of α6β2*- and α4β2*-nAChRs.18 6,7,8,9,10-Tetrahydro-6,10-methano-6H-pyyrazino[2,3-h][3]benzazepine (varenicline, 4) (Figure 1) has EC50 values of 0.007 and 0.086 μM at α6β2*-and α4β2*-nAChR-mediated [3H]DA release from rat striatal synaptosomes, respectively, and thus, was functionally more potent at the α6β2-nAChR.19 Varenicline acted as a partial agonist in these studies with maximal efficacies of 49 and 24% at the α6β2- and α4β2-nAChRs, respectively.19 In monkeys, varenicline potently inhibited striatal α6β2*- and α4β2*-nAChR with Ki values of 0.13 and 0.19 nM.19 In functional studies it stimulated α6β2*- and α4β2*-nAChR-mediated [3H]DA-release from monkey striatal synaptosomes with EC50 values of 0.14 and 0.026 μM, respectively. The Emax was 13 and 42% for the α6β2*- and α4β2*-nAChRs, respectively. 1,2-bis-N-Cystinylethane (5, CC4) is another selective partial agonist at α4β2/α6β2-nAChR. CC4-induced CPP and its self-administration show an inverted U dose response curve. Pretreatment with nonreinforcing doses of CC4 significantly reduced nicotine-induced self-administration and CPP without affecting motor function.14 The receptor binding affinity and functional efficacy and selectivity of 6 and 7 at α6β2*- relative to α4β2- and α3β4-nAChR were reported.13 Compound 6 had Ki values of 1.8, 1100, and 8 nM at α4β2-, α7-, and α6β2*-nAChRs while 7 had Ki values of 1.2, 9100, and 17.3 nm. Using DA release studies, compound 6 had an EC50 = 90 nM (Emax = 104%) and 7 had an EC50 = 100 nM (Emax = 77%). The α3β4*/α6β2* EC50 ratio for 6 and 7 were 26.7 and 410, respectively, and the α3β4*/α4β2* EC50 ratio for 6 and 7 were 5.3 and 22.7, respectively. Very recently, 3-cyclopropylcarbonyl-3,6-dazabicyclo[3.1.1]heptane (8, TC-8831) was reported to have Ki = 3.0 and 21 nM at α4β2- and α6/α3β2β3-nAChRs, respectively.20 Using calcium-flux functional studies, 8 had EC50 = 80 nM (Emax = 100%) at the α6/α3β2β3-nAChR. Previous studies had shown that compound 8 ameliorated L-dopa induced dyskinesia (LID) without reducing antiparkinsonian benefits in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned nonhuman primates with established LID.21 Compound 8 also reduced abnormal involuntary movements (AIMs) in rats.22 In another study, N,N′-decane-diyl-bis-3-picolinium 9 (bPiDI) with selectivity for α6β2*-nAChRs over α4β2*-nAChRs has been shown to reduce nicotine SA in rats.23 Even though studies from these compounds and a few others have provided useful information about the pharmacological properties of compounds interacting at the α6*-nAChR, few of the compounds were a full agonist at the α6*-nAChR with any degree of selectivity. In this study, we report that both (+)- and (−)-PHT are α6β2-nAChR agonists. (+)-PHT is more potent and more selective for α6β2*-nAChR than (−)-PHT.
PHT is a conformationally rigid analogue of nicotine that showed high affinity for nAChRs using various radiolabeled nicotinic ligands.6–8 In 2006, we reported the synthesis and pharmacological characterization of (+)- and (−)-PHT.9 We found that PHT and (+)- and (−)-PHT had Ki values of 6.2, 1.29, and 346 nM, respectively, for inhibition of [3H]epibatidine binding to nAChRs. Thus, (+)-PHT has a 266-times higher affinity than (−)-PHT for inhibition of [3H]epibatidine binding to nAChRs. Even though (+)-PHT has a Ki = 1.29 nM at nAChRs, it has no antinociceptive activity in mouse tail-flick or hot-plate tests even when given intrathecally (10 μg/mouse). (+)-PHT did show weak activity in mouse hypothermia and locomotor-activity tests, but the effects were not reversed by the nicotinic antagonist mecamylamine. Not surprisingly, (−)-PHT with a Ki = 346 nM for inhibition of [3H]epibatidine binding was inactive as an agonist in the mouse tail-flick, hot-plate, hypothermia, and locomotor-activity behavioral tests. Like (+)-PHT, it was also inactive when given intrathecally (10 μg/mouse) in the tail-flick test. Surprisingly, (−)-PHT was highly potent in antagonizing nicotine-induced antinociception in the tail-flick test (AD50 = 0.07 μg/kg) and also antagonized the antinociceptive effects of nicotine in the hot-plate test (AD50 = 0.8 μg/kg). In an in vitro functional test, (+)-PHT behaved as a weak partial agonist at α4β2-nAChR with an intrinsic activity of 17% versus a full agonist acetylcholine (1 mM) and EC50 = 5.5 μm. (−)-PHT did not activate currents at α4β2-nAChR. Neither compound activated or inhibited α3β4-nAChR expressing cells. In nicotine drug discrimination studies, PHT and its enantiomers failed to fully generalize nicotine at doses that did not affect the rate of responding in rat (0.3–56 μg/kg). In addition, they failed to antagonize nicotine discrimination. Our in vivo results with the CPP test show that (+)- but not (−)-PHT possesses reward-like effects at the range of doses tested, reflecting perhaps the difference of potency of these compounds at α6β2*-nAChR and their ability to release DA in the striatum. Pretreatment with (+)- and (−)-PHT significantly reduced nicotine preference, suggesting antagonistic properties of these compounds on nicotine reward and motivation. These results are in line with the partial agonism and antagonistic properties of (+)and (−)-PHT at α4β2 expressed nAChRs, respectively. The higher potency of (−)-PHT in blocking nicotine CPP than (−)-PHT was also observed in their ability of blocking the acute effects of nicotine.9
Studies showing that α6*-nAChR knockout mice did not self-administer nicotine which could be restored by re-expression of the α6 subunit in the mesolimbic dopamine system provided additional evidence that the α6*-nAChR was involved in nicotine-induced reward.24 In addition, intra-cerebroventricular injection of α-Ctx MII in mice dose-dependently attenuated the rewarding effect of nicotine in the CPP model without reducing locomotor behavior.25 α-Ctx MII also attenuated the affective signs of nicotine withdrawal but not the physical signs and attenuated mecamylamine-precipitated CPA.25 In addition, α-Ctx MII produced anxiolytic effects in mice withdrawn from nicotine after chronic exposure. The microinjection of α-Ctx MII directly into the VTA or nucleus accumbens shell attenuated established nicotine self-administration in rats26 without nonspecific changes in food response.27 These studies indicate that blockade of α6β2*-nAChRs reduces the rewarding effects of nicotine in CPP, attenuates nicotine intake in an SA paradigm, reduces anxiety associated with nicotine withdrawal, and underscores the role of α6β2*-nAChRs in nicotine abuse and, thus, suggests the α6β2*-nAChR ligands may have potential as pharmacotherapies for smoking cessation.28
In summary, the high potency and full agonist activity of (+)and (−)-PHT at α6*-nAChR contrasts with the partial agonist activity observed for α4*-nAChR. Our in vitro studies suggest that (+)- and (−)-PHT are more efficacious than varenicline in releasing striatal DA. Similar to varenicline, (+)- and (−)-PHT are partial agonists at α4β2*-nAChRs. The partial activity at α4*-nAChR is further reduced following deletion of the α5 nAChR subunit, suggesting both compounds may be more active, although still partial agonists, at the α4β2α5-nAChR subtype. (+)-PHT, in particular, may be a useful tool for studying α6-nAChR systems. Our in vivo studies using CPP as a test of nicotine reward showed that (+)- but not (−)-PHT is rewarding, albeit to a lesser extent than nicotine. This difference in CPP response probably reflects their difference in potency at α6*-nAChR subtypes. The ability of (+)- and (−)-PHT to block nicotine-induced CPP is reflective of their partial agonistic properties at α4β2*-nAChRs. Importantly, (+)- and (−)-PHT are novel lead structures for the development of selective small molecule-based agonists and antagonists and radioligands for studying the various receptors in the α6*-nAChR subfamily. In addition, the rigid structure of PHT and its enantiomers combined with their unexpected in vitro and in vivo pharmacological properties make them intriguing probes for learning more about the pharmacophores for various nAChRs. Finally, our in vivo and in vitro findings show that (+)- and (−)-PHT are highly useful pharmacological probes for studying various nAChR subtypes and support the possible development of PHT enantiomers or future analogues as possible smoking cessation agents.
METHODS
Materials
(+)- and (−)-PHT were synthesized using previously reported methods.9
Binding Studies
Binding Ki values were determined from inhibition of [125I]epibatidine binding to membranes prepared from mouse cortex (α4β2 sites only) or membranes prepared from striatum and olfactory tubercle from α4KO mice (α6β2 sites only).29 The production, care, and use of mice used for the binding assays and the functional assays described below was approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder and meet the guidelines of the National Institutes of Health.
In Vitro Efficacy Studies
(+)- and (−)-PHT were tested for agonist and antagonist activity at rat α4β2-, α3β4, and α7-nAChR expressed in Xenopus laevis oocytes and assayed in an electrophysiology assay as previously described.30 The care and use of X. laevis frogs in this study was approved by the University of Miami Animal Research Committee and meet the guidelines of the National Institutes of Health.
[3H]Dopamine Release
The [3H]dopamine release studies were conducted using previously reported methods.10,18 Concentration–effect curves were constructed for (+)- and (−)-PHT in the absence (total release) and the presence of 50 nM α-conotoxin MII (α-Ctx MII) to inhibit α6β2*-nAChR subtypes. [3H]dopamine release in synatosomes prepared from α5 null mutant mice was measured to evaluate the role of the α5 subunit (as a component of α4β2α5-nAChR subtype). These mice were originally generated at and obtained from Baylor College of Medicine, Houston, TX, and null mutants were obtained from mating of α5 heterozygotes.31 The difference in activity between [3H]dopamine release measured in the absence and presence of α-Ctx MII provides the measure of the response mediated by α6β2*nAChRs and release measured in the presence of α-Ctx MII provides the measure of the response mediated by α4β2*-nAChRs. Maximal release and ED50 values were calculated by nonlinear least-squares fits of the data to the Michaelis–Menten equation. Relative efficacy was determined by comparison to the release mediated by stimulation with 10 μM nicotine.
In Vitro Off-Target Binding
Both (+)- and (−)-PHT were evaluated for their in vitro receptor binding and functional properties at the following receptors: 5HT1A, 5HT2A, 5HT2C, DAD1, DAD2, DAD3, and DAD4, μ, δ, and κ opioid receptors and DAT, SERT, and NET as well as their profiles in a 62 radioligand/enzyme assay (NovaScreen) at 100 nM and 10 000 nM concentrations in duplication. The studies and data were provided through the National Institute on Drug Abuse (NIDA) Addiction Treatment Discovery Program.
Nicotine Conditioned Place Preference (CPP) Studies
Naive male 8–10 week-old ICR mice (Harlan Laboratories; Indianapolis, IN) served as subjects. They were housed five per cage in a 21 °C humidity controlled facility with ad libitum access to food and water. The animal facility was approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. (−)-Nicotine hydrogen tartrate salt was purchased from Sigma Chemical Company (Milwaukee, WI), and it was dissolved in physiological saline (0.9% sodium chloride). Nicotine was injected s.c. at a volume of 10 mL/kg body weight. The pH of the nicotine solution was checked and neutralized if necessary. All doses are expressed as the free base of the drug. Nicotine CPP was conducted using an unbiased design as previously described.32 In brief, separate groups of male ICR adult mice (n = 8 per group) were handled for 3 days prior to initiation of CPP testing. The CPP apparatus consisted of a three-chambered box with a white compartment, a black compartment, and a center gray compartment. The black and white compartments also have different floor textures to help the mice further differentiate between the two environments. On day 1, mice were placed in the gray center compartment for a 5 min habituation period, followed by a 15 min test period to determine baseline responses. A prepreference score was recorded and used to randomly pair each mouse with either the black or white compartment. Drug-paired sides were randomized so that an even number of mice received drug on the black and white side. Over the next 3 days, mice were conditioned for 20 min twice a day with conditioning sessions no less than 5 h apart. The saline group received saline on both sides of the boxes and drug groups received nicotine (0.5 mg/kg, s.c.) on one side of the box and saline on the opposite side. Animals in the drug group received drug each day. On test day (day 5), mice were exposed to the chambers, and day 1 procedure was repeated. In addition, mice were pretreated with vehicle (s.c.), (+)- and (−)-PHT at different doses 15 min prior nicotine. Data were expressed as time spent on the drug-paired side postconditioning minus time spent on the drug-paired side preconditioning. A positive number indicated a preference for the drug-paired side, whereas a negative number indicated an aversion to the drug-paired side. A number at or near zero indicated no preference for either side.
Acknowledgments
Funding
This research was supported by the National Institute on Drug Abuse Grants DA12001, P30 DA015663, and DA003194. The off-target studies were performed by the contractor of the Drug Discovery program of NIDA/DPMCDA.
ABBREVIATIONS
- ACh
acetylcholine
- nAChR
nicotinic acetylcholine receptor
- α-CtXMII
α-conotoxin MII
- CC4
1,2-bis-N-cystinylethane
- CPP
conditional place preference
- CPA
conditional place adversion
- SA
self-administration
- α4KO
alpha 4 knockout
- 5HT1A, 5HT2A, and 5HT2C
serotonin-1A, 2A, and 2C receptors, respectively
- DAD1, DAD2, DAD3, and DAD4
dopamine D1, D2, D3, and D4 receptors, respectively
- DAT
dopamine transporter
- SERT
serotonin transporter
- NET
norepinephrine transporter
Footnotes
Author Contributions
F.I.C., M.I.D., C.W.L., and M.J.M. participated in research design. A.H.C., C.R.W., and A.J. conducted experiments. F.I.C., H.A.N., S.W.M., A.H.C., C.W.L., C.R.W., M.J.M., A.J., and M.I.D. performed data analysis. F.I.C., H.A.N., S.W.M., C.W.L., M.J.M., and M.I.D. wrote or contributed to the writing of the manuscript.
Notes
The authors declare no competing financial interest.
References
- 1.Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013;137:22–54. doi: 10.1016/j.pharmthera.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- 4.Gotti C, Moretti M, Zanardi A, Gaimarri A, Champtiaux N, Changeux JP, Whiteaker P, Marks MJ, Clementi F, Zoli M. Heterogeneity and selective targeting of neuronal nicotinic acetylcholine receptor (nAChR) subtypes expressed on retinal afferents of the superior colliculus and lateral geniculate nucleus: identification of a new native nAChR subtype α3β2(α5 or β3) enriched in retinocollicular afferents. Mol Pharmacol. 2005;68:1162–1171. doi: 10.1124/mol.105.015925. [DOI] [PubMed] [Google Scholar]
- 5.Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology. 2000;39:2570–2590. doi: 10.1016/s0028-3908(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 6.Kanne DB, Abood LG. Synthesis and biological characterization of pyridohomotropanes. Structure-activity relationships of conformationally restricted nicotinoids. J Med Chem. 1988;31:506–509. doi: 10.1021/jm00398a004. [DOI] [PubMed] [Google Scholar]
- 7.Kanne DB, Ashworth DJ, Cheng MT, Mutter LC, Abood LG. Synthesis of the first highly potent bridged nicotinoid. 9-Azabicylo[4.2.l]nona[2,3-c]pyridine (Pyrido[3,4-b]-homotropane) J Am Chem Soc. 1986;108:7864–7865. doi: 10.1021/ja00284a078. [DOI] [PubMed] [Google Scholar]
- 8.Kanne DB, Tomizawa M, Durkin KA, Casida JE. 6′-Methylpyrido[3,4-b]norhomotropane: synthesis and outstanding potency in relation to the alpha4beta2 nicotinic receptor pharmacophore model. Bioorg Med Chem Lett. 2005;15:877–881. doi: 10.1016/j.bmcl.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 9.Carroll FI, Hu X, Navarro HA, Deschamps J, Abdrakhmanova GR, Damaj MI, Martin BR. Synthesis and pharmacological characterization of nicotinic acetylcholine receptor properties of (+)- and (−)-pyrido-[3,4-b]homotropanes. J Med Chem. 2006;49:3244–3250. doi: 10.1021/jm060122n. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. Analogs of α-conotoxin MII are selective for α6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- 11.Dash B, Li MD, Lukas RJ. Roles for N-terminal Extracellular Domains of Nicotinic Acetylcholine Receptor (nAChR) β3 Subunits in Enhanced Functional Expression of Mouse α6β2β3-and α6β4β3-nAChRs. J Biol Chem. 2014;289:28338–28351. doi: 10.1074/jbc.M114.566018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grady SR, Salminen O, McIntosh JM, Marks MJ, Collins AC. Mouse striatal dopamine nerve terminals express alpha4alpha5beta2 and two stoichiometric forms of alpha4beta2*-nicotinic acetylcholine receptors. J Mol Neurosci. 2010;40:91–95. doi: 10.1007/s12031-009-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivera BM, Quik M, Vincler M, McIntosh JM. Subtype-selective conopeptides targeted to nicotinic receptors: Concerted discovery and biomedical applications. Channels (Austin) 2008;2:143–152. doi: 10.4161/chan.2.2.6276. [DOI] [PubMed] [Google Scholar]
- 14.Breining SR, Bencherif M, Grady SR, Whiteaker P, Marks MJ, Wageman CR, Lester HA, Yohannes D. Evaluation of structurally diverse neuronal nicotinic receptor ligands for selectivity at the α6(*) subtype. Bioorg Med Chem Lett. 2009;19:4359–4363. doi: 10.1016/j.bmcl.2009.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [(3)H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- 16.Dowell C, Olivera BM, Garrett JE, Staheli ST, Watkins M, Kuryatov A, Yoshikami D, Lindstrom JM, McIntosh JM. Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. J Neurosci. 2003;23:8445–8452. doi: 10.1523/JNEUROSCI.23-24-08445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valera S, Bertrand S, Rollema H, Hurst R, Bertrand D. Effects of Nicotine and Varenicline at α6 Containing nAChRs: Relevance for Nicotine Dependence. Society for Neuroscience Meeting; San Diego, CA. 2010. Poster No. 476.12/NN6. [Google Scholar]
- 18.Marks MJ, Wageman CR, Grady SR, Gopalakrishnan M, Briggs CA. Selectivity of ABT-089 for α4β2* and α6β2* nicotinic acetylcholine receptors in brain. Biochem Pharmacol. 2009;78:795–802. doi: 10.1016/j.bcp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordia T, Hrachova M, Chin M, McIntosh JM, Quik M. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012;342:327–334. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strachan JP, Kombo DC, Mazurov A, Heemstra R, Bhatti BS, Akireddy R, Murthy S, Miao L, Jett JE, Speake J, Bencherif M. Identification and pharmacological characterization of 3,6-diazabicyclo[3.1.1]heptane-3-carboxamides as novel ligands for the alpha4beta2 and alpha6/alpha3beta2beta3 nicotinic acetylcholine receptors (nAChRs) Eur J Med Chem. 2014;86C:60–74. doi: 10.1016/j.ejmech.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Mallela A, Sohn D, Carroll FI, Bencherif M, Letchworth S, Quik M. Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson’s disease. J Pharmacol Exp Ther. 2013;347:225–234. doi: 10.1124/jpet.113.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quik M, Campos C, Bordia T, Strachan JP, Zhang J, McIntosh JM, Letchworth S, Jordan K. alpha4beta2 Nicotinic receptors play a role in the nAChR-mediated decline in L-dopa-induced dyskinesias in parkinsonian rats. Neuropharmacology. 2013;71:191–203. doi: 10.1016/j.neuropharm.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wooters TE, Smith AM, Pivavarchyk M, Siripurapu KB, McIntosh JM, Zhang Z, Crooks PA, Bardo MT, Dwoskin LP. bPiDI: a novel selective alpha6beta2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. Br J Pharmacol. 2011;163:346–357. doi: 10.1111/j.1476-5381.2011.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunzell DH, McIntosh JM, Papke RL. Diverse strategies targeting alpha7 homomeric and alpha6beta2* heteromeric nicotinic acetylcholine receptors for smoking cessation. Ann NY Acad Sci. 2014;1327:27–45. doi: 10.1111/nyas.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 30.Ondachi P, Castro A, Luetje CW, Damaj MI, Mascarella SW, Navarro HA, Carroll FI. Synthesis and Nicotinic Acetylcholine Receptor In Vitro and In Vivo Pharmacological Properties of 2′-Fluoro-3′-(substituted phenyl)deschloroepibatidine Analogues of 2′-Fluoro-3′-(4-nitrophenyl)deschloroepibatidine. J Med Chem. 2012;55:6512–6522. doi: 10.1021/jm300575y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- 32.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]