Abstract
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases. Although many researchers have attempted to explain the origins of AD, developing an effective strategy in AD clinical therapy is difficult. Recent studies have revealed a potential link between AD and circRNA-associated-ceRNA networks. However, few genome-wide studies have identified the potential circRNA-associated-ceRNA pairs involved in AD. In this study, we systematically explored the circRNA-associated-ceRNA mechanism in a 7-month-old senescence-accelerated mouse prone 8 (SAMP8) model brain through deep RNA sequencing. We obtained 235 significantly dysregulated circRNA transcripts, 30 significantly dysregulated miRNAs, and 1,202 significantly dysregulated mRNAs. We then constructed the most comprehensive circRNA-associated-ceRNA networks in SAMP8 brain. GO analysis revealed that these networks were involved in regulating the development of AD from various angles, for instance, axon terminus (GO: 0043679) and synapse (GO: 0045202). Following rigorous selection, we discovered that the circRNA-associated-ceRNA networks in this AD mouse model were mainly involved in the regulation of Aβ clearance (Hmgb2) and myelin function (Dio2). This research is the first to provide a systematic dissection of circRNA-associated-ceRNA profiling in SAMP8 mouse brain. The selected circRNA-associated-ceRNA networks can profoundly affect the diagnosis and therapy of AD in the future.
Keywords: circRNA-associated-ceRNA, SAMP8, Alzheimer’s disease
circRNA inhibits the function of miRNA as miRNA sponges through the ceRNA network. Zhang et al. explored mouse brain genome-wide circRNA-associated-ceRNA networks between 7-month-old SAMP8 and SAMR1 models through deep RNA-seq and listed two ceRNA (circRNA-associated-ceRNA)-related genes (Hmgb2 and Dio2) that were most likely involved in AD.
Introduction
Circular RNA (circRNA) has become a research focus in the field of non-coding RNA. By contrast to linear RNA, circRNA forms a covalently closed-loop structure without 5′-3′ polarity and poly(A) tail.1, 2 circRNA is more resistant to exonuclease than linear transcript and is therefore more stable in cells.3, 4 circRNA inhibits the function of microRNA (miRNA) as miRNA sponges through the competing endogenous RNA (ceRNA) network.5 The circRNA-associated-ceRNA network may play a key role in many disease processes. Huang et al.6 reported that circRNA MYLK competitively binds with miRNA-29a-3p and thus increases expression of the target genes DNMT3B, VEGFA, and ITGB1, which are involved in the progression of bladder cancer. Fan et al.7 summarized the function and role of circRNA as a biomarker in cardiovascular diseases. circRNA-ZNF609 functions as a ceRNA to regulate AKT3 expression by sponging miR-150-5p in Hirschsprung’s disease.8 As a new form of post-transcriptional control, this network has a great potential implication in disease research. In addition, growing evidence suggests that circRNAs are highly enriched in mammalian brain tissues and are often derived from the genes specific for neuronal function.9 Given these findings, exploring the role of the circRNA-associated-ceRNA network in neurodegenerative disorders such as Alzheimer’s disease (AD) is necessary.
AD is the most common form of dementia, characterized by age-dependent memory loss and impairments of multiple cognitive functions.10, 11, 12 Although many research efforts have tried to explain the origins of AD, developing an effective strategy in AD clinical therapy is difficult. However, the potential link between AD and circRNA-associated-ceRNA networks provides a new hint in combating this life-threatening disease. Lukiw13 reported that ciRS-7 acts as a competing endogenous miRNA sponge to inhibit miRNA-7 functions in AD-affected brain. Furthermore, Zhao et al.14 observed the network of ciRS-7-miRNA-7-UBE2A in sporadic AD (SAD) neocortex and hippocampal CA1. To date, only two cases have been reported in this field. Additional research and attention should be paid to this problem.
In the current study, we elucidated circRNA-associated-ceRNA networks in the brain of senescence-accelerated mouse prone 8 (SAMP8) at the 7-month-old stage using deep RNA sequencing (RNA-seq). SAMP8 has an age-related spontaneous deterioration in learning and memory abilities and is often treated as an important SAD model.15, 16 Senescence-accelerated mouse resistant 1 (SAMR1) is widely used as a control strain.17 Moreover, RNA-seq is an advanced approach that determines the differential expression profiles underlying phenotypic differences.18, 19 This study is the first to identify circRNA-associated-ceRNA networks in SAD model mice SAMP8, and our data will serve as useful resources for developing therapeutic targets or novel diagnostics in the future.
Results
Memory and Learning Ability Evaluation in the SAMP8 Mouse Model
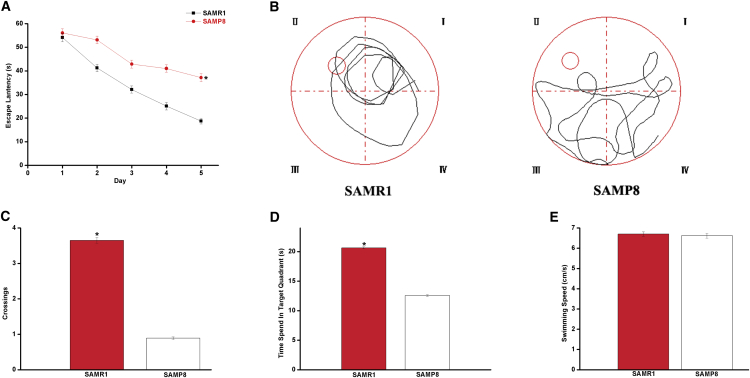
We used the Morris water maze (MWM) test to evaluate the learning and memory ability in SAMP8 and SAMR1 of 7-month-old mice. As shown in Figure 1A, the mean escape latency of SAMR1 mice significantly decreased compared with that of SAMP8 mice (p < 0.05). After the place navigation test, we used the spatial probe test to detect memory retention and spatial exploration ability. As shown in Figure 1B, the SAMP8 mice randomly swam in the tank without knowing the target location, whereas the SAMR1 mice preferentially searched for the target quadrant. Moreover, the number of crossings and the time spent in the target quadrant significantly decreased in the SAMP8 mice compared with those in the SAMR1 mice (p < 0.05, Figures 1C and 1D). We did not observe any significant difference in the swimming speed of the two groups (p > 0.05, Figure 1E), implying that the cognitive dysfunction of SAMP8 mice was not due to the motor and visual impairments. Compared with the SAMR1 mice of the same age, the 7-month-old SAMP8 mice exhibited severe cognitive impairments, which are the core clinical features observed in AD patients.
Figure 1.
Memory and Learning Ability Evaluation in the SAMP8 Mouse Model
We used the MWM test to evaluate the learning and memory ability in SAMP8 and SAMR1 of 7-month-old mice. (A) Mean escape latency in the hidden platform test. (B) Swimming paths in the probe trial test. (C) Number of crossings in the probe trial test. (D) Time spent in the target quadrant in the probe trial test. (E) Average swimming speeds of mice in the visible-platform test. The data were presented as the mean ± SEM; *p < 0.05.
Overview of circRNA-Seq
A total of 235,801,234 raw reads (111,956,771 for SAMP8 and 123,844,463 for SAMR1) were generated. We removed the ploy-N-containing, low-quality, and adapter-containing reads from the raw data. A total of 225,282,945 clean reads (118,620,626 for SAMR1 and 106,662,319 for SAMP8) were found in the two groups. The high-quality clean data were mapped to the mouse reference sequence by TopHat v.2.0.9,20 and the unmapped reads were subsequently selected. Based on the theory of find_circ software,3 34,096 circRNAs were detected. These circRNAs were used for the subsequent analysis.
Overview of miRNA-Seq
A total of 72,201,113 raw reads (35,207,345 for SAMP8 and 36,993,768 for SAMR1) were generated. After removal of low-quality and adapter sequences, 71,044,988 clean reads (36,446,427 for SAMR1 and 34,598,561 for SAMP8) were obtained. We filtered the preceding results based on length (18–35 nt). Most selected reads were 22 nt in length for both groups (Figures S1A and S1B). These filtered reads were mapped to the mouse reference sequence by Bowtie,21 and the mapping rates were approximately 93.67% and 93.51% for the SAMP8 and SAMR1 mice, respectively. These mapped tags were annotated and classified by aligning with non-coding small RNAs (sRNAs) (rRNA, tRNA, small nuclear RNA [snRNA], and small nucleolar RNA [snoRNA]) in GenBank, repeat-associated RNA, exon- and intron-associated RNAs, and miRBase v.20.0. The miREvo22 and miRDeep223 software were integrated to predict the novel miRNA. Finally, 1,298 matured miRNAs (1,226 known and 72 novel) were detected. These miRNAs were used for the subsequent analysis.
Overview of mRNA-Seq
A total of 619,062,690 raw reads (251,783,324 for SAMP8 and 367,279,366 for SAMR1) were generated. After discarding the reads with adapters, poly-N > 10%, and any other possible contaminants, 599,985,802 clean reads (244,770,474 for SAMP8 and 355,215,328 for SAMR1) were obtained. The clean reads were mapped to the mouse reference genome, and the mapping rates were approximately 85.73% and 83.55% for the SAMP8 and SAMR1 mice, respectively. The cufflink results indicated that 74,885 protein-coding transcripts were identified. These mRNAs were used for the subsequent analysis.
Differential Expression Analysis: SAMP8 versus SAMR1
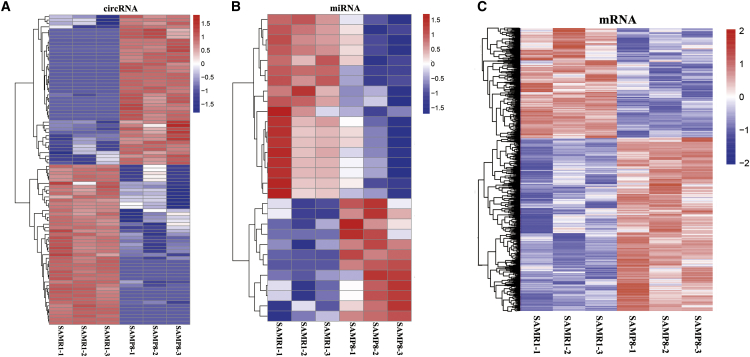
In transcripts per million (TPM), we first identified 235 significantly dysregulated circRNA transcripts between the two groups (p < 0.01, Table S2), with 94 upregulated and 141 downregulated transcripts in SAMP8 mice relative to those in SAMR1 mice. Cluster analysis of differentially expressed circRNAs was conducted with a heatmap (Figure 2A). Thirty significantly dysregulated miRNAs were also detected between the two groups based on TPM (p < 0.01, Table S3). Twelve dysregulated miRNAs were upregulated in the SAMP8 mice, whereas 18 were upregulated in the SAMR1 mice. Cluster analysis of differentially expressed miRNAs was revealed by a heatmap (Figure 2B). We used fragments per kilobase of exons per million fragments mapped (FPKM) to estimate the expression level of the mRNA transcripts. A total of 1,202 significantly dysregulated mRNA transcripts were identified: 716 were upregulated and 486 were downregulated in SAMP8 mice (p < 0.01, Table S4). The cluster analysis of differentially expressed mRNAs is shown in Figure 2C.
Figure 2.
Cluster Analysis Using a Heatmap
(A) Cluster analysis of differentially expressed circRNAs. (B) Cluster analysis of differentially expressed miRNAs. (C) Cluster analysis of differentially expressed mRNAs. Red indicates increased expression, and blue indicates decreased expression.
qPCR Confirmation
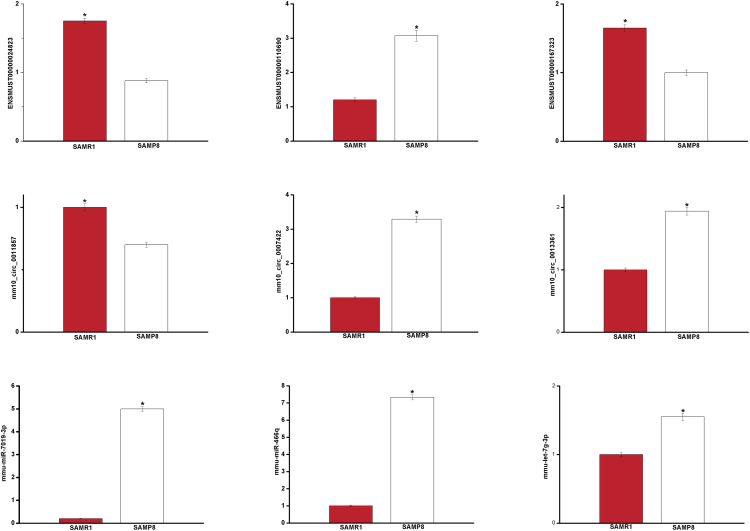
We sought to confirm the differential expression identified in our RNA-seq experiments using qPCR. Nine differentially expressed transcripts were randomly selected: three circRNAs, three miRNAs, and three mRNAs. As shown in Figure 3, all selected transcripts were detected in SAMP8 and SAMR1 brains and exhibited significant differential expressions. In summary, a high level of consistency was observed between the qPCR results and the RNA-seq data.
Figure 3.
Validation of Transcript Expression by qPCR between SAMP8 and SAMR1 Mice
Mouse β-actin and U6 genes were used as housekeeping internal control. Transcript expression was quantified relative to the expression level of β-actin using the comparative cycle threshold (ΔCT) method. The data were presented as the mean ± SEM (n = 3); *p < 0.05.
Construction of a circRNA-Associated-ceRNA Network
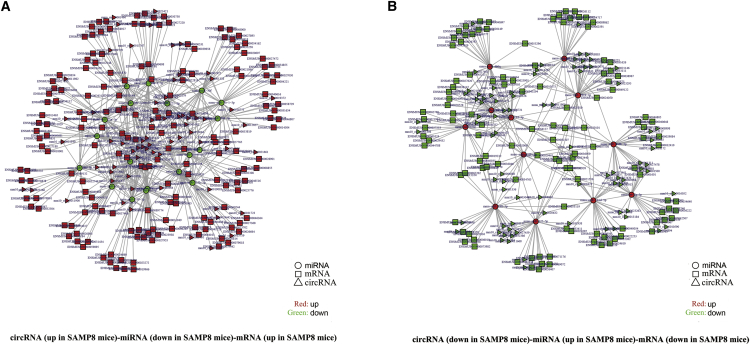
According to ceRNA hypothesis, RNA transcripts effectively communicate with one another. The members of ceRNA compete for the same miRNA response elements (MREs) to regulate one another. In this study, we pioneered the identification of a ceRNA network in the SAMP8 brain through our RNA-seq data. We selected 235 circRNAs and 1,202 mRNAs that were differentially expressed and shared a common binding site of MRE (30 significantly dysregulated miRNAs). The ceRNA network covered two cases (Figures 4A and 4B): one was circRNA (up in SAMP8 mice)-miRNA (down in SAMP8 mice)-mRNA (up in SAMP8 mice), and the other one was circRNA (down in SAMP8 mice)-miRNA (up in SAMP8 mice)-mRNA (down in SAMP8 mice). Additional details are listed in Tables S5 and S6. These RNA interactions may supply a novel perspective for the pathogenesis of AD.
Figure 4.
circRNA-Associated-ceRNA Networks in SAMP8 Mice
The ceRNA networks were based on circRNA-miRNA and miRNA-mRNA interactions. (A) circRNA (up in SAMP8 mice)-miRNA (down in SAMP8 mice)-mRNA (up in SAMP8 mice). (B) circRNA (down in SAMP8 mice)-miRNA (up in SAMP8 mice)-mRNA (down in SAMP8 mice).
Functional Annotation: GO
A circRNA-associated-ceRNA network executes functions that are embodied in related mRNA genes. Gene Ontology (GO) analyses were performed on these genes. Through the GO survey, 159 GO terms were significantly enriched (corrected p < 0.05, Table S7). The top three terms were intracellular (GO: 0005622), intracellular part (GO: 0044424), and metabolic process (GO: 0008152). Several cognition-associated terms were also observed, such as axon terminus (GO: 0043679), synapse (GO: 0045202), neuron projection terminus (GO: 0044306), axon part (GO: 0033267), and long-term synaptic depression (GO: 0060292). In summary, the circRNA-associated-ceRNA network participates in the pathological progress of AD from different angles.
Association Study
In this study, three limiting factors were established to further understand the most possible relationship between circRNA-associated-ceRNA networks and AD. First, we selectively analyzed pairs in which the circRNAs, miRNAs, and their target genes should be significantly and differentially expressed between the SAMP8 and the SAMR1 mice (corrected p < 0.05). Second, the concentrations of the selected circRNAs, miRNAs, and their target genes in the mouse brain should be in a certain order of magnitude. Lastly, the selected pairs should be associated with AD. The fulfilled pairs were detected according to the three preceding requirements. For example, mm10_circ_0027491, mmu_circ_0001293, mm10_circ_0027459, mmu_circ_0000967, and mm10_circ_0027483 were ceRNA of mmu-miR-122-5p targeting Dio2. The expression of Dio2 in AD patients was lower than that in non-AD people.24 Dio2 activates the thyroid hormone by converting the pro-hormone thyroxine (T4) to bioactive 3,3′,5-triiodothyronine (T3).25 An increase in thyroxine activates myelination.26 Myelin sheath loss in AD is a marked morphological component of the disease.27 Additional results are listed in Table 1. Therefore, we predicted that the previously mentioned circRNA-associated-ceRNA networks are possibly involved in the adjustment of AD.
Table 1.
circRNA-Associated-ceRNA Networks that Are Most Likely Involved in AD Pathogenesis
| circRNA | Log2 Fold Change (SAMP8 versus SAMR1) | Corrected p Value | miRNA | Log2 Fold Change (SAMP8 versus SAMR1) | Corrected p Value | transcript_id | gene_id | gene_name | Log2 Fold Change (SAMP8 versus SAMR1) | Corrected p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| mm10_circ_0027491 | −2.84 | 3.83E-08 | mmu-miR-122-5p | 1.18 | 0.045558 | ENSMUST00000082432 | ENSMUSG00000007682 | Dio2 | −0.37 | 0.0227923 |
| mmu_circ_0001293 | −7.23 | 8.74E-06 | ||||||||
| mm10_circ_0027459 | −2.21 | 0.004122 | ||||||||
| mmu_circ_0000967 | −1.65 | 0.0064198 | ||||||||
| mm10_circ_0027483 | −3.36 | 0.0073264 | ||||||||
| mm10_circ_0027470 | −6.46 | 0.0003266 | mmu-let-7g-3p | 0.86 | 0.0166 | ENSMUST00000067925 | ENSMUSG00000054717 | Hmgb2 | −0.64 | 0.045214 |
| mm10_circ_0011311 | −3.03 | 0.0068371 | ||||||||
| mm10_circ_0018430 | −3.65 | 0.038051 |
To make the fold change values easier to work with, a log2 transformation is applied.
Discussion
AD is a chronic neurodegenerative disease that usually starts slowly and worsens over time.28 This illness brings huge pain and inconvenience to the patients, including problems with language, behavioral issues, loss of motivation, inability for self-care, and heavy family burdens. However, few therapeutic options are available to prevent or reverse AD because of its complex pathogenesis. The traditional treatment of AD may not be useful in warding off this illness. For example, the protocols targeting the main neuropathological hallmarks in AD (β-amyloid [Aβ] and neurofibrillary tangles) have been unsuccessful.29 Many studies on AD focused on the epigenetic regulation of its pathogenesis and the potential targets for therapy. The dynamic changes of DNA methylation and long non-coding RNAs (lncRNAs) in brain contribute to AD, as reported by our previous studies.30, 31 However, the occurrence of circRNAs in AD remains largely unknown. The studies of Lukiw13 and Zhao et al.14 are the only explorations in this respect. The first evidence about the existence of circRNAs was declared in 197632 and was long dismissed as low abundance splicing errors with no function. RNA-seq,18, 19 an up-to-date technology, propels circRNA into the spotlight as a popular topic in the RNA field. Research has shown that the most important function of circRNA is to act as miRNA sponges. circRNAs harbor MREs that can compete with mRNAs for the limited pool of cellular miRNAs and thus affect the level of competing RNAs.5 circRNAs are more enriched in mammalian brain tissues,9 implying their important roles in neurodegenerative diseases. In our study, we used RNA-seq to systematically analyze circRNA, miRNA, and mRNA profiles in the 7-month-old SAMP8 mice brain. The 7-month-old SAMP8 mice exhibited the cognitive, behavioral, and neuropathological alterations observed in aged humans and thus are an appropriate animal model to understand the pathogenic mechanisms of AD, especially SAD.15, 16 Our MWM experiment confirmed this result. By contrast, the transgenic mouse models of AD, such as 3xTg-AD (APP/PS1/Tau) and 2xTg-AD (APP/PS1), may simply represent uncommon familial AD (FAD). The circRNA-associated-ceRNA networks were then constructed based on the analysis of the relationship. According to the results, these selected circRNA-associated-ceRNA networks may offer hope of new cues for AD.
Previous research indicated that AD is a multifactorial disease, and many theories, including Aβ deposition, oxidative stress, tau neuropathology, synapse injury, mitochondrial dysfunction, neuron loss, and immune system dysfunction tried to explain the origin of this illness.33 Many genes are closely related with these theories. Thus, we first explained the significant difference in the gene expression between SAMP8 and SAMR1 brains. A total of 1,202 significantly dysregulated mRNA transcripts were identified. These mRNA transcripts are related to AD pathogenesis. For instance, the Cd274 gene participates in the immunological process.34 The Cd74 gene suppresses Aβ deposition.35 In the next two sections, we discussed differential circRNA and miRNA expressions between SAMP8 and SAMR1 mice. According to the statistics, 235 significantly dysregulated circRNA transcripts and 30 significantly dysregulated miRNAs were detected. circRNA and miRNA molecules may be key regulators of diverse AD course. circRNA and protein-coding mRNA function as ceRNAs and super-sponges to regulate the expression of mRNA. Based on this theory, we constructed the most comprehensive miRNA-circRNA and protein-coding mRNA interaction networks of SAMP8 and SAMR1 mice brain. GO analyses revealed that the pathological process of AD may be regulated by these networks from different aspects, including synapse (GO: 0045202), neuron projection terminus (GO: 0044306), axon part (GO: 0033267), and long-term synaptic depression (GO: 0060292).
Strict limitative requirements were applied to select the most possible circRNA-associated-ceRNA networks that are involved in the occurrence and development of AD. Ultimately, two eligible pairs were identified. Earlier in the article, we discussed a network that participates in the control of the Dio2 gene.24, 25, 26, 27 Another one is the Hmgb2 gene. mm10_circ_0027470, mm10_circ_0011311, mm10_circ_0018430, mm10_circ_0009478, mm10_circ_0010326, and mmu_circ_0001442 were ceRNAs of mmu-let-7g-3p targeting Hmgb2. The Hmgb2 gene encoded a member of the non-histone chromosomal high-mobility group protein family.36 Yamanaka et al.37 found that Hmgb2 contributes to the expression of the Lrp1 gene. Lrp1 is implicated in the systemic clearance of Aβ, and the level of Lrp1 expression is critical for AD progression.38 The circRNA-associated-ceRNA networks in AD are highly complex and diverse. Our ongoing efforts will provide more fundamental information for understanding this regulatory mechanism in AD. This endeavor will be an enormous challenge in the future.
In addition, we sought to confirm the differential expressions of the randomly selected transcripts (three circRNAs, three miRNAs, and three mRNAs) identified in RNA-seq experiments using qPCR. The real-time qPCR data agreed with the RNA-seq assay. All of these results confirm that our resultant transcripts were of high quality.
We elucidated the brain circRNA-associated-ceRNA profiles of SAMP8 and SAMR1 mice using deep RNA-seq analysis. Our findings further expanded our knowledge on ceRNA biology and contributed to the understanding of their regulation roles in AD pathogenesis. These novel networks are potential biomarkers or therapeutic targets in AD. Regardless, this strategy should provide a valuable resource for the clinical diagnosis, treatment, and prevention of AD.
Materials and Methods
Preparation of Tissues
Three-month-old SAMP8 mice (male, n = 45) and SAMR1 mice (male, n = 45) were purchased from WTLH Biotechnology. The mice were housed one per cage with standard specific conditions (25°C, 50% humidity, 12 hr light/dark cycle, and pathogen free). The mice were provided free diet for 4 months (until they were 7 months old). Three randomly selected animals from each group were administered general anesthesia. We collected their cerebral cortex for RNA-seq. In addition, we randomly selected 12 animals from each group for the MWM test.
All animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals39 and were approved by the Institutional Animal Use and Care Committee of Beijing Normal University.
MWM Test
Spatial learning and memory were evaluated through the MWM test according to standard procedures.40 One day before the experiment, the mice were familiarized with the MWM environment in the morning and afternoon. In the place navigation test (days 1 to 6), a platform was placed in a set quadrant and submerged 1 cm below the water level. The mice were placed in the maze at four assigned points (southeast, northeast, southwest, and northwest) and allowed to swim for 90 s in every training session. Escape latency was designated as the length of time needed by the mouse to reach the platform. When a mouse failed to reach the platform within 90 s, we guide it toward the platform, and the escape latency was recorded as 90 s. In both cases, the mice were allowed to rest on the platform for 15 s and subsequently placed in the home cage. The mice were subjected to two trials per day for 6 consecutive days. In the spatial probe test (day 7), the platform was withdrawn. The mice were released from the opposite quadrant and allowed to swim freely for 60 s. The swimming trajectory and the number of crossings of the platform within 1 min were recorded. The visible platform test was performed on the day following the spatial probe test (day 8). We raised the platform above the water surface, and each mouse was subjected to four trials. All experiments were conducted at the same time each day. Furthermore, the investigator was unaware of the mouse genotypes until all tests were completed.
RNA Extraction and Qualification
In this study, the total RNA of each sample was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Briefly, 1% agarose gels were used to monitor RNA degradation and contamination. RNA purity was checked using the NanoPhotometer spectrophotometer (Implen). RNA concentration was measured using the Qubit RNA Assay Kit in the Qubit 2.0 Fluorometer (Life Technologies). RNA integrity was evaluated by the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 System (Agilent Technologies).
RNA-Seq
The details of the method for mRNA-seq were previously described.41 For miRNA-seq and circRNA-seq, the detailed methods are described in Supplemental Materials and Methods.
Expression Analysis
First, we calculated the FPKM of the transcripts using Cuffdiff (v.2.1.1) to evaluate the expression level of protein-coding genes in each sample.42 The expression levels of miRNAs and circRNAs were estimated by TPM through the following criteria.43 Transcripts with p value < 0.01 were described as differentially expressed between SAMP8 and SAMR1:
ceRNA Network Analysis
The expression levels of circRNAs, miRNAs, and mRNAs showed significant difference between SAMP8 and SAMR1 and thus were analyzed. The potential MREs were searched on the sequences of circRNAs and mRNAs. We used miRanda (http://www.microrna.org/microrna/) to predict miRNA binding seed sequence sites, and overlapping of the same miRNA binding site on both circRNAs and mRNA represented circRNA-miRNA-mRNA interaction.
GO Enrichment Analyses
The GO database was used to analyze the circRNA-miRNA-enriched genes. GO analysis was performed by the GOseq R package.44 GO terms with corrected p < 0.05 were considered significantly enriched.
Real-Time qPCR Validation
The real-time qPCR reaction was performed using the SYBR Green assay (Cat. No. GMRS-001, GenePharma) in a Light Cycler 480 real-time PCR system (Roche Applied Science) with the following conditions: 95°C for 3 min, followed by 40 cycles of 95°C for 12 s and 62°C for 40 s. Each reaction involved 7.44 μL of H2O, 0.4 μL of Taq DNA polymerase, 0.08 μL of each primer, 2 μL of cDNA, and 10 μL of 2× RealMasterMix (GenePharma). The specific quantitative primers were designed using oligo7 software and are listed in Table S1. The primers of β-actin (for circRNA and mRNA) and U6 (for miRNA) were designed as an endogenous control. Each experiment was performed in triplicate.
Statistical Analysis
Statistical analyses were performed with SPSS 20.0 software. All data were expressed as the mean ± SEM. p < 0.05 was statistically significant. The escape latency in the MWM test was compared through two-way ANOVA. Student’s t test was used to compare the qPCR results and the remaining data of the MWM test.
Author Contributions
S.Z. and W.Z. designed the experiments. S.Z. and D.Z. performed the experiments. S.Z. analyzed the data and wrote the paper. S.Z., D.Z., Hong Li, Hejian Li, C.F., and W.Z. contributed reagents, materials, and analysis tools.
Conflicts of Interest
We declared that no competing interests exist.
Acknowledgments
We thank all contributors to this study. This work was supported by the Beijing Joint Project for the Central-Affiliated University (2017-01), the Key New Drug Creation and Development Program of China (2012ZX09103-201), the Fundamental Research Funds for Central Universities (2015KJJCA05), and a grant from the National Natural Science Foundation of China (81274118).
Footnotes
Supplemental Information includes Supplemental Materials and Methods, one figure, and seven tables and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.06.009.
Accession Numbers
The accession number for the mRNA-seq, miRNA-seq, and circRNA-seq data reported in this paper are SRA: SRP096779, SRP107885, and SRP107902.
Supplemental Information
References
- 1.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L.L., Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 4.Salzman J., Chen R.E., Olsen M.N., Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 6.Huang M., Zhong Z., Lv M., Shu J., Tian Q., Chen J. Comprehensive analysis of differentially expressed profiles of lncRNAs and circRNAs with associated co-expression and ceRNA networks in bladder carcinoma. Oncotarget. 2016;7:47186–47200. doi: 10.18632/oncotarget.9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan X., Weng X., Zhao Y., Chen W., Gan T., Xu D. Circular RNAs in cardiovascular disease: an overview. BioMed Res. Int. 2017;2017:5135781. doi: 10.1155/2017/5135781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng L., Chen G., Zhu Z., Shen Z., Du C., Zang R., Su Y., Xie H., Li H., Xu X. Circular RNA ZNF609 functions as a competitive endogenous RNA to regulate AKT3 expression by sponging miR-150-5p in Hirschsprung’s disease. Oncotarget. 2017;8:808–818. doi: 10.18632/oncotarget.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You X., Vlatkovic I., Babic A., Will T., Epstein I., Tushev G., Akbalik G., Wang M., Glock C., Quedenau C. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 2015;18:603–610. doi: 10.1038/nn.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H., Del Trecidi K. Neuroanatomy and pathology of sporadic Alzheimer’s disease. Adv. Anat. Embryol. Cell Biol. 2015;215:1–162. [PubMed] [Google Scholar]
- 11.Roy D.S., Arons A., Mitchell T.I., Pignatelli M., Ryan T.J., Tonegawa S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531:508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns A., Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 13.Lukiw W.J. Circular RNA (circRNA) in Alzheimer’s disease (AD) Front. Genet. 2013;4:307. doi: 10.3389/fgene.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y., Alexandrov P.N., Jaber V., Lukiw W.J. Deficiency in the ubiquitin conjugating enzyme UBE2A in Alzheimer’s disease (AD) is linked to deficits in a natural circular miRNA-7 sponge (circRNA; ciRS-7) Genes (Basel) 2016;7:116. doi: 10.3390/genes7120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang L., Li S., Xing Z., Li J., Su Y., Fan P., Wang L., Cui H. Dihydrotestosterone treatment delays the conversion from mild cognitive impairment to Alzheimer’s disease in SAMP8 mice. Horm. Behav. 2014;65:505–515. doi: 10.1016/j.yhbeh.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem. Res. 2009;34:639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- 17.Castillo C.A., Albasanz J.L., León D., Jordán J., Pallàs M., Camins A., Martín M. Age-related expression of adenosine receptors in brain from the senescence-accelerated mouse. Exp. Gerontol. 2009;44:453–461. doi: 10.1016/j.exger.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Gerstein M., Snyder M. RNA-seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marguerat S., Bähler J. RNA-seq: from technology to biology. Cell. Mol. Life Sci. 2010;67:569–579. doi: 10.1007/s00018-009-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen M., Shen Y., Shi S., Tang T. miREvo: an integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinformatics. 2012;13:140. doi: 10.1186/1471-2105-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedländer M.R., Mackowiak S.D., Li N., Chen W., Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphries C.E., Kohli M.A., Nathanson L., Whitehead P., Beecham G., Martin E., Mash D.C., Pericak-Vance M.A., Gilbert J. Integrated whole transcriptome and DNA methylation analysis identifies gene networks specific to late-onset Alzheimer’s disease. J. Alzheimers Dis. 2015;44:977–987. doi: 10.3233/JAD-141989. [DOI] [PubMed] [Google Scholar]
- 25.Buettner C., Harney J.W., Larsen P.R. The role of selenocysteine 133 in catalysis by the human type 2 iodothyronine deiodinase. Endocrinology. 2000;141:4606–4612. doi: 10.1210/endo.141.12.7831. [DOI] [PubMed] [Google Scholar]
- 26.Calza L., Fernandez M., Giuliani A., Aloe L., Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2002;99:3258–3263. doi: 10.1073/pnas.052704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan X., Jickling G.C., Ander B.P., Liu D., Stamova B., Cox C., Jin L.W., DeCarli C., Sharp F.R. Myelin injury and degraded myelin vesicles in Alzheimer’s disease. Curr. Alzheimer Res. 2014;11:232–238. doi: 10.2174/1567205011666140131120922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 29.Selkoe D.J. Resolving controversies on the path to Alzheimer’s therapeutics. Nat. Med. 2011;17:1060–1065. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S., Qin C., Cao G., Guo L., Feng C., Zhang W. Genome-wide analysis of DNA methylation profiles in a senescence-accelerated mouse prone 8 brain using whole-genome bisulfite sequencing. Bioinformatics. 2017;33:1591–1595. doi: 10.1093/bioinformatics/btx040. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S., Qin C., Cao G., Xin W., Feng C., Zhang W. Systematic analysis of long noncoding RNAs in the senescence-accelerated mouse prone 8 brain using RNA sequencing. Mol. Ther. Nucleic Acids. 2016;5:e343. doi: 10.1038/mtna.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanger H.L., Klotz G., Riesner D., Gross H.J., Kleinschmidt A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong R.A. What causes Alzheimer’s disease? Folia Neuropathol. 2013;51:169–188. doi: 10.5114/fn.2013.37702. [DOI] [PubMed] [Google Scholar]
- 34.Saresella M., Calabrese E., Marventano I., Piancone F., Gatti A., Farina E., Alberoni M., Clerici M. A potential role for the PD1/PD-L1 pathway in the neuroinflammation of Alzheimer’s disease. Neurobiol. Aging. 2012;33:624.e11–624.e22. doi: 10.1016/j.neurobiolaging.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda S., Matsuda Y., D’Adamio L. CD74 interacts with APP and suppresses the production of Abeta. Mol. Neurodegener. 2009;4:41. doi: 10.1186/1750-1326-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murugesapillai D., McCauley M.J., Maher L.J., 3rd, Williams M.C. Single-molecule studies of high-mobility group B architectural DNA bending proteins. Biophys. Rev. 2016;9:17–40. doi: 10.1007/s12551-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanaka Y., Faghihi M.A., Magistri M., Alvarez-Garcia O., Lotz M., Wahlestedt C. Antisense RNA controls LRP1 Sense transcript expression through interaction with a chromatin-associated protein, HMGB2. Cell Rep. 2015;11:967–976. doi: 10.1016/j.celrep.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martiskainen H., Haapasalo A., Kurkinen K.M., Pihlajamäki J., Soininen H., Hiltunen M. Targeting ApoE4/ApoE receptor LRP1 in Alzheimer’s disease. Expert Opin. Ther. Targets. 2013;17:781–794. doi: 10.1517/14728222.2013.789862. [DOI] [PubMed] [Google Scholar]
- 39.Clark J.D., Gebhart G.F., Gonder J.C., Keeling M.E., Kohn D.F. Special report: the 1996 guide for the care and use of laboratory animals. ILAR J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- 40.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S., Zhu D., Li H., Zhang H., Feng C., Zhang W. Analyses of mRNA profiling through RNA sequencing on a SAMP8 mouse model in response to ginsenoside Rg1 and Rb1 treatment. Front. Pharmacol. 2017;8:88. doi: 10.3389/fphar.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L., Chen J., Li Z., Li X., Hu X., Huang Y., Zhao X., Liang C., Wang Y., Sun L. Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS ONE. 2010;5:e15224. doi: 10.1371/journal.pone.0015224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young M.D., Wakefield M.J., Smyth G.K., Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.